AP39, a Mitochondrial-Targeted H2S Donor, Improves Porcine Islet Survival in Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement, Preservation, and Islet Isolation of Porcine Pancreas
2.2. Culture of Isolated Porcine Islets
2.3. Islet Evaluation
2.4. Annexin V/PI Staining
2.5. In Vitro Assessment
2.6. In Vivo Assessment
2.7. Statistical Analysis
3. Results
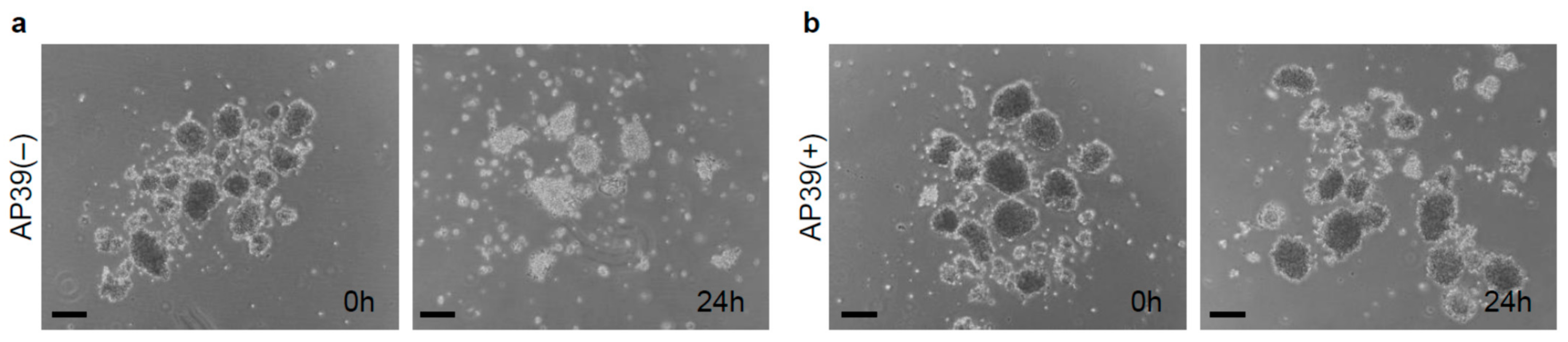
3.1. Culture of Isolated Islets with AP39
3.2. Annexin V/PI Staining
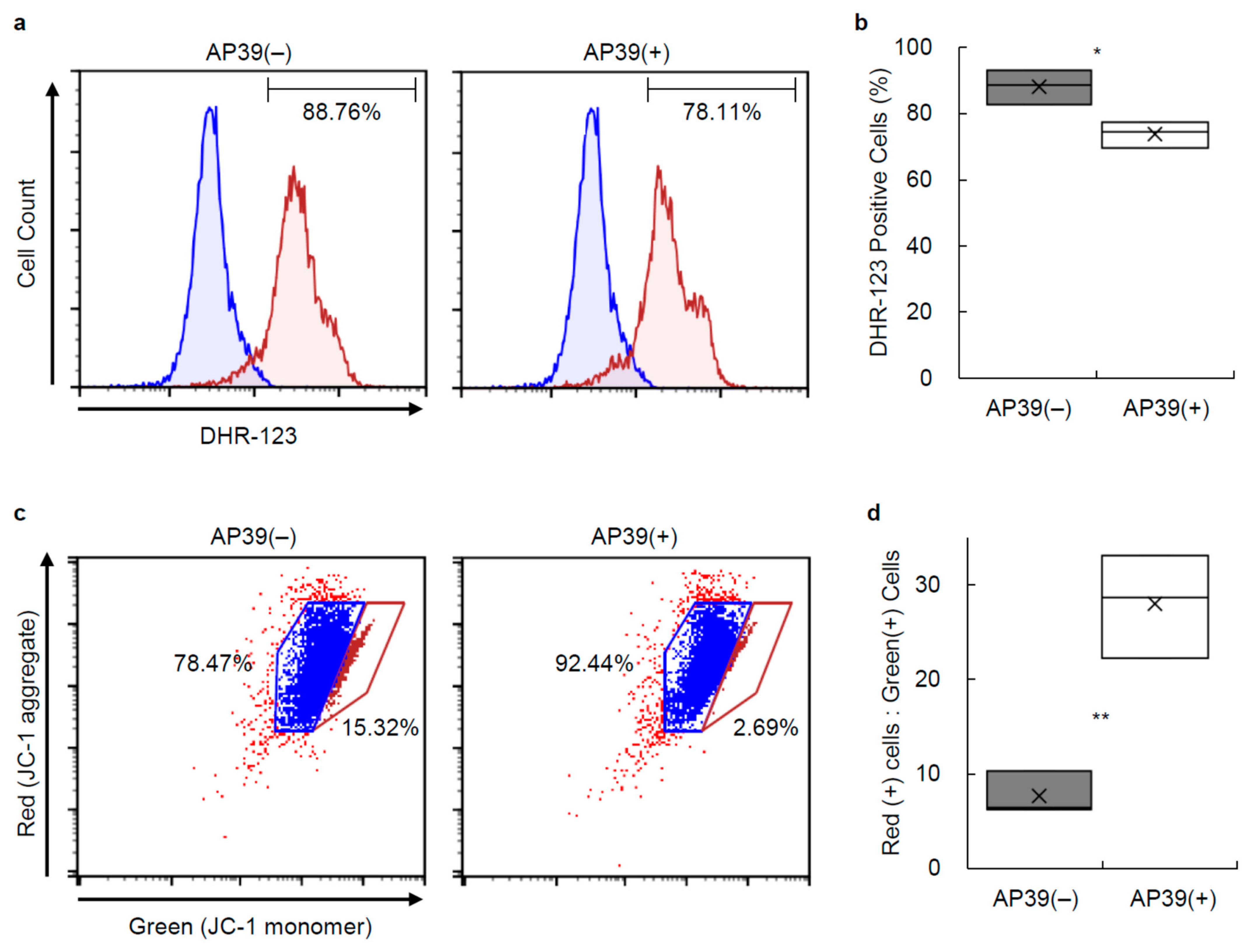
3.3. Production of ROS in Porcine Islets
3.4. Mitochondrial Membrane Permeability and Proton Pumping in Porcine Islets
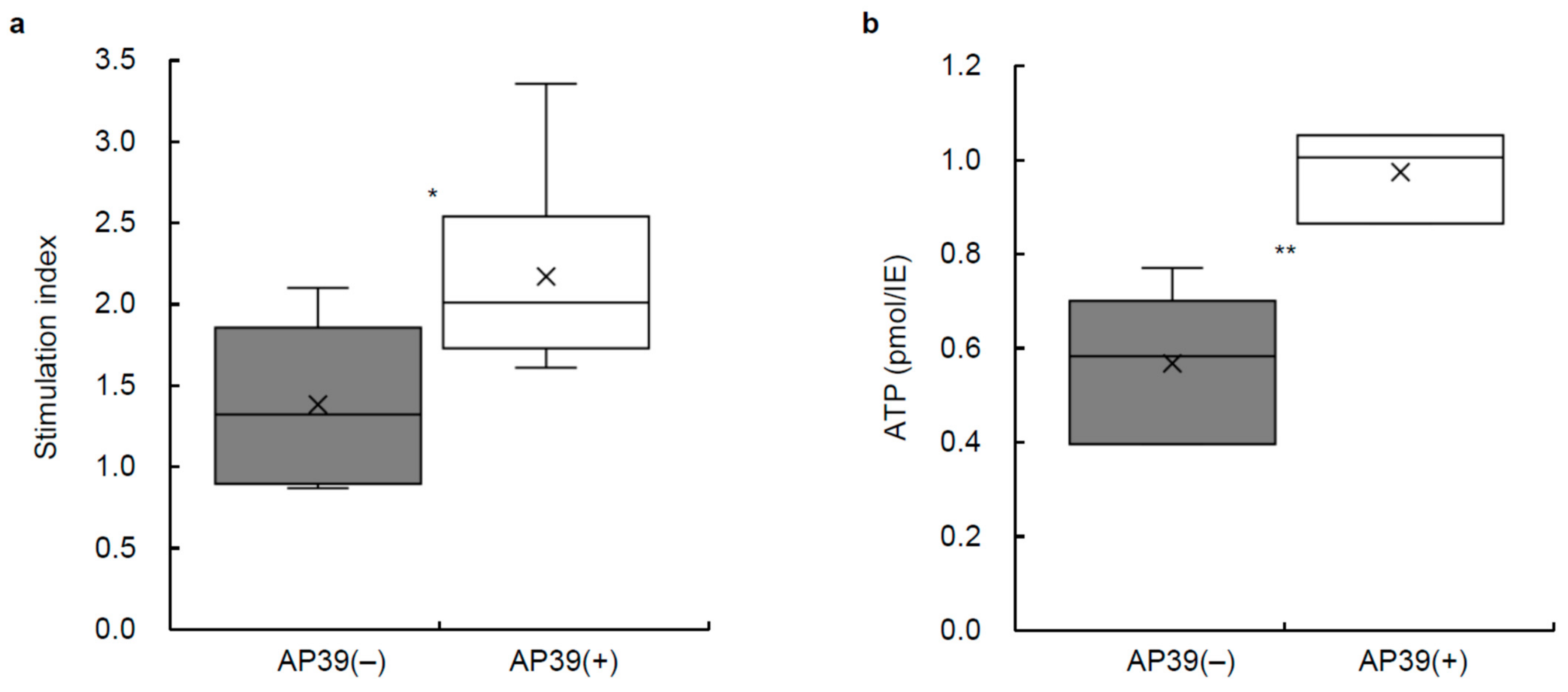
3.5. Stimulation Index of Cultured Islets Treated with or without AP39
3.6. ATP Content of Cultured Islets Treated with or without AP39
3.7. In Vivo Assessment of Cultured Islets Treated with or without AP39
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H. Clinical Islet Transplantation Covered by Health Insurance in Japan. J. Clin. Med. 2022, 11, 3977. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Kandaswamy, R.; Harmon, J.V.; Ansite, J.D.; Clemmings, S.M.; Sakai, T.; Paraskevas, S.; Eckman, P.M.; Sageshima, J.; Nakano, M.; et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am. J. Transplant. 2004, 4, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Froud, T.; Ricordi, C.; Baidal, D.A.; Hafiz, M.M.; Ponte, G.; Cure, P.; Pileggi, A.; Poggioli, R.; Ichii, H.; Khan, A.; et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 2005, 5, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Matsumoto, S. Evaluation of islet transplantation from non-heart beating donors. Am. J. Transplant. 2006, 6, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Senior, P.; O’Gorman, D.; Richer, B.; Salam, A.; Shapiro, A.M. Risk factors for islet loss during culture prior to transplantation. Transpl. Int. 2008, 21, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Kedinger, M.; Haffen, K.; Grenier, J.; Eloy, R. In vitro culture reduces immunogenicity of pancreatic endocrine islets. Nature 1977, 270, 736–738. [Google Scholar] [CrossRef]
- Keymeulen, B.; Gillard, P.; Mathieu, C.; Movahedi, B.; Maleux, G.; Delvaux, G.; Ysebaert, D.; Roep, B.; Vandemeulebroucke, E.; Marichal, M.; et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc. Natl. Acad. Sci. USA 2006, 103, 17444–17449. [Google Scholar] [CrossRef]
- Ichii, H.; Sakuma, Y.; Pileggi, A.; Fraker, C.; Alvarez, A.; Montelongo, J.; Szust, J.; Khan, A.; Inverardi, L.; Naziruddin, B.; et al. Shipment of human islets for transplantation. Am. J. Transplant. 2007, 7, 1010–1020. [Google Scholar] [CrossRef]
- Noguchi, H.; Yamada, Y.; Okitsu, T.; Iwanaga, Y.; Nagata, H.; Kobayashi, N.; Hayashi, S.; Matsumoto, S. Secretory unit of islet in transplantation (SUIT) and engrafted islet rate (EIR) indexes are useful for evaluating single islet transplantation. Cell Transplant. 2008, 17, 121–128. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Zhu, J.X.; Kalbfleisch, M.; Yang, Y.X.; Bihari, R.; Lobb, I.; Davison, M.; Mok, A.; Cepinskas, G.; Lawendy, A.R.; Sener, A. Detrimental effects of prolonged warm renal ischaemia-reperfusion injury are abrogated by supplemental hydrogen sulphide: An analysis using real-time intravital microscopy and polymerase chain reaction. BJU Int. 2012, 110, E1218–E1227. [Google Scholar] [CrossRef]
- Bos, E.M.; Wang, R.; Snijder, P.M.; Boersema, M.; Damman, J.; Fu, M.; Moser, J.; Hillebrands, J.L.; Ploeg, R.J.; Yang, G.; et al. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013, 24, 759–770. [Google Scholar] [CrossRef]
- Lobb, I.; Zhu, J.; Liu, W.; Haig, A.; Lan, Z.; Sener, A. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can. Urol. Assoc. J. 2014, 8, E413–E418. [Google Scholar] [CrossRef]
- Johansen, D.; Ytrehus, K.; Baxter, G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—Evidence for a role of K ATP channels. Basic Res. Cardiol. 2006, 101, 53–60. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef]
- Ikeda, K.; Marutani, E.; Hirai, S.; Wood, M.E.; Whiteman, M.; Ichinose, F. Mitochondria-targeted hydrogen sulfide donor AP39 improves neurological outcomes after cardiac arrest in mice. Nitric Oxide 2015, 49, 90–96. [Google Scholar] [CrossRef]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress In Vitro and in Acute Renal Injury In Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Nishime, K.; Miyagi-Shiohira, C.; Kuwae, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Saitoh, I.; Watanabe, M.; Noguchi, H. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am. J. Transplant. 2021, 21, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Miyagi-Shiohira, C.; Kuwae, K.; Nishime, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Yamasaki, I.; Shinzato, M.; Saitoh, I.; et al. Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation. J. Clin. Med. 2022, 11, 4290. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Saitoh, I.; Watanabe, M. Novel cell-permeable p38-MAPK inhibitor efficiently prevents porcine islet apoptosis and improves islet graft function. Am. J. Transplant. 2020, 20, 1296–1308. [Google Scholar] [CrossRef]
- Sakai-Yonaha, M.; Miyagi-Shiohira, C.; Kuwae, K.; Tamaki, Y.; Nishime, K.; Yonaha, T.; Saitoh, I.; Watanabe, M.; Noguchi, H. Pancreas Preservation in Modified Histidine-lactobionate Solution Is Superior to That in University of Wisconsin Solution for Porcine Islet Isolation. Transplantation 2022, 106, 1770–1776. [Google Scholar] [CrossRef]
- Ellis, C.; Lyon, J.G.; Korbutt, G.S. Optimization and Scale-Up Isolation and Culture of Neonatal Porcine Islets: Potential for Clinical Application. Cell Transplant. 2016, 25, 539–547. [Google Scholar] [CrossRef]
- Kuwae, K.; Miyagi-Shiohira, C.; Hamada, E.; Tamaki, Y.; Nishime, K.; Sakai, M.; Yonaha, T.; Makishi, E.; Saitoh, I.; Watanabe, M.; et al. Excellent Islet Yields after 18-h Porcine Pancreas Preservation by Ductal Injection, Pancreas Preservation with MK Solution, Bottle Purification, and Islet Purification Using Iodixanol with UW Solution and Iodixanol with MK Solution. J. Clin. Med. 2019, 8, 1561. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M. Modified cell-permeable JNK inhibitors efficiently prevents islet apoptosis and improves the outcome of islet transplantation. Sci. Rep. 2018, 8, 11082. [Google Scholar] [CrossRef]
- Noguchi, H.; Ueda, M.; Hayashi, S.; Kobayashi, N.; Okitsu, T.; Iwanaga, Y.; Nagata, H.; Nakai, Y.; Matsumoto, S. Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function. Cell Transplant. 2008, 17, 69–81. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef]
- Noguchi, H.; Ueda, M.; Nakai, Y.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Kobayashi, N.; Nakamura, T.; Wada, H.; et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am. J. Transplant. 2006, 6, 496–504. [Google Scholar] [CrossRef]
- Noguchi, H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. J. Clin. Med. 2020, 10, 10. [Google Scholar] [CrossRef]
- Ricordi, C.; Gray, D.W.; Hering, B.J.; Kaufman, D.B.; Warnock, G.L.; Kneteman, N.M.; Lake, S.P.; London, N.J.; Socci, C.; Alejandro, R. Islet isolation assessment in man and large animals. Acta Diabetol. Lat. 1990, 27, 185–195. [Google Scholar] [CrossRef]
- Bank, H.L. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Dev. Biol. 1988, 24, 266–273. [Google Scholar] [CrossRef]
- Faust, A.; Rothe, H.; Schade, U.; Lampeter, E.; Kolb, H. Primary nonfunction of islet grafts in autoimmune diabetic nonobese diabetic mice is prevented by treatment with interleukin-4 and interleukin-10. Transplantation 1996, 62, 648–652. [Google Scholar] [CrossRef]
- Brandhorst, H.; Theisinger, B.; Guenther, B.; Johnson, P.R.; Brandhorst, D. Pancreatic L-Glutamine Administration Protects Pig Islets from Cold Ischemic Injury and Increases Resistance Toward Inflammatory Mediators. Cell Transplant. 2016, 25, 531–538. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Li, H.; Xue, M.; Ji, A.; Li, Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 186908. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Juriasingani, S.; Zhang, M.Y.; Sener, A. H2S donor molecules against cold ischemia-reperfusion injury in preclinical models of solid organ transplantation. Pharmacol. Res. 2021, 172, 105842. [Google Scholar] [CrossRef]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Hu, L.F.; Lu, M.; Wu, Z.Y.; Wong, P.T.; Bian, J.S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009, 75, 27–34. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef]
- Ackermann, M.; Kubitza, M.; Hauska, G.; Piña, A.L. The vertebrate homologue of sulfide-quinone reductase in mammalian mitochondria. Cell Tissue Res. 2014, 358, 779–792. [Google Scholar] [CrossRef]
- Abdelli, S.; Ansite, J.; Roduit, R.; Borsello, T.; Matsumoto, I.; Sawada, T.; Allaman-Pillet, N.; Henry, H.; Beckmann, J.S.; Hering, B.J.; et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004, 53, 2815–2823. [Google Scholar] [CrossRef][Green Version]
- Ammendrup, A.; Maillard, A.; Nielsen, K.; Aabenhus Andersen, N.; Serup, P.; Dragsbaek Madsen, O.; Mandrup-Poulsen, T.; Bonny, C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 2000, 49, 1468–1476. [Google Scholar] [CrossRef]
- Noguchi, H. Regulation of c-Jun NH (2)-Terminal Kinase for Islet Transplantation. J. Clin. Med. 2019, 8, 1763. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Xu, G.; Fujii, N.; Kim, S.; Bonner-Weir, S.; Weir, G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002, 277, 30010–30018. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Moritz, W.; Bodmer, E.; Dindo, D.; Kugelmeier, P.; Lehmann, R.; Gassmann, M.; Groscurth, P.; Weber, M. Central necrosis in isolated hypoxic human pancreatic islets: Evidence for postisolation ischemia. Cell Transplant. 2005, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | n = 6 |
|---|---|
| Pancreas size (g) | 105.8 ± 7.0 |
| Operation time (min) | 7.2 ± 1.1 |
| Warm ischemic time (min) | 28.2 ± 1.1 |
| Cold ischemic time (min) | 1114.2 ± 13.9 |
| Phase I period (min) | 11.0 ± 0.4 |
| Phase II period (min) | 38.8 ± 4.0 |
| Undigested tissue (g) | 8.8 ± 1.7 |
| Characteristic | n = 6 |
|---|---|
| IE before purification | 544,440 ± 89,511 |
| IE after purification | 458,933 ± 96,123 |
| Embedded islets (%) | 11.5 ± 3.3 |
| Viability (%) | 96.6 ± 0.4 |
| Purity (%) | 57.3 ± 4.4 |
| Post-purification recovery (%) 1 | 82.2 ± 5.5 |
| Score | 9.4 ± 0.1 |
| AP39(−) | AP39(+) | |
| No. Transplanted Mice | 12 | 12 |
| No. Cured Mice | 0 | 7 |
| % | 0 | 58.3 |
| p-Value vs. AP39(−) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinzato, M.; Miyagi-Shiohira, C.; Kuwae, K.; Nishime, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Yamasaki, I.; Otsuka, R.; Saitoh, I.; et al. AP39, a Mitochondrial-Targeted H2S Donor, Improves Porcine Islet Survival in Culture. J. Clin. Med. 2022, 11, 5385. https://doi.org/10.3390/jcm11185385
Shinzato M, Miyagi-Shiohira C, Kuwae K, Nishime K, Tamaki Y, Yonaha T, Sakai-Yonaha M, Yamasaki I, Otsuka R, Saitoh I, et al. AP39, a Mitochondrial-Targeted H2S Donor, Improves Porcine Islet Survival in Culture. Journal of Clinical Medicine. 2022; 11(18):5385. https://doi.org/10.3390/jcm11185385
Chicago/Turabian StyleShinzato, Misaki, Chika Miyagi-Shiohira, Kazuho Kuwae, Kai Nishime, Yoshihito Tamaki, Tasuku Yonaha, Mayuko Sakai-Yonaha, Ikuo Yamasaki, Ryusei Otsuka, Issei Saitoh, and et al. 2022. "AP39, a Mitochondrial-Targeted H2S Donor, Improves Porcine Islet Survival in Culture" Journal of Clinical Medicine 11, no. 18: 5385. https://doi.org/10.3390/jcm11185385
APA StyleShinzato, M., Miyagi-Shiohira, C., Kuwae, K., Nishime, K., Tamaki, Y., Yonaha, T., Sakai-Yonaha, M., Yamasaki, I., Otsuka, R., Saitoh, I., Watanabe, M., & Noguchi, H. (2022). AP39, a Mitochondrial-Targeted H2S Donor, Improves Porcine Islet Survival in Culture. Journal of Clinical Medicine, 11(18), 5385. https://doi.org/10.3390/jcm11185385







