Abstract
The decline in cardiac contractility due to damage or loss of cardiomyocytes is intensified by changes in the extracellular matrix leading to heart remodeling. An excessive matrix response in the ischemic cardiomyopathy may contribute to the elevated fibrotic compartment and diastolic dysfunction. Fibroproliferation is a defense response aimed at quickly closing the damaged area and maintaining tissue integrity. Balance in this process is of paramount importance, as the reduced post-infarction response causes scar thinning and more pronounced left ventricular remodeling, while excessive fibrosis leads to impairment of heart function. Under normal conditions, migration of progenitor cells to the lesion site occurs. These cells have the potential to differentiate into myocytes in vitro, but the changed micro-environment in the heart after infarction does not allow such differentiation. Stem cell transplantation affects the extracellular matrix remodeling and thus may facilitate the improvement of left ventricular function. Studies show that mesenchymal stem cell therapy after infarct reduces fibrosis. However, the authors did not specify whether they meant the reduction of scarring as a result of regeneration or changes in the matrix. Research is also necessary to rule out long-term negative effects of post-acute infarct stem cell therapy.
1. Introduction
Ischemic heart disease is one of the most common causes of death worldwide [1]. Its most dramatic and dangerous manifestation is myocardial infarction, commonly known asa heart attack. In 2018, cardiological organizations (European Society of Cardiology [ESC], American College of Cardiology Foundation [ACCF], American Heart Association [AHA], World Heart Federation [WHF]), based on objective markers of myocardial necrosis, agreed on the 4th universal definition of myocardial infarction. The essence of the diagnosis was the occurrence of acute myocardial damage and demonstration of its ischemic basis [2]. Left ventricular systolic dysfunction is the most common, but not the only, cause of post-infarction heart failure. Primary decline in contractility due to damage or loss of cardiomyocytes is supported and enhanced by changes in the heart’s extracellular matrix (ECM), leading to heart remodeling [3,4]. The term ‘matrix’ itself refers to the tissue component and more precisely to the substance produced by the cells and filling the space between them. An excessive ECM response in the case of ischemic cardiomyopathy may contribute to an elevated fibrotic compartment and finally diastolic dysfunction [5,6].
The objective of the literature review was to determine whether treatment with mesenchymal stem cells after myocardial infarction has a negative, neutral or beneficial effect on diastolic function and regulation of the extracellular matrix.
1.1. Mechanisms Underlying Diastolic Dysfunction after MI
Increased fibrosis was observed in both the infarct zone (IZ) and the non-infarct zone (NIZ). In the infarct zone, successive reparative fibrosis (also called corrective, replacement fibrosis) was observed [5,7]. In the process, connective tissue fills the gaps after dead myocytes and is limited to one area of the tissue. In the non-infarct zone, there has been observed reactive, disseminated hypertrophy, connected with interstitial fibrosis. This process causes excessive and diffuse accumulation of connective tissue in interstitial and perivascular spaces [3,8]. During the reactive fibrosis, no loss of myocytes is observed, and the scope of the remodeling process comprises the perivascular space and interstitial tissue. This type of fibrosis occurs more frequently in patients with heart failure caused by hypertension, hypertrophic cardiomyopathy or aortic stenosis than after a myocardial infarction [9].
Fibroproliferation is a tissue defense aimed at quickly closing the damaged area and maintaining the integrity of the heart. Balance in this process is of paramount importance. The reduced post-infarction response causes scar thinning and more pronounced left ventricular remodeling, while excessive fibrosis (as mentioned above) leads to impairment of heart function. The process of excessive fibrosis results from increased collagen synthesis, which outweighs unchanged or reduced degradation [3,10,11]. Fibroblasts and myofibroblasts separate extracellular procollagen chains that connect with each other and cross-link to form collagen fibrils [5,12]. Two types of collagen dominate the heart: 80% type I collagen, which makes the heart muscle resistant to deformation due to the thickness of the fibers, and type III collagen (11%), whose thin fibers give the heart elasticity. The remaining forms of collagen account for 9% of its total amount [5,13,14]. Collagen cross-linking increases myocardial tensile strength and collagen fibers’ resistance to degradation by matrix metalloproteinases [3,15,16]. The pathological change in the collagen matrix is caused by a disorder of homeostasis of pro- and anti-fibrotic factors (cytokines, chemokines, growth factors, proteases, hormones, reactive forms of oxygen) [17]. Imbalance in collagen metabolism occurs in myofibroblasts, which are formed from fibroblasts as a result of the expression of alpha-smooth muscle actin (characteristic of smooth muscle cells), as well as the appearance of an extensive, synthetically active reticuloendoplasmic area [3,4,18]. Myocardial infarction causes successive changes in connective tissue, the pathophysiologically of which can be divided into four stages. Pathophysiological stages of the healing process after a myocardial infarction are presented in Figure 1.
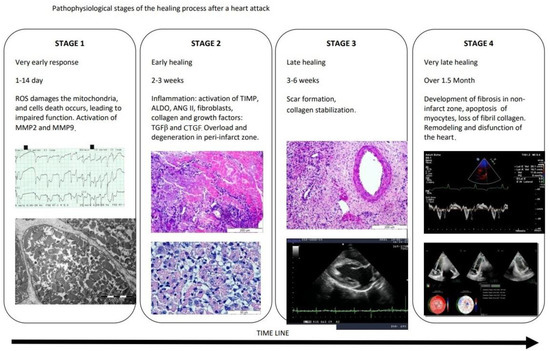
Figure 1.
Pathophysiological stages of the healing process after a myocardial infarction. ROS—reactive oxygen species, MMP2—metalloproteinase-2, MMP9—metalloproteinase-9, TIMP—tissue inhibitors of matrix metalloproteinase, ALDO—aldosterone, ANGII—angiotensin II, TGF-β—transforming growth factor beta, CTGF—connective tissue growth factor.
Cardiac insult rapidly activates the matrix metalloproteinases (MMPs), leading to ECM fragmentation. These fragments act as bioactive pro-inflammatory matrikines [19]. In the very early, acute phase of the evolution of myocardial infarction (which covers 1 day), an increase in ECM-degrading proteases–matrix metalloproteinases (MMPs)–was observed. The increased activity of metalloproteinases, including MMP-2 and MMP-9, is preceded by an increase in transcription of MMP genes into pro-MMP, which is stimulated by IL-1, platelet-derived growth factor (PDGF) and TNF-α. This process is inhibited by TGF-β, retinoids, heparin and corticosteroids [5,20]. It is worth emphasizing the role of CD68+ macrophages, which, under ischemic conditions, enhance fibrosis and angiogenesis, and produce MMPs [21]. Macrophages also remove dead cells and ECM debris (in the first, inflammatory phase of infarction healing) and induce the release of anti-inflammatory mediators and proliferative factors, thus allowing the transition to the proliferative phase [22]. After 2 weeks, there are increased levels of tissue (endogenous) MMP inhibitors (TIMP), profibrotic aldosterone and angiotensin II, collagen, fibroblasts and growth factors (TGF beta and CTGF). The time frames observed for these events are well established in the mouse model, but studies in dog models show differences, such as a prolonged cell infiltration period and slower granulation formation after MI [23]. Late healing follows a period of relative collagen stabilization and leads to scar formation after about 3–6 weeks. This final, maturation phase varies in response to loading stimuli, including metabolic dysfunction, pressure load, genetic factors and aging [24].In this phase, the fibroblasts become quiescent, and the ECM collagen is cross-linked [25].The anti-inflammatory suppression of inflammation in the infarction zone does not apply to non-infarct segments where increased wall loading can locally activate cardiomyocytes, macrophages and fibroblasts, elevating MMPs and triggering chronic remodeling of the ECM [26].Earlier studies showing the association of MMPs (MMP2, MMP9, MMP14) with the development of post-infarction cardiomyopathy suggested that MMPs are responsible for this phenomenon [27].However, subsequent observations indicated that myocardial segments displaying decreased ECM activity did not regain function after revascularization [28].Thus, it seems that activation of the interstitial environment, enrichment of the number of growth factors, cells and matrix proteins may be necessary for regeneration of ischemic areas of the myocardium; however, actions dependent on ECM and fibroblasts may not be sufficient to activate the regeneration program [24].
Fibroproliferation in the infarct zone ispresented in Figure 2.
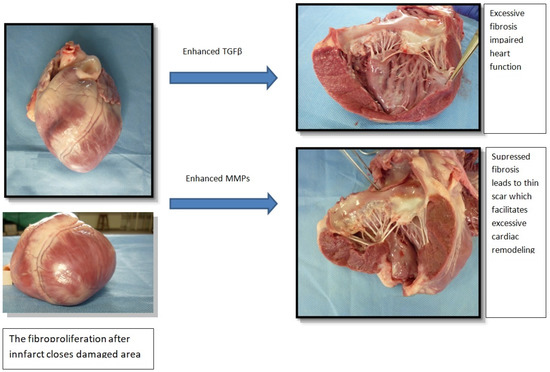
Figure 2.
Fibroproliferation in the infarct zone. TGF-β—transforming growth factor beta, MMP—metalloproteinase.
In the last stage, 1.5 months and later, fibrosis also develops in the NIZ zone [5,29]. In the following weeks, ECM degradation in the non-infarct zone (NIZ), loss of fibril collagen, and loss of myocytes due to apoptosis contribute to progressive global expansion and dysfunction of the left ventricle [5,29,30]. Notably, the infarct zone may widen as border zone (BZ) cells also contain cells at risk of death. This was proven by the increased level of T17-PLB phosphorylation and caspase-3 activation [31]. Long-term stimulation with pro-inflammatory cytokines results in the formation of diffuse small inflammation, scars and the transition to further remodeling processes. Collectively, data show that the ECM plays an important role in these processes [5,6,32]. Evidence suggests that some patients with preserved systolic function and diastolic dysfunction have normal collagen volume fractions despite myocardial stiffness, comparable to those with increased collagen volume fractions. In these patients, fibrosis coexists with comorbidities (e.g., diabetes) that affect the disease process [33]. Clinical and physiological features of diastolic dysfunction are altered after MI. Different pathophysiological responses in the IZ, BZ and NIZ result in a different clinical picture. Moreover, this picture has changed in recent years, as more and more people survived the myocardial infarction. They develop post-infarction heart failure in consequence of modern intervention procedures [34]. The gold standard in the diagnosis and assessment of cardiac fibrosis is the histopathological evaluation of myocardial biopsy samples. However, less invasive methods are constantly being sought. A number of circulating biomarkers have been proposed and analyzed, to observe myocardial injury, natriuretic peptides (ANP, BNP, NT-proBNP), troponin, and to analyze fibrinolytic activity: metalloproteinase-1 (MMP-1) and growth factors, e.g., transforming growth factor beta 1 (TGF-β1), promoting transactivation of myofibroblasts and ECM synthesis [3,35].
1.2. Diagnosis of Diastolic Dysfunction
Ultrasonography is a very helpful diagnostic tool. It enables the monitoring of morphological and functional changes of the heart muscle, along with the assessment of the regional systolic and diastolic functions of the left ventricle. Assessment of left ventricular diastolic dysfunction (LVDD), according to the guidelines of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI), is mainly based on six indicators: E-wave, E/A ratio, septal or lateral e′, mean E/e′, left atrial index volume (LAVI) and peak tricuspid velocity (TRpV) [36,37]. These indicators are presented in Figure 3.
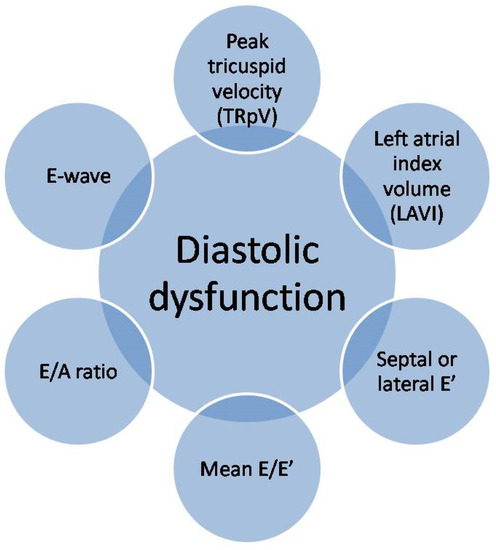
Figure 3.
Echocardiographic assessment of left ventricular diastolic dysfunction.
However, other means, such as cardiac MRI and cardiac catheterization, can be more accurate in terms of quantitative assessment and accurate diagnosis of diastolic dysfunction and assessment of cardiac fibrosis. The diastolic function is closely related to the heart rate and rhythm, atrial function (mainly systolic), ventricular compliance, preload and atrioventricular valve function. Diastolic dysfunction is therefore a reflection of impaired left ventricular relaxation, resulting from its increased stiffness (advanced stages) and increased filling pressure (even more advanced stages) [36,38].
1.3. Stem Cell Therapy
The development of regenerative medicine and stem cell treatment of coronary heart disease has become a matter of interest for many scientists. It has been observed that, after myocardial infarction, the number of circulating endothelial progenitors CD34+ and CD133+ KDR and mesenchymal cells increases [39]. It has been demonstrated that, under normal conditions, the migration of progenitor cells to the lesion site and differentiation into heart cells occur, provided that the injury is minor [17,40,41]. In 2015, it was emphasized that in optimal conditions adult cardiac and marrow cells expressing protein C-kit (c-kit+) are able to generate new myocytes. These cells have the potential to differentiate into heart myocytes in vitro, but the changed micro-environment in the heart, being a result of infarction, doesnot allow such differentiation [42]. Mesenchymal stem cells (MSCs) enable physical contact between living cardiomyocytes, and they are the stimulants necessary to support the repair of the heart muscle. Moreover, as shown in the Central Illustration (Figure 4), mesenchymal stem cells seem to be a promising therapeutic agent for cardiac regeneration in myocardial diastolic dysfunction.
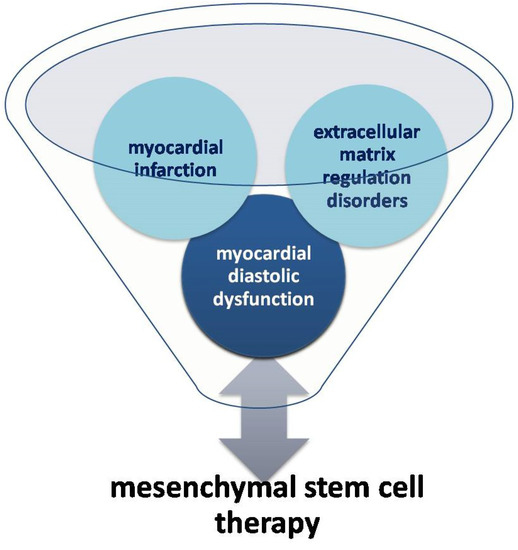
Figure 4.
Relationship between mesenchymal stem cell therapy and myocardial diastolic dysfunction (Central Illustration).
1.4. Impact of Stem Cell Therapy on Diastolic Function of the Heart
The key factor affecting the diastolic dysfunction of the myocardium as a result of myocardial infarction and dysregulation of the ECM in the context of MSC therapy is the type of stem cells supplied. Some stem cells seem to have greater differentiation capacity, while other may have greater paracrine activity or greater potential to stimulate neovascularization. It was reported that MSCs obtained from the subcutaneous white fat tissue of the abdomen of a pig (adipose tissue-derived mesenchymal stem cells [ATSC]), injected into a pre-planned area, improved left ventricular systolic function and reduced the scope of the infarction [43]. It also modulated the reconstruction of the local intercellular matrix, despite the fact that myocytes in BZ began to break down into necrotic tissues. Microscopic study helped to reveal that after ATSC injection myocytes as well as the vascular system remained undisturbed. Immunofluorescence staining proved spaces sparing the basal membrane with intact, smooth muscle layers and endothelium of blood vessels, ensuring blood flow. Another benefit of therapy (at the structural as well as metabolic level) is the observed increase in the number of formed vessels and better-preserved cardiomyocyte as well as Purkinje fibers [44]. Of note, most of the experiments that have strongly supported the beneficial effects of stem cell therapy have been carried out on young animals (rodents or pigs). It was a reasonable concern whether the response of heart tissue in elderly persons would be different due to the progressive decline of the heart’s ability to react with age [45]. However, clinical trials have dispelled these doubts by confirming that stem cell therapy profoundly increases functional recovery after myocardial infarction [46].
Many studies indicate a low immunogenicity of MSCs, thanks to which a single transplant is well tolerated by the recipient organism. However, repeated administration of MSCs may result in the production of all antibodies. Von Bonin et al., reported that the transplant of MSCs, which had contact with fetal bovine serum (FBS), induced the production of antibodies against FBS in the blood of the recipient. It is worth emphasizing that FBS is often used in the MSC culture medium. Thus, MSCs are dualistic in nature.
1.5. Influence of Stem Cell Therapy on Cardiac Fibrosis
Research shows that, thanks to the use of the latest pharmacological treatment methods on people with MI, we are able to eliminate clinical symptoms, but we are not able to eliminate fibrosis [3,47]. The resulting scar protects the heart muscle against rupture. On the other hand, chronic diffuse or focal reactive myocardial fibrosis resulting from pressure or volume overload exacerbates functional disorders [3,18]. Stem cell transplantation affects the remodeling of the extracellular matrix significantly, and this may contribute to the improvement of LV function. Most studies show that stem cell therapy after MI, combined with the administration of mesenchymal stem cells, reduces fibrosis. However, the authors of these studies did not specify whether they meant the reduction of post-infarction scar as a result of regeneration or changes in the extracellular matrix [46]. MSCs appear to significantly reduce the expression of type I and III collagen and significantly suppress the activity of the type III collagen promoter [47]. They reduce the expression of MMP-2 and MMP-9 proteins and increase the level of matrix modulating factors, such as metalloproteinase 2 (MMP-2), tissue inhibitors of matrix metalloproteinase (TIMP)-1 and TIMP-2, as well as matrix proteins thrombospondin-1 and tenascin C [48]. Paracrine substances derived from MSCs play a key role in reducing fibrosis, promoting neovascularization, modulating the extracellular matrix, cytoprotection, and inhibiting apoptosis and inflammation [49,50,51]. Some of the most important paracrine factors released by adult stem cells are growth factors. These are vascular endothelial growth factor VEGF, stromal cell origin factor 1 (SDF-1, also known as the CXC chemokine 1 motif CXCL12) and insulin-like growth factor-1 (IGF-1) [49,52,53,54,55,56].
Despite significant progress in this method of treatment, cell transplants still have an insufficient degree of implantation in the damaged heart and insufficient survival in the area of damage. In the years 2010–2020 several randomized and multicenter studies of the treatment of ischemic heart disease were carried out, using mainly autologous bone marrow-derived mononuclear cells, but also selected bone marrow cells. All these studies showed no additional benefit over standard therapy [57,58,59,60,61,62,63,64,65]. We still do not fully understand why clinical outcomes improve with combined stem cell MSC and CSC therapy without improving LV function or reducing scar size [66]. Some authors have suggested that the functional benefits of heart cell therapy result from an acute inflammatory wound healing response that rejuvenates the post-infarction area of the heart. This conclusion was based on the observation that there is an interesting effect of improving the work of the heart after administering cells killed by freezing and thawing. These cells induced regional macrophages CCR2+ and CX3CR1+ and provided functional rejuvenation of the heart following ischemia–reperfusion injury. This selective macrophage response altered the activity of cardiac fibroblasts, reduced the extracellular matrix content in the border zone and improved the mechanical properties of the damaged area [67].
1.6. Application of Fibroblasts in Stem Cell Therapy
An adult heart muscle has more fibroblasts compared to resident heart progenitor cells (cardiac fibroblasts make up more than 50% of all cells in the heart). Therefore, the concept was developed to increase the endogenous regenerative potential of an adult heart, performed by reprogramming fibroblasts with the use of retroviruses into ESC-like cells (referred to as induced pluripotential stem cells [iPS]) and then into new cardiomyocytes [61]. Using gene cocktails such as c-MYC, OCT3/4, SOX2 KLF4 or OCT3/4, SOX2, NANOG, LIN28, it was possible to reprogram human fibroblasts back intopluripotent stem cells [68,69]. Subsequent studies have shown similar results using fewer genes [70,71] and developed a non-viral methodology to avoid the potentially mutagenic effects of integrating viral delivery methods [72]. Today, generation of iPS cell lines is faster and cheaper, and promises to replace conventional sources of pluripotential stem cells.
1.7. Post-Infarction Treatment of Diastolic Dysfunction
Excessive cardiac fibrosis, associated with diastolic dysfunction, is still a clinical problem. There has been no uniform approach to remodeling therapy for several decades. Preventing post-infarction ECM remodeling seems to be very important, because it prevents such dangerous consequences as left ventricular dilatation and rupture [5,6,15,73]. The standard pharmacotherapy includes inhibitors of the angiotensin I convertase enzymes (ACE-I), aldosterone antagonists, blockers of the angiotensin II receptors and the β-adrenergic receptor blockers [5,32,74,75]. Patients are heterogeneous in pathophysiology and therapeutic response. The severity and characteristics of post-MI remodeling depend not only on the size of the acute MI, but also on age, sex, genetic background and concomitant disease. Some patients with hypertension or diabetes may show prolonged pro-inflammatory signaling after infarction, leading to dilative remodeling and systolic dysfunction. These patients may benefit from anti-inflammatory therapy [76]. In other diabetic patients, the hypertrophic and fibrotic reactions are more pronounced, leading to diastolic dysfunction. This type of unfavorable remodeling is associated with overactivity of the TGF-β system [77]. The identification of such patient subpopulations, using appropriate biomarkers or molecular imaging techniques, allows more effective therapies to be designed for MI patients [78].
1.8. Conclusions
In conclusion, cellular therapy for heart regeneration has much room for improvement before being considered an affordable, widely available, safe and effective clinical approach. It seems that studies should be carried out at this stage on animal models, as the effects of both non-hematopoietic and hematopoietic stem cells may differ in vitro and in vivo. Long-term research performed to rule out long-term negative effects of post-acute MI stem cell therapy, including diastolic failure, will also be necessary.
Author Contributions
Writing—original draft preparation, P.P.-M., R.P., U.P., L.K., M.P., A.J., M.M., A.G., J.K. and J.G.; writing—review and editing, P.P.-M., R.P., U.P., L.K., M.P., A.J., M.M., A.G., J.K. and J.G.; visualization, P.P.-M., U.P. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 1 May 2017).
- Budaj, A.; Beręsewicz, A. Ischemic Heart Disease; Interna Szczeklika Wyd. 10.; Gajewski, P., Szczeklik, A., Eds.; MedycynaPraktyczna: Kraków, Poland, 2019; pp. 168–226. ISBN 9788374305686. [Google Scholar]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2019, 19, 177–191. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast- mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jugdutt, B.I. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Anversa, P.; Armstrong, P.W.; Brilla, C.G.; Burnett, J.C.; Cruickshank, J.M.; Devereux, R.B.; Giles, T.D.; Korsgaard, N.; Leier, C.V.; et al. Remodeling and reparation of the cardiovascular system. J. Am. Coll. Cardiol. 1992, 20, 3–16. [Google Scholar] [CrossRef]
- Jugdutt, B.I.; Joljart, M.J.; Khan, M.I. Rate of collagen deposition during healing after myocardial infarction in the rat and dog models: Mechanistic insights into ventricular remodeling. Circulation 1996, 94, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Pick, R.; Jalil, J.E.; Janicki, J.S.; Carroll, E.P. Patterns of myocardial fibrosis. J. Mol. Cell Cardiol. 1989, 21, 121–131. [Google Scholar] [CrossRef]
- Karolko, B.; Przewłocka-Kosmala, M. Fibrosis markers in heart failure. Folia Cardiol. 2017, 12, 245–253. [Google Scholar]
- Kong, P.; Christia, P.; Frangogiannis, N. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Li, A.H.; Liu, P.P.; Villarreal, F.J.; Garcia, R.A. Dynamic changes in myocardial matrix and relevance to disease: Translational perspectives. Circ. Res. 2014, 114, 916–927. [Google Scholar] [CrossRef]
- Olivetti, G.; Capasso, J.M.; Sonnenblick, E.H.; Anversa, P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ. Res. 1990, 67, 23–34. [Google Scholar] [CrossRef]
- Alberts, B.; Bray, D.; Lewis, J.; Raff, M.; Roberts, K.; Watson, J.D. Molecular Biology of the Cell, 3rd ed.; Garland Publishing: New York, NY, USA, 1994. [Google Scholar]
- Phillips, C.; Wenstrup, R.J. Biosynthetic and genetic disorders of collagen. In Wound Healing: Biochemical and Clinical Aspects; Cohen, I.K., Diegelmann, R.F., Lindblad, W.J., Eds.; WB Saunders Co.: Philadelphia, PA, USA, 1992; pp. 152–176. [Google Scholar]
- Factor, S.M.; Robinson, T.F.; Dominitz, R.; Cho, S.H. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. Am. J. Cardiovasc. Pathol. 1987, 1, 91–97. [Google Scholar] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagenstructure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Kajstura, J.; Torella, D.; Urbanek, K.; Heleniak, H.; Colussi, C.; Di Meglio, F.; Nadal-Ginard, B.; Frustaci, A.; Leri, A.; et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 2003, 93, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef]
- Ceauşu, Z.; Popa, M.; Socea, B.; Gorecki, G.P.; Costache, M.; Ceauşu, M. Influence of the microenvironment dynamics on extracellular matrix evolution under hypoxic ischemic conditionns in the myocardium. Exp. Ther. Med. 2022, 23, 199. [Google Scholar] [CrossRef]
- Etoh, T.; Joffs, C.; Deschamps, A.M.; Davis, J.; Dowdy, K.; Hendrick, J.; Baicu, S.; Mukherjee, R.; Manhaini, M.; Spinale, F.G. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am. J. Physiol. 2001, 281, H987–H994. [Google Scholar] [CrossRef]
- Chen, B.; Frangogiannis, N.G. The role of macrophages in nonischemic heart failure. JACC Basic Transl. Sci. 2018, 3, 245–248. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Dewald, O.; Ren, G.; Duerr, G.D.; Zoerlein, M.; Klemm, C.; Gersch, C.; Tincey, S.; Michael, L.H.; Entman, M.L.; Frangogiannis, N. Of mice and dogs: Species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 2004, 164, 665–677. [Google Scholar] [CrossRef]
- Frangogiannis, N. The extracellular matrix in ischemic and nonischemicheart failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Hulsmans, M.; Lavine, K.J.; Moreira, M.B.; Heidt, T.; Courties, G.; Sun, Y.; Iwamoto, Y.; Tricot, B.; Khan, O.F.; et al. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ. Res. 2016, 119, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, D.; Sigusch, H.H.; Hensse, J.; Tyagi, S.C.; Körfer, R.; Figulla, H.R. Cardiac remodelling in end stage heart failure: Upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002, 88, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Shimoni, S.; Chang, S.M.; Ren, G.; Dewald, O.; Gersch, C.; Shan, K.; Aggeli, C.; Reardon, M.; Letsou, G.V.; et al. Active interstitial remodeling: An important process in the hibernating human myocardium. J. Am. Coll. Cardiol. 2002, 39, 1468–1474. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Prevention of ventricular remodelling post myocardial infarction: Timing and duration of therapy. Can. J. Cardiol. 1993, 9, 103–114. [Google Scholar]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Duran, J.M.; Taghavi, S.; Berretta, R.M.; Makarewich, C.A.; Iii, T.S.; Starosta, T.; Udeshi, F.; George, J.C.; Kubo, H.; Houser, S.R. A characterization and targeting of the infarct border zone in a swine model of myocardial infarction. Clin. Transl. Sci. 2012, 5, 416–421. [Google Scholar] [CrossRef]
- Latini, R.; Maggioni, A.P.; Flather, M.; Sleight, P.; Tognoni, G. ACE inhibitor use in patients with myocardial infarction: Summary of evidence from clinical trials. Circulation 1995, 92, 3132–3137. [Google Scholar] [CrossRef]
- Gladden, J.D.; Linke, W.A.; Redfield, M.M. Heart failure with preserved ejection fraction. Pflug. Arch. Eur. J. Physiol. 2014, 466, 1037–1053. [Google Scholar] [CrossRef]
- Velagaleti, R.S.; Pencina, M.J.; Murabito, J.; Wang, T.; Parikh, N.I.; D’Agostino, R.B.; Levy, D.; Kannel, W.B.; Vasan, R.S. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 2008, 118, 2057–2262. [Google Scholar] [CrossRef]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San José, G.; Querejeta, R.; Díez, J. Circulating biomarkers of myocardial fibrosis: The need for a reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Kossaify, A.; Nasr, M. Diastolic Dysfunction and the New Recommendations for Echocardiographic Assessment of Left Ventricular Diastolic Function: Summary of Guidelines and Novelties in Diagnosis and Grading. J. Diagn. Med. Sonogr. 2019, 35, 317–325. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.-I.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, X.; Ramil, J.M.; Rikka, S.; Kim, L.; Lee, Y.; Gude, N.A.; Thistlethwaite, P.A.; Sussman, M.A.; Gottlieb, R.A.; et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularisation resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 2010, 121, 675–683. [Google Scholar] [CrossRef]
- Wojakowski, W.; Tendera, M.; Cybulski, W.; Zuba-Surma, E.K.; Szade, K.; Florczyk, U.; Kozakowska, M.; Szymula, A.; Krzych, Ł.; Paslawska, U.; et al. Effects of intracoronary delivery of allogenic bone marrow-derived stem cells expressing heme oxygenase-1 on the reperfusion injury in experimental myocardial infarction. Thromb. Hemost. 2012, 108, 464–475. [Google Scholar]
- Hatzistergos, K.E.; Takeuchi, L.M.; Saur, D.; Seidler, B.; Dymecki, S.M.; Mai, J.J.; White, I.A.; Balkan, W.; Kanashiro-Takeuchi, R.M.; Schally, A.V.; et al. cKit+ cardiac progenitors of neural crest origin. Proc. Natl. Acad. Sci. USA 2015, 112, 13051–13056. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, Y.S.; Kang, W.S.; Lee, K.H.; Cho, M.; Hong, M.H.; Lim, K.S.; Jeong, M.H.; Ahn, Y. Intramyocardial Injection of Stem Cells in Pig Myocardial Infarction Model: The First Trial in Korea. J. Korean Med. Sci. 2017, 32, 1708–1712. [Google Scholar] [CrossRef]
- Gómez-Heras, S.G.; Largo, C.; Larrea, J.L.; Vega-Clemente, L.; Flores, M.C.; Ruiz-Pérez, D.; García-Olmo, D.; García-Arranz, M. Main histological parameters to be evaluated in an experimental model of myocardial infarcttreated by stem cells on pigs. Peer J. 2019, 7, e7160. [Google Scholar] [CrossRef]
- Sopko, N.A.; Turturice, B.A.; Becker, M.E.; Brown, C.R.; Dong, F.; Popović, Z.B.; Penn, M.S. Bone marrow support of the heart in pressure overload is lost with aging. PLoS ONE 2010, 5, e15187. [Google Scholar] [CrossRef] [PubMed]
- Dimmler, S.; Zeiher, A.M. Wanted! The best cel for cardiac regeneration. Editorial comment. JACC 2004, 44, 2. [Google Scholar]
- Querejeta, R.; López, B.; González, A.; Sánchez, E.; Larman, M.; MartínezUbago, J.L.; Díez, J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardialfibrosis. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Kaur, S.; Isenberg, J.S. Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer. Antioxid. Redox Signal. 2017, 27, 874–911. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-N.; Cores, J.; Huang, K.; Cui, X.-L.; Luo, L.; Zhang, J.-Y.; Li, T.-S.; Qian, L.; Cheng, K. Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem. Cells Transl. Med. 2018, 7, 354–359. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Chimenti, I.; Smith, R.R.; Li, T.-S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marbán, E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef]
- Rota, M.; Padin-Iruegas, M.E.; Misao, Y.; De Angelis, A.; Maestroni, S.; Ferreira-Martins, J.; Fiumana, E.; Rastaldo, R.; Arcarese, M.L.; Mitchell, T.S.; et al. Local activation or implantation of cardiac progenitor cells rescues scarred infracted myocardium improving cardiac function. Circ. Res. 2008, 103, 107–116. [Google Scholar] [CrossRef]
- Lefer, D.J.; Marbán, E. Is Cardioprotection Dead? Circulation 2017, 136, 98–109. [Google Scholar] [CrossRef]
- Tang, X.-L.; Rokosh, G.; Sanganalmath, S.K.; Yuan, F.; Sato, H.; Mu, J.; Dai, S.; Li, C.; Chen, N.; Peng, Y.; et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010, 121, 293–305. [Google Scholar] [CrossRef]
- Klarenbosch, B.R.; Chamuleau, S.A.J.; Teske, A.J. Deformation imaging to assess global and regional effects of cardiac regenerative therapy in ischaemic heart disease: A systematic review. J. Tissue Eng. Regen. Med. 2019, 13, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A.I.; Salem, A.M.; Saad, A.; Afifi, A.M.; Afify, A.Y.; Afify, H.; Salem, H.S.; Ghanem, E.; Abdel-Daim, M.M. Mesenchymal Stem Cell Therapy for Doxorubicin-Induced Cardiomyopathy: Potential Mechanisms, Governing Factors, and Implications of the Heart Stem Cell Debate. Front. Pharmacol. 2019, 10, 635. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Ellis, S.G.; Pepine, C.J.; Willerson, J.T.; Zhao, D.X.M.; Forder, J.R.; Byrne, B.J.; Hatzopoulos, A.K.; Penn, M.S.; et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The late TIME randomized trial. JAMA 2011, 306, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Willerson, J.T.; Pepine, C.J.; Henry, T.D.; Ellis, S.G.; Zhao, D.X.M.; Silva, G.V.; Lai, D.; Thomas, J.D.; Kronenberg, M.W.; et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA 2012, 307, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Zhao, D.X.M.; Ellis, S.G.; Forder, J.R.; Anderson, R.D.; Hatzopoulos, A.K.; Penn, M.S.; et al. Effect of the use and timing of bone marrow mononuclear cel delivery on left ventricular function after acute myocardial infarction: The tIME randomized trial. JAMA 2012, 308, 2380–2389. [Google Scholar] [CrossRef]
- Sürder, D.; Manka, R.; Cicero, V.L.; Moccetti, T.; Rufibach, K.; Soncin, S.; Turchetto, L.; Radrizzani, M.; Astori, G.; Schwitter, J.; et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction. Circulation 2016, 127, 1968–1979. [Google Scholar] [CrossRef]
- Choudry, F.; Hamshere, S.; Saunders, N.; Veerapen, J.; Bavnbek, K.; Knight, C.; Pellerin, D.; Locca, D.; Westwood, M.; Rakhit, R.; et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: The REGENERATE-AMI clinical trial†. Eur. Heart J. 2016, 37, 256–263. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A randomized, double-blind, placebocontrolled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ. Res. 2017, 120, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Müller-Ehmsen, J.; Tschöpe, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Plasencia, A.C.; Gilaberte, I.; Belmans, A.; Santos, M.E.F.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ. Res. 2016, 123, 579–589. [Google Scholar] [CrossRef]
- Nicolau, J.C.; Furtado, R.H.; Silva, S.A.; Rochitte, C.E.; Rassi, A.; Moraes, J.B., Jr.; Quintella, E.; Costantini, C.R.; Korman, A.P.; Mattos, M.A.; et al. Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double-blind randomized trial. Clin. Cardiol. 2018, 41, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Mitrani, R.D.; Hare, J.M.; Pepine, C.J.; Perin, E.C.; Willerson, J.T.; Traverse, J.H.; Henry, T.D.; Yang, P.C.; Murphy, M.P.; et al. Cardiovascular Cell Therapy Research Network (CCTRN). Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, singly or in combination, in patients with ischemic heart failure: The CCTRN CONCERT-HF study. Heart Fail. Eur. J. 2021, 23, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefits of heart stem cell therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Huangfu, D.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Snitow, M.; Chen, A.E.; Melton, D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008, 26, 795–797. [Google Scholar] [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef]
- O’Doherty, R.; Greiser, U.; Wang, W. Nonviral methods for inducing pluripotency to cells. Biomed. Res. Int. 2013, 2013, 705902. [Google Scholar] [CrossRef]
- Caulfield, J.B.; Borg, T.K. The collagen network of the heart. Lab Investig. 1979, 40, 364–372. [Google Scholar]
- Zannad, F.; Alla, F.; Dousset, B.; Perez, A.; Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the Randomized ALdactone Evaluation Study (RALES). Circulation 2000, 102, 2700–2706. [Google Scholar] [CrossRef]
- Cohn, J.N.; Tognoni, G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef]
- Abbate, A.; Kontos, M.C.; Grizzard, J.D.; Biondi-Zoccai, G.G.; Van Tassell, B.W.; Robati, R.; Roach, L.M.; Arena, R.A.; Roberts, C.S.; Varma, A.; et al. VCU-ART Investigators. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 2010, 105, 1371–1377.e1. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Cavalera, M.; Wang, J.; Russo, I.; Shinde, A.; Kong, P.; Gonzalez-Quesada, C.; Rai, V.; Dobaczewski, M.; Lee, D.W.; et al. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ. Heart Fail. 2015, 8, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Saha, P.; Datla, S.R.; Mellacheruvu, P.; Gunasekaran, M.; Guru, S.A.; Fu, X.; Chen, L.; Bolli, R.; Sharma, S.; et al. Transplanted allogeneic cardiac progenitor cells secrete GDF-15 and stimulate an active immune remodeling process in the ischemic myocardium. J. Transl. Med. 2022, 20, 323. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).