Patterns of Outpatient Phecodes Predating the Diagnosis of Systemic Lupus Erythematosus in Taiwanese Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Identification of Main Diagnosis of Outpatient Medical Visits
2.3. Statistical Analysis
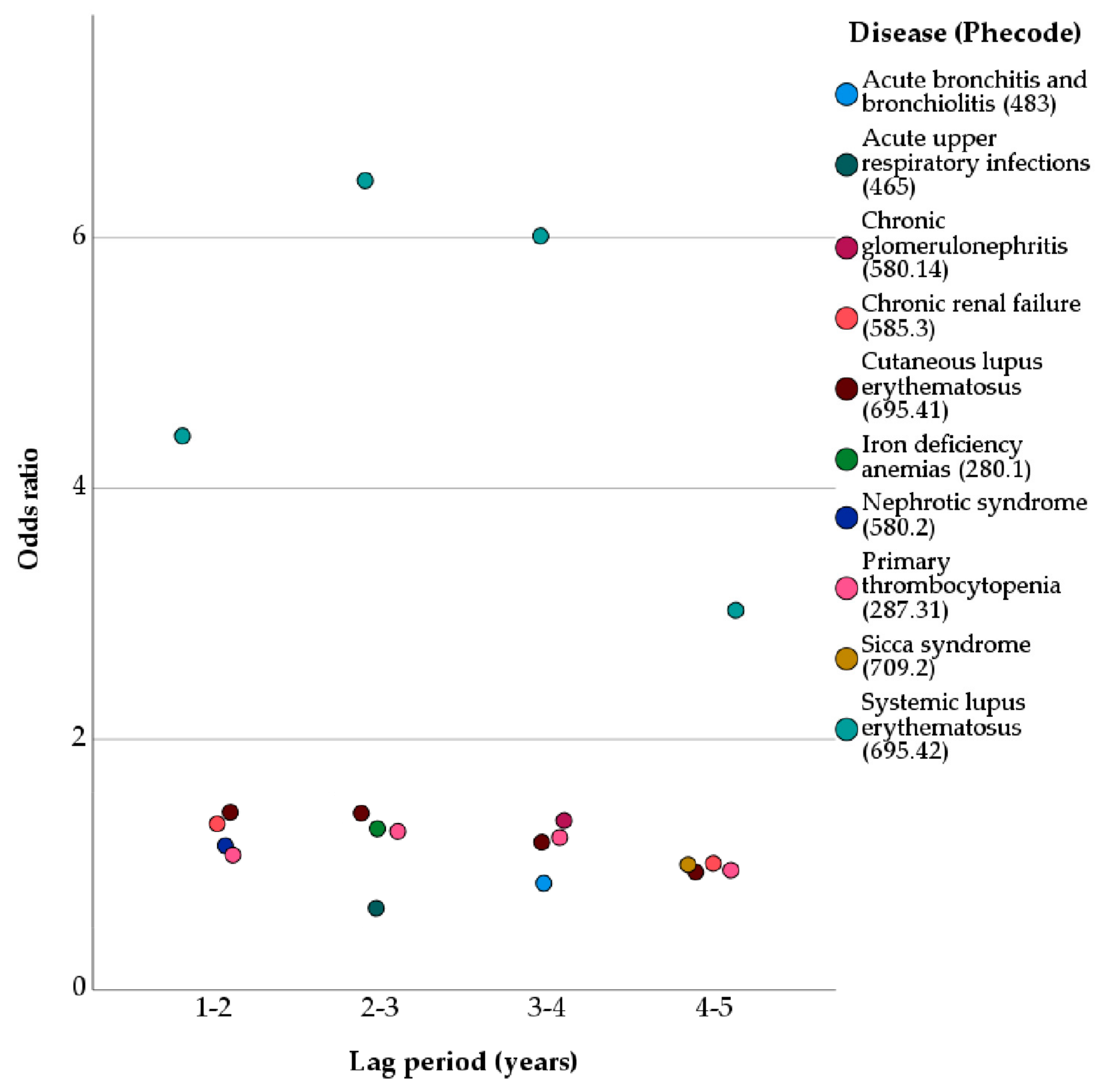
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, M.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Costenbader, K.H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2016, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [PubMed]
- Leong, P.Y.; Huang, J.Y.; Chiou, J.Y.; Bai, Y.C.; Wei, J.C. The prevalence and incidence of systemic lupus erythematosus in Taiwan: A nationwide population-based study. Sci. Rep. 2021, 11, 5631. [Google Scholar] [CrossRef]
- Jönsen, A.; Hjalte, F.; Willim, M.; Carlsson, K.S.; Sjöwall, C.; Svenungsson, E.; Leonard, D.; Bengtsson, C.; Rantapää-Dahlqvist, S.; Pettersson, S.; et al. Direct and indirect costs for systemic lupus erythematosus in Sweden. A nationwide health economic study based on five defined cohorts. Semin. Arthritis Rheum. 2016, 45, 684–690. [Google Scholar] [CrossRef]
- Zhu, T.Y.; Tam, L.S.; Li, E.K. Cost-of-illness studies in systemic lupus erythematosus: A systematic review. Arthritis Care Res. 2011, 63, 751–760. [Google Scholar] [CrossRef]
- Adamichou, C.; Bertsias, G. Flares in systemic lupus erythematosus: Diagnosis, risk factors and preventive strategies. Mediterr. J. Rheumatol. 2017, 28, 4–12. [Google Scholar] [CrossRef]
- Gomez, A.; Qiu, V.; Cederlund, A.; Borg, A.; Lindblom, J.; Emamikia, S.; Enman, Y.; Lampa, J.; Parodis, I. Adverse health-related quality of life outcome despite adequate clinical response to treatment in systemic lupus erythematosus. Front. Med. 2021, 8, 651249. [Google Scholar] [CrossRef]
- Bernatsky, S.; Boivin, J.F.; Joseph, L.; Manzi, S.; Ginzler, E.; Gladman, D.D.; Urowitz, M.; Fortin, P.R.; Petri, M.; Barr, S.; et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2550–2557. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Lam, N.C.; Ghetu, M.V.; Bieniek, M.L. Systemic lupus erythematosus: Primary care approach to diagnosis and management. Am. Fam. Physician 2016, 94, 284–294. [Google Scholar]
- Bertsias, G.K.; Pamfil, C.; Fanouriakis, A.; Boumpas, D.T. Diagnostic criteria for systemic lupus erythematosus: Has the time come? Nat. Rev. Rheumatol. 2013, 9, 687–694. [Google Scholar] [CrossRef]
- Ozbek, S.; Sert, M.; Paydas, S.; Soy, M. Delay in the diagnosis of SLE: The importance of arthritis/arthralgia as the initial symptom. Acta Med. Okayama 2003, 57, 187–190. [Google Scholar]
- Kernder, A.; Richter, J.G.; Fischer-Betz, R.; Winkler-Rohlfing, B.; Brinks, R.; Aringer, M.; Schneider, M.; Chehab, G. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: Cross sectional analysis of the LuLa cohort. Lupus 2021, 30, 431–438. [Google Scholar] [CrossRef]
- Kapsala, N.N.; Nikolopoulos, D.S.; Flouda, S.P.; Chavatza, A.P.; Tseronis, D.D.; Aggelakos, M.D.; Katsimbri, P.P.; Bertsias, G.K.; Fanouriakis, A.C.; Boumpas, D.T. From first symptoms to diagnosis of systemic lupus erythematosus: Mapping the journey of patients in an observational study. Clin. Exp. Rheumatol. 2022, 40, 1–8. [Google Scholar] [CrossRef]
- Morgan, C.; Bland, A.R.; Maker, C.; Dunnage, J.; Bruce, I.N. Individuals living with lupus: Findings from the LUPUS UK Members Survey 2014. Lupus 2018, 27, 681–687. [Google Scholar] [CrossRef]
- Oglesby, A.; Korves, C.; Laliberté, F.; Dennis, G.; Rao, S.; Suthoff, E.D.; Wei, R.; Duh, M.S. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl. Health Econ. Health Policy 2014, 12, 179–190. [Google Scholar] [CrossRef]
- Rees, F.; Doherty, M.; Lanyon, P.; Davenport, G.; Riley, R.D.; Zhang, W.; Grainge, M.J. Early clinical features in systemic lupus erythematosus: Can they be used to achieve earlier diagnosis? A risk prediction model. Arthritis Care Res. 2017, 69, 833–841. [Google Scholar] [CrossRef]
- Lai, N.S.; Tsai, T.Y.; Koo, M.; Huang, K.Y.; Tung, C.H.; Lu, M.C. Patterns of ambulatory medical care utilization and rheumatologist consultation predating the diagnosis of systemic lupus erythematosus: A national population-based study. PLoS ONE 2014, 9, e101485. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Denny, J.C.; Ritchie, M.D.; Basford, M.A.; Pulley, J.M.; Bastarache, L.; Brown-Gentry, K.; Wang, D.; Masys, D.R.; Roden, D.M.; Crawford, D.C. PheWAS: Demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics 2010, 26, 1205–1210. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, S.; Shia, B.C.; Lee, T.S. An epidemiological human disease network derived from disease co-occurrence in Taiwan. Sci. Rep. 2018, 8, 4557. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hung, Y.T.; Chuang, Y.L.; Chen, Y.J.; Weng, W.S.; Liu, J.S.; Liang, K.Y. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. (In Chinese) [Google Scholar]
- Wei, W.Q.; Bastarache, L.A.; Carroll, R.J.; Marlo, J.E.; Osterman, T.J.; Gamazon, E.R.; Cox, N.J.; Roden, D.M.; Denny, J.C. Evaluating Phecodes, Clinical Classification Software, and ICD-9-CM Codes for Phenome-Wide Association Studies in the Electronic Health Record. PLoS ONE 2017, 12, e0175508. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R, 2nd ed.; Springer: New York, NY, USA, 2021; pp. 225–288. [Google Scholar]
- Zhu, F.X.; Huang, J.Y.; Ye, Z.; Wen, Q.Q.; Wei, J.C. Risk of systemic lupus erythematosus in patients with idiopathic thrombocytopenic purpura: A population-based cohort study. Ann. Rheum. Dis. 2020, 79, 793–799. [Google Scholar] [CrossRef]
- Bultink, I.E.; Lems, W.F. Systemic lupus erythematosus and fractures. RMD Open 2015, 1, e000069. [Google Scholar] [CrossRef]
- Jung, J.H.; Soh, M.S.; Ahn, Y.H.; Um, Y.J.; Jung, J.Y.; Suh, C.H.; Kim, H.A. Thrombocytopenia in systemic lupus erythematosus: Clinical manifestations, treatment, and prognosis in 230 patients. Medicine 2016, 95, e2818. [Google Scholar] [CrossRef]
- Bagavant, H.; Fu, S.M. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2009, 21, 489–494. [Google Scholar] [CrossRef]
- Santacruz, J.C.; Mantilla, M.J.; Rueda, I.; Pulido, S.; Rodriguez-Salas, G.; Londono, J. A practical perspective of the hematologic manifestations of systemic lupus erythematosus. Cureus 2022, 14, e22938. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Rayes, H.A.; Lazova, T.; Anderson, N.; Bonilla, D.; Su, J.; Touma, Z. Herpes zoster in SLE: Prevalence, incidence and risk factors. Lupus Sci. Med. 2022, 9, e000574. [Google Scholar] [CrossRef] [PubMed]
- Dammacco, R. Systemic lupus erythematosus and ocular involvement: An overview. Clin. Exp. Med. 2018, 18, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Pavlov-Dolijanovic, S.; Damjanov, N.S.; Vujasinovic Stupar, N.Z.; Marcetic, D.R.; Sefik-Bukilica, M.N.; Petrovic, R.R. Is there a difference in systemic lupus erythematosus with and without Raynaud’s phenomenon? Rheumatol. Int. 2013, 33, 859–865. [Google Scholar] [CrossRef]
- Benli, M.; Batool, F.; Stutz, C.; Petit, C.; Jung, S.; Huck, O. Orofacial manifestations and dental management of systemic lupus erythematosus: A review. Oral Dis. 2021, 27, 151–167. [Google Scholar] [CrossRef]
- Cuchacovich, R.; Gedalia, A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum. Dis. Clin. N. Am. 2009, 35, 75–93. [Google Scholar] [CrossRef]
- Alani, H.; Henty, J.R.; Thompson, N.L.; Jury, E.; Ciurtin, C. Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren’s syndrome (secondary Sjögren’s syndrome) focusing on autoimmune rheumatic diseases. Scand. J. Rheumatol. 2018, 47, 141–154. [Google Scholar] [CrossRef]
- Alharbi, S.; Ahmad, Z.; Bookman, A.A.; Touma, Z.; Sanchez-Guerrero, J.; Mitsakakis, N.; Johnson, S.R. Epidemiology and survival of systemic sclerosis-systemic lupus erythematosus overlap syndrome. J. Rheumatol. 2018, 45, 1406–1410. [Google Scholar] [CrossRef]
- Pasoto, S.G.; Adriano de Oliveira Martins, V.; Bonfa, E. Sjögren’s syndrome and systemic lupus erythematosus: Links and risks. Open Access Rheumatol. 2019, 11, 33–45. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M. Autoimmune rheumatic diseases: One or many diseases? J. Transl. Autoimmun. 2021, 4, 100129. [Google Scholar] [CrossRef]
- Lu, M.C.; Fa, W.H.; Tsai, T.Y.; Koo, M.; Lai, N.S. Increased utilisation of eye disorder-related ambulatory medical services prior to the diagnosis of Sjögren’s syndrome in female patients: A longitudinal population-based study in Taiwan. BMJ Open 2014, 4, e003862. [Google Scholar] [CrossRef]
- Lu, M.C.; Jheng, C.H.; Tsai, T.Y.; Koo, M.; Lai, N.S. Increased dental visits in patients prior to diagnosis of primary Sjögren’s syndrome: A population-based study in Taiwan. Rheumatol. Int. 2014, 34, 1555–1561. [Google Scholar] [CrossRef]
- Peng, X.; Lu, Y.; Wei, J.; Lin, T.; Lu, Q.; Liu, Q.; Ting, W.J. A cohort study of T helper 17 cell-related cytokine levels in tear samples of systemic lupus erythematosus and Sjögren’s syndrome patients with dry eye disease. Clin. Exp. Rheumatol. 2021, 39, 159–165. [Google Scholar] [CrossRef]
- Yen, J.C.; Hsu, C.A.; Li, Y.C.; Hsu, M.H. The prevalence of dry eye syndrome’s and the likelihood to develop Sjögren’s syndrome in Taiwan: A population-based study. Int. J. Environ. Res. Public Health 2015, 12, 7647–7655. [Google Scholar] [CrossRef]
- Akpek, E.K.; Bunya, V.Y.; Saldanha, I.J. Sjögren’s syndrome: More than just dry eye. Cornea 2019, 38, 658–661. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, W.Y.; Tsai, M.C.; Fu, L.S. Systemic lupus erythematosus and thyroid disease—Experience in a single medical center in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 480–486. [Google Scholar] [CrossRef]
- Lee, C.; Chen, S.F.; Yang, Y.C.; Hsu, C.Y.; Shen, Y.C. Association between Graves’ disease and risk of incident systemic lupus erythematosus: A nationwide population-based cohort study. Int. J. Rheum. Dis. 2021, 24, 240–245. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Perricone, C.; Cipriano, E.; Massaro, L.; Natalucci, F.; Capalbo, G.; Leccese, I.; Bogdanos, D.; Spinelli, F.R.; Alessandri, C.; et al. Joint involvement in systemic lupus erythematosus: From pathogenesis to clinical assessment. Semin. Arthritis Rheum. 2017, 47, 53–64. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, diagnosis and management of obstetric antiphospholipid syndrome: A comprehensive review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef]
- Tarr, T.; Lakos, G.; Bhattoa, H.P.; Szegedi, G.; Shoenfeld, Y.; Kiss, E. Primary antiphospholipid syndrome as the forerunner of systemic lupus erythematosus. Lupus 2007, 16, 324–328. [Google Scholar] [CrossRef]
- Chen, H.H.; Lin, C.H.; Chao, W.C. Risk of Systemic lupus erythematosus in patients with anti-phospholipid syndrome: A population-based study. Front. Med. 2021, 8, 654791. [Google Scholar] [CrossRef] [PubMed]
- Navarra, S.V.; Leynes, M.S.N. Infections in systemic lupus erythematosus. Lupus 2010, 19, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Guilabert, A.; Pallarés, L.; Cerveral, R.; Ramos-Casals, M.; Bové, A.; Ingelmo, M.; Font, J. Infections in systemic lupus erythematosus: A prospective and controlled study of 110 patients. Lupus 2006, 15, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Olivares, N.; Ruiz-Arruza, I.; Martinez-Berriotxoa, A.; Egurbide, M.V.; Aguirre, C. Predictors of major infections in systemic lupus erythematosus. Arthritis Res. Ther. 2009, 11, R109. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Zhu, M.; Xu, A. Heart involvement in systemic lupus erythematosus: A systemic review and meta-analysis. Clin. Rheumatol. 2016, 35, 2437–2448. [Google Scholar] [CrossRef]
- Shaban, A.; Leira, E.C. Neurological complications in patients with systemic lupus erythematosus. Curr. Neurol. Neurosci. Rep. 2019, 19, 97. [Google Scholar] [CrossRef]
- Karremah, M.F.; Hassan, R.Y.; Faloudah, A.Z.; Alharbi, L.K.; Shodari, A.F.; Rahbeeni, A.A.; Alharazi, N.K.; Binjabi, A.Z.; Cheikh, M.M.; Manasfi, H.; et al. From symptoms to diagnosis: An observational study of the journey of SLE patients in Saudi Arabia. Open Access Rheumatol. 2022, 14, 103–111. [Google Scholar] [CrossRef]
| Variable | n (%) | p Value | |
|---|---|---|---|
| Systemic lupus erythematosus 547 (20.0) | Control 2188 (80.0) | ||
| Age group, years | >0.999 | ||
| 20–29 | 170 (31.1) | 680 (31.1) | |
| 30–39 | 161 (29.4) | 644 (29.4) | |
| 40–49 | 127 (23.2) | 508 (23.2) | |
| 50–59 | 89 (16.3) | 356 (16.3) | |
| Mean age, years (SD) | 36.8 (10.6) | 36.8 (10.6) | >0.999 |
| Socioeconomic status | >0.999 | ||
| Low | 182 (33.3) | 728 (33.3) | |
| Medium | 136 (24.9) | 544 (24.9) | |
| High | 229 (41.9) | 916 (41.9) | |
| Urbanization level | >0.999 | ||
| Urban | 140 (25.6) | 560 (25.6) | |
| Suburban | 135 (24.7) | 540 (24.7) | |
| Rural | 272 (49.7) | 1088 (49.7) | |
| Disease Phenotype (Phecode) | Years Prior to Definitive Diagnosis of SLE | ||||||
|---|---|---|---|---|---|---|---|
| 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | 7–8 | |
| Systemic lupus erythematosus (695.42) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cutaneous lupus erythematosus (695.41) | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Herpes zoster (053) | 6 | ||||||
| Other benign neoplasm of uterus (218.2) | 7 | ||||||
| Graves’ disease (242.1) | 3 | ||||||
| Chronic lymphocytic thyroiditis (245.21) | 10 | 10 | |||||
| Disorders involving the immune mechanism (279) | 6 | ||||||
| Iron deficiency anemias, unspecified or not due to blood loss (280.1) | 4 | 6 | |||||
| Thrombocytopenia (287.3) | 7 | ||||||
| Primary thrombocytopenia (287.31) | 5 | 5 | 4 | 3 | 4 | ||
| Visual disturbances (368) | 10 | ||||||
| Conjunctivitis, infectious (369.5) | 10 | ||||||
| Raynaud’s syndrome (443.1) | 7 | 8 | |||||
| Acute upper respiratory infections of multiple or unspecified sites (465) | 3 ↓ | 7 ↓ | |||||
| Acute bronchitis and bronchiolitis (483) | 7 ↓ | ||||||
| Dental caries (521.1) | 3 | ||||||
| Chronic glomerulonephritis, not otherwise specified (580.14) | 9 | 5 | |||||
| Nephrotic syndrome without mention of glomerulonephritis (580.2) | 4 | ||||||
| Chronic renal failure (585.3) | 3 | 8 | 4 | ||||
| Calculus of ureter (594.3) | 9 | ||||||
| Inflammatory diseases of uterus, except cervix (614.4) | 5 | ||||||
| Miscarriage; stillbirth (634) | 6 | ||||||
| Other local infections of skin and subcutaneous tissue (686) | 9 | ||||||
| Localized superficial swelling, mass, or lump (687.2) | 8 | ||||||
| Sicca syndrome (709.2) | 5 | ||||||
| Systemic sclerosis (709.3) | 6 | ||||||
| Unspecified diffuse connective tissue disease (709.7) | 8 | ||||||
| Arthropathy, not otherwise specified (716.9) | 9 | 8 | |||||
| Displacement of intervertebral disc (722.1) | 3 | ||||||
| Fracture of unspecified bones (809) | 3 | ||||||
| Internal derangement of knee (835) | 4 | ||||||
| Number of non-zero coefficient | 22 | 46 | 40 | 25 | 8 | 4 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.-C.; Hsu, C.-W.; Koo, M. Patterns of Outpatient Phecodes Predating the Diagnosis of Systemic Lupus Erythematosus in Taiwanese Women. J. Clin. Med. 2022, 11, 5406. https://doi.org/10.3390/jcm11185406
Lu M-C, Hsu C-W, Koo M. Patterns of Outpatient Phecodes Predating the Diagnosis of Systemic Lupus Erythematosus in Taiwanese Women. Journal of Clinical Medicine. 2022; 11(18):5406. https://doi.org/10.3390/jcm11185406
Chicago/Turabian StyleLu, Ming-Chi, Chia-Wen Hsu, and Malcolm Koo. 2022. "Patterns of Outpatient Phecodes Predating the Diagnosis of Systemic Lupus Erythematosus in Taiwanese Women" Journal of Clinical Medicine 11, no. 18: 5406. https://doi.org/10.3390/jcm11185406
APA StyleLu, M.-C., Hsu, C.-W., & Koo, M. (2022). Patterns of Outpatient Phecodes Predating the Diagnosis of Systemic Lupus Erythematosus in Taiwanese Women. Journal of Clinical Medicine, 11(18), 5406. https://doi.org/10.3390/jcm11185406








