Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study
Abstract
1. Introduction
2. Subjects and Methods
2.1. Patient Information and Data Collection
2.2. Measurements of Bone Mineral Density
2.3. Statistics
3. Results
3.1. Characteristics of the Studied Populations
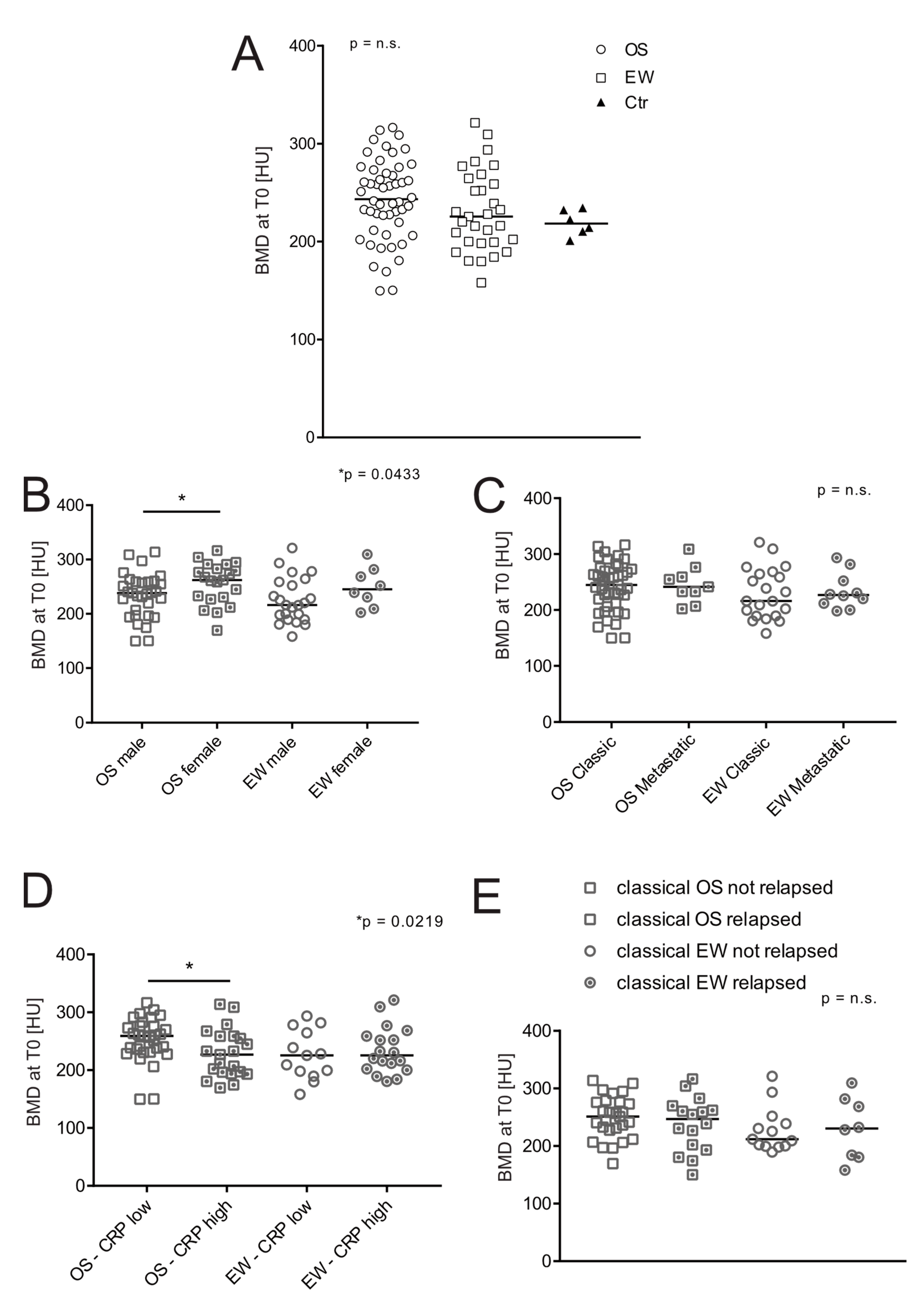
3.2. BMD at the Onset
3.3. BMD at the Completion of Chemotherapy
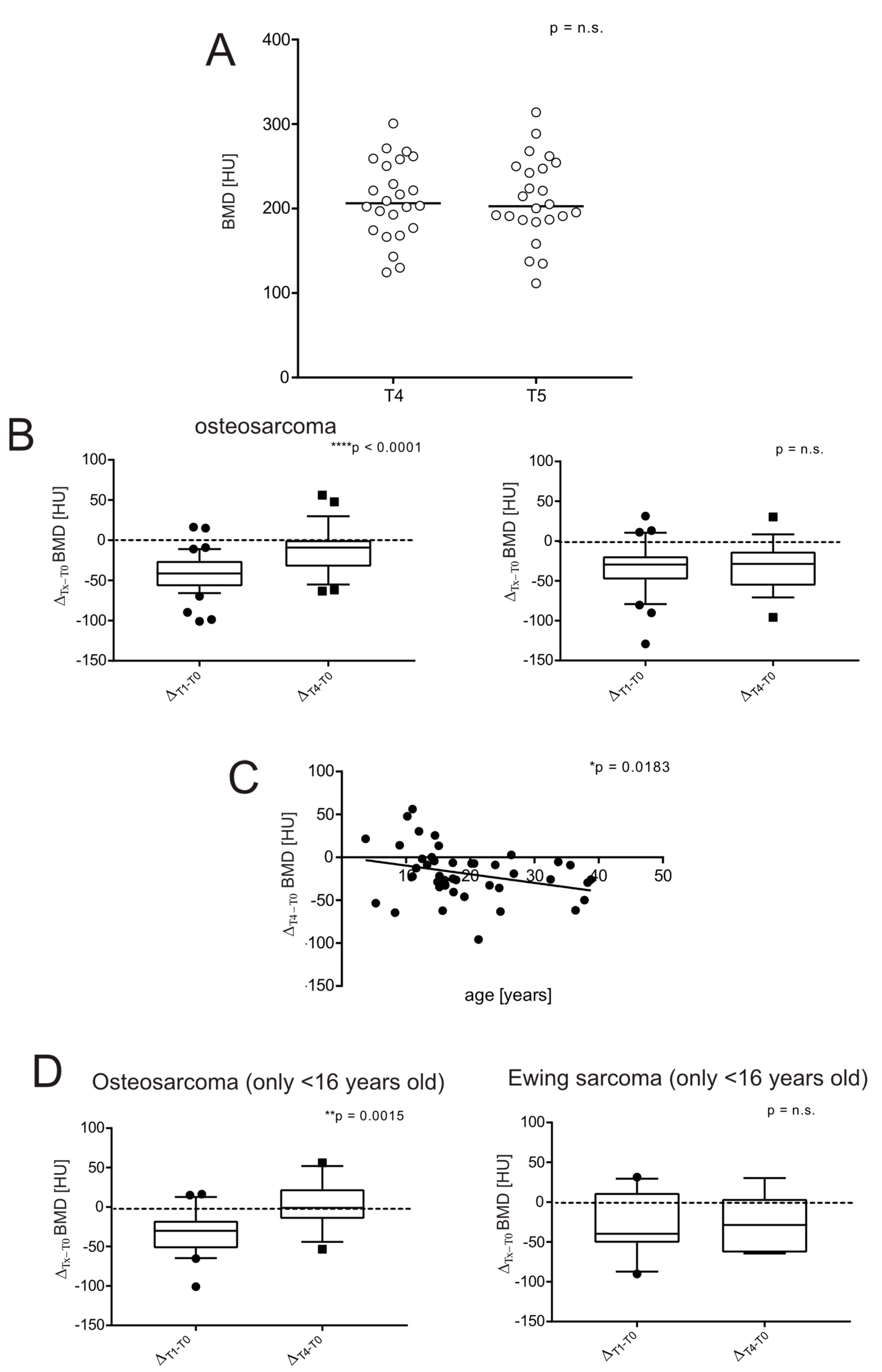
3.4. BMD in Sarcoma Survivors
3.5. Severe Short-Term Bone Loss Is Predictive of a Low Likelihood of Long-Term Recovery in Bone Sarcoma Survivors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chemaitilly, W.; Cohen, L.E.; Mostoufi-Moab, S.; Patterson, B.C.; Simmons, J.H.; Meacham, L.R.; van Santen, H.M.; Sklar, C.A. Endocrine Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Halton, J.M.; Atkinson, S.A.; Fraher, L.; Webber, C.; Gill, G.J.; Dawson, S.; Barr, R.D. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J. Bone Miner. Res. 1996, 11, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Diel, I.J. Osteoporosis due to cancer treatment: Pathogenesis and management. J. Clin. Oncol. 2000, 18, 1570–1593. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lim, J.S. Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management. Korean J. Pediatr. 2013, 56, 60–67. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21 (Suppl. S7), vii320–vii325. [Google Scholar] [CrossRef]
- Grunewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Alava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing sarcoma. Nat. Rev. Dis. Primers 2018, 4, 5. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Wilson, C.L.; Ness, K.K. Bone mineral density deficits and fractures in survivors of childhood cancer. Curr. Osteoporos. Rep. 2013, 11, 329–337. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Chevalley, T.; Ferrari, S.; Rizzoli, R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009, 51 (Suppl. S1), S5–S17. [Google Scholar] [CrossRef]
- Kaste, S.C.; Ahn, H.; Liu, T.; Liu, W.; Krasin, M.J.; Hudson, M.M.; Spunt, S.L. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr. Blood Cancer 2008, 50, 1032–1038. [Google Scholar] [CrossRef]
- Muller, C.; Winter, C.C.; Rosenbaum, D.; Boos, J.; Gosheger, G.; Hardes, J.; Vieth, V. Early decrements in bone density after completion of neoadjuvant chemotherapy in pediatric bone sarcoma patients. BMC Musculoskelet. Disord 2010, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Cho, W.H.; Lee, J.A.; Kim, D.H.; Seo, J.H.; Lim, J.S. Bone mineral density change during adjuvant chemotherapy in pediatric osteosarcoma. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Krepler, P.; Koschat, M.A.; Grampp, S.; Dominkus, M.; Kotz, R. Bone mineral density in long-term survivors of highly malignant osteosarcoma. J. Bone Joint Surg. Br. 2003, 85, 231–237. [Google Scholar] [CrossRef]
- Ruza, E.; Sierrasesumaga, L.; Azcona, C.; Patino-Garcia, A. Bone mineral density and bone metabolism in children treated for bone sarcomas. Pediatr. Res. 2006, 59, 866–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pirker-Fruhauf, U.M.; Friesenbichler, J.; Urban, E.C.; Obermayer-Pietsch, B.; Leithner, A. Osteoporosis in children and young adults: A late effect after chemotherapy for bone sarcoma. Clin. Orthop. Relat. Res. 2012, 470, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski-Masker, K.; Kaste, S.C.; Hudson, M.M.; Esiashvili, N.; Mattano, L.A.; Meacham, L.R. Bone mineral density deficits in survivors of childhood cancer: Long-term follow-up guidelines and review of the literature. Pediatrics 2008, 121, e705–e713. [Google Scholar] [CrossRef]
- Hobusch, G.M.; Noebauer-Huhmann, I.; Krall, C.; Holzer, G. Do long term survivors of ewing family of tumors experience low bone mineral density and increased fracture risk? Clin. Orthop. Relat. Res. 2014, 472, 3471–3479. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Ward, L.M. Skeletal Morbidity in Children and Adolescents during and following Cancer Therapy. Horm. Res. Paediatr. 2019, 91, 137–151. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Lee, L.J.; del Rio, A.M.; Lauder, T.; Bruce, R.J.; Summers, R.M.; Pooler, B.D.; Binkley, N. Simultaneous screening for osteoporosis at CT colonography: Bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J. Bone Miner. Res. 2011, 26, 2194–2203. [Google Scholar] [CrossRef]
- Marcucci, G.; Beltrami, G.; Tamburini, A.; Body, J.J.; Confavreux, C.B.; Hadji, P.; Holzer, G.; Kendler, D.; Napoli, N.; Pierroz, D.D.; et al. Bone health in childhood cancer: Review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann. Oncol. 2019, 30, 908–920. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Radiology 2003, 226, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001, 323, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.; Guyatt, G.H.; Sackett, D.L. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994, 271, 703–707. [Google Scholar] [CrossRef]
- Ferrari, S.; Bertoni, F.; Mercuri, M.; Picci, P.; Giacomini, S.; Longhi, A.; Bacci, G. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: An analysis of 300 patients treated at the Rizzoli Institute. Ann. Oncol. 2001, 12, 1145–1150. [Google Scholar] [CrossRef]
- Bachrach, L.K. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol. Metab. 2001, 12, 22–28. [Google Scholar] [CrossRef]
- Gilsanz, V.; Gibbens, D.T.; Carlson, M.; Boechat, M.I.; Cann, C.E.; Schulz, E.E. Peak trabecular vertebral density: A comparison of adolescent and adult females. Calcif. Tissue Int. 1988, 43, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Jt. Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Allin, K.H.; Nordestgaard, B.G. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab. Sci. 2011, 48, 155–170. [Google Scholar] [CrossRef]
- Heikkila, K.; Ebrahim, S.; Lawlor, D.A. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J. Epidemiol. Community Health 2007, 61, 824–833. [Google Scholar] [CrossRef]
- Nakamura, T.; Grimer, R.; Gaston, C.; Francis, M.; Charman, J.; Graunt, P.; Uchida, A.; Sudo, A.; Jeys, L. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur. J. Cancer 2013, 49, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.B.; Oliveira, P.D.; Goncalves, H.; Oliveira, I.O.; Assuncao, M.C.F.; Tovo-Rodrigues, L.; Ferreira, G.D.; Wehrmeister, F.C. Are cytokines (IL-6, CRP and adiponectin) associated with bone mineral density in a young adult birth cohort? BMC Musculoskelet Disord. 2018, 19, 427. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Longhi, A.; Salerno, M.; Halleen, J.M.; Perut, F.; Granchi, D.; Ferrari, S.; Bertoni, F.; Giunti, A.; Baldini, N. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int. J. Oncol. 2008, 33, 1231–1238. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Rogowska, E.; Szamotulska, K.; Chelchowska, M.; Rowicka, G.; Rychlowska-Pruszynska, M. Decreased bone mineral density and alteration in biochemical bone metabolism markers in children affected by bone tumors after completion of therapy. Neoplasma 2015, 62, 288–294. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- eviQ, a Free Resources of Evidence-Based, Consensus Driven Cancer Treatment Protocol. Available online: https://www.eviq.org.au/medical-oncology/sarcoma/bone-sarcoma (accessed on 6 August 2022).
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef]
- Vestergaard, P. Skeletal effects of drugs to treat cancer. Curr. Drug Saf. 2008, 3, 173–177. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, D.H.; Lee, J.A.; Kim, D.H.; Cho, J.; Cho, W.H.; Lee, S.Y.; Jeon, D.G. Young age at diagnosis, male sex, and decreased lean mass are risk factors of osteoporosis in long-term survivors of osteosarcoma. J. Pediatr. Hematol. Oncol. 2013, 35, 54–60. [Google Scholar] [CrossRef]
- May, K.P.; West, S.G.; McDermott, M.T.; Huffer, W.E. The effect of low-dose methotrexate on bone metabolism and histomorphometry in rats. Arthritis Rheum. 1994, 37, 201–206. [Google Scholar] [CrossRef]
- Mandel, K.; Atkinson, S.; Barr, R.D.; Pencharz, P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2004, 22, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Shandala, T.; Shen Ng, Y.; Hopwood, B.; Yip, Y.C.; Foster, B.K.; Xian, C.J. The role of osteocyte apoptosis in cancer chemotherapy-induced bone loss. J. Cell. Physiol. 2012, 227, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Luo, L.; Liu, S.; Guan, Z.; Zhang, Q.; Li, X.; Tao, K. The Role of Depletion of Gut Microbiota in Osteoporosis and Osteoarthritis: A Narrative Review. Front. Endocrinol. 2022, 13, 847401. [Google Scholar] [CrossRef]
- Kelly, J.; Damron, T.; Grant, W.; Anker, C.; Holdridge, S.; Shaw, S.; Horton, J.; Cherrick, I.; Spadaro, J. Cross-sectional study of bone mineral density in adult survivors of solid pediatric cancers. J. Pediatr. Hematol. Oncol. 2005, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Bodeen, G.; Brett, A.; Brown, J.K.; Binkley, N. Comparison of femoral neck BMD evaluation obtained using Lunar DXA and QCT with asynchronous calibration from CT colonography. J. Clin. Densitom. 2015, 18, 5–12. [Google Scholar] [CrossRef]
- Bartenschlager, S.; Dankerl, P.; Chaudry, O.; Uder, M.; Engelke, K. BMD accuracy errors specific to phantomless calibration of CT scans of the lumbar spine. Bone 2022, 157, 116304. [Google Scholar] [CrossRef]
- Chevalley, T.; Rizzoli, R. Acquisition of peak bone mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101616. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Le Deley, M.C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Anderton, J.; Moroz, V.; Marec-Bérard, P.; Gaspar, N.; Laurence, V.; Martín-Broto, J.; Sastre, A.; Gelderblom, H.; Owens, C.; Kaiser, S.; et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours-EURO EWING 2012 Protocol. Trials 2020, 21, 96. [Google Scholar] [CrossRef]


| Characteristics | OS (n = 52) | EW (n = 31) | Control (n = 6) |
|---|---|---|---|
| Sex | n (%) | n (%) | n (%) |
| Male | 31 (59.6) | 23 (74.2) | 4 (66.7) |
| Female | 21 (40.4) | 8 (25.8) | 2 (33.3) |
| Age (years) | Median (range) | Median (range) | Median (range) |
| Total | 17.4 (3.7–44.2) | 20.2 (8.4–38.3) | 20.3 (8.8–23.6) |
| Male | 19.1 (5.3–44.2) | 20.2 (8.4–36.3) | 20.3 (19.5–23.6) |
| Female | 15.2 (3.7–36.4) | 20.3 (11.0–38.3) | 15.2 (8.8–21.7) |
| Localization | |||
| Lower limbs | 40 | 19 | - |
| Axial | 3 | 11 | - |
| Upper limbs | 9 | 2 | - |
| Biochemical markers | mean ± SEM | mean ± SEM | - |
| ALP [U/L], | 313.5 ± 65.3 | 126.3 ± 13.8 | - |
| LDH [U/L] | 461.6 ± 34.2 | 430.5 ± 27.1 | - |
| CRP [mg/100 mL] | 1.8 ± 0.7 | 3.3 ± 0.8 | - |
| Metastatic at diagnosis | |||
| N | 9 | 10 | |
| % | 17% | 32% | |
| Months from T0 (mean ± SEM) at T1 | 11.0 ± 0.4 | 11.3 ± 0.3 | - |
| n of censored patients at T1 | 49 | 30 | - |
| Months from T0 (mean ± SEM) at T2 | 23.1 ± 0.5 | 23.3 ± 1.0 | - |
| n of censored patients at T2 | 37 | 21 | - |
| Months from T0 (mean ± SEM) at T3 | 35.0 ± 0.6 | 34.4 ± 0.4 | - |
| n of censored patients at T3 | 36 | 16 | - |
| Months from T0 (mean ± SEM) at T4 | 47.6 ± 0.5 | 46.7 ± 0.7 | - |
| n of censored patients at T4 | 28 | 16 | - |
| Months from T0 (mean ± SEM) at T5 | 58.9 ± 0.9 | 58.5 ± 10.1 | - |
| n of censored patients at T5 | 14 | 10 | - |
| Prognostic Performance | OS | EW |
|---|---|---|
| Sample size | ||
| Total | 25 | 15 |
| Positive group | 7 (28%) | 3 (20%) |
| Negative group | 18 (72%) | 12 (80%) |
| Area under the ROC curve | 0.786 ± 0.114 (fair) | 1 ± 0 (excellent) |
| 95% Confidence interval | 0.577 to 0.923 | 0.782 to 1.00 |
| p value | 0.012 (*) | <0.0001 (****) |
| Sensitivity | ||
| Value | 57.14% | 100% |
| 95% CI | 18.41% to 90.10% | 29.24% to 100% |
| Specificity | ||
| Value | 83.33% | 83.33% |
| 95% CI | 58.58% to 96.42% | 51.59% to 97.91% |
| Prevalence | ||
| Value | 28% | 20% |
| 95% CI | 12.07% to 49.39% | 4.33% to 48.09% |
| Accuracy | ||
| Value | 76.00% | 86.67% |
| 95% CI | 54.87% to 90.64% | 59.54% to 98.34% |
| Positive predictive value | ||
| Value | 57.14% | 60.00% |
| 95% CI | 28.33% to 81.81% | 29.74% to 84.17% |
| Negative predictive value | ||
| Value | 83.33% | 100% |
| 95% CI | 67.47% to 92.34% | - |
| Likelihood ratio test + | ||
| Value | 3.43 | 6.00 |
| 95% CI | 1.02 to 11.57 | 1.69 to 21.26 |
| Likelihood ratio test − | ||
| Value | 0.51 | 0.00 |
| 95% CI | 0.21 to 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avnet, S.; Falzetti, L.; Bazzocchi, A.; Gasperini, C.; Taddei, F.; Schileo, E.; Baldini, N. Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study. J. Clin. Med. 2022, 11, 5412. https://doi.org/10.3390/jcm11185412
Avnet S, Falzetti L, Bazzocchi A, Gasperini C, Taddei F, Schileo E, Baldini N. Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study. Journal of Clinical Medicine. 2022; 11(18):5412. https://doi.org/10.3390/jcm11185412
Chicago/Turabian StyleAvnet, Sofia, Luigi Falzetti, Alberto Bazzocchi, Chiara Gasperini, Fulvia Taddei, Enrico Schileo, and Nicola Baldini. 2022. "Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study" Journal of Clinical Medicine 11, no. 18: 5412. https://doi.org/10.3390/jcm11185412
APA StyleAvnet, S., Falzetti, L., Bazzocchi, A., Gasperini, C., Taddei, F., Schileo, E., & Baldini, N. (2022). Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study. Journal of Clinical Medicine, 11(18), 5412. https://doi.org/10.3390/jcm11185412








