Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends
Abstract
:1. Introduction
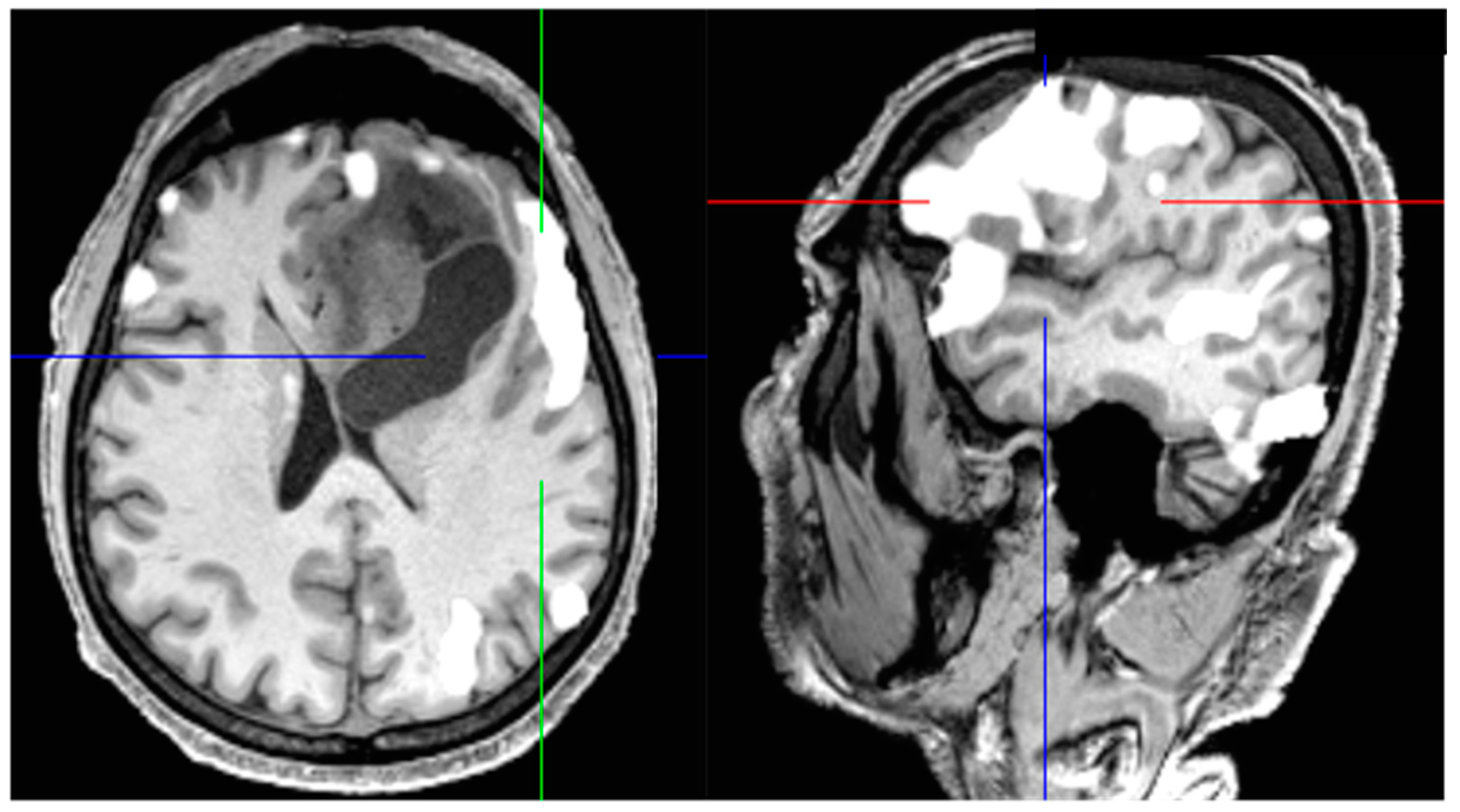
2. Extent of Resection and Residual Tumor Volume: Agreements and Controversies
3. Fluorescence-Guided Surgery: An Indispensable Innovation
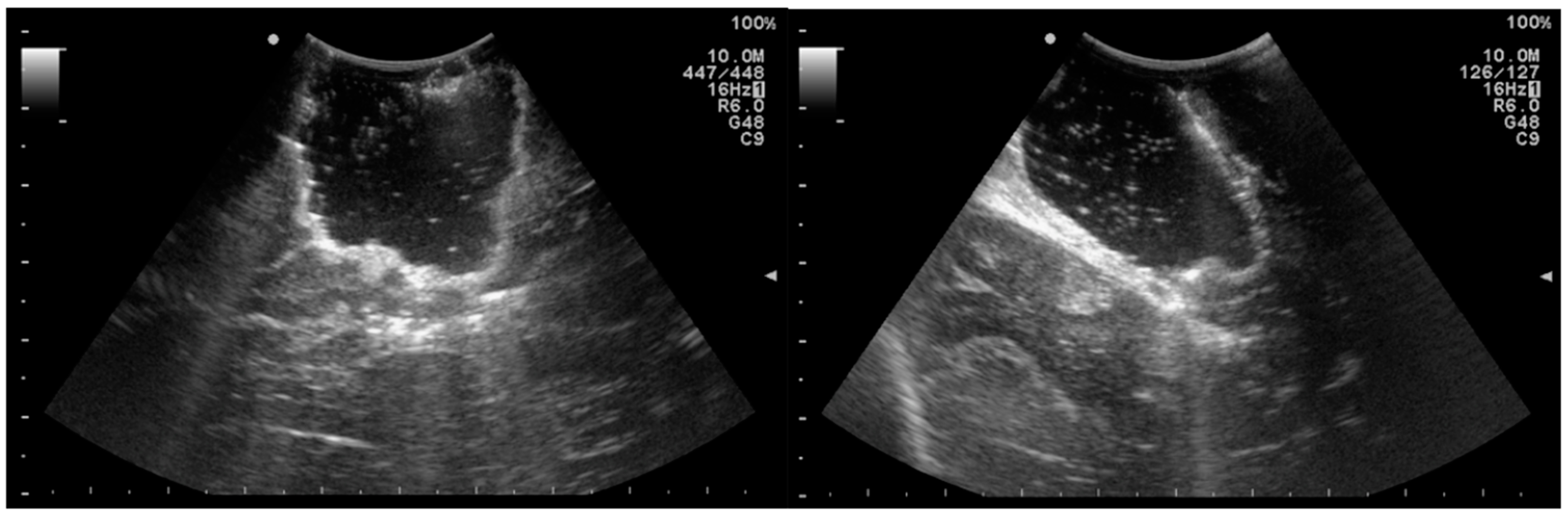
4. Intraoperative Ultrasound: A Widely Available and Inexpensive Tool
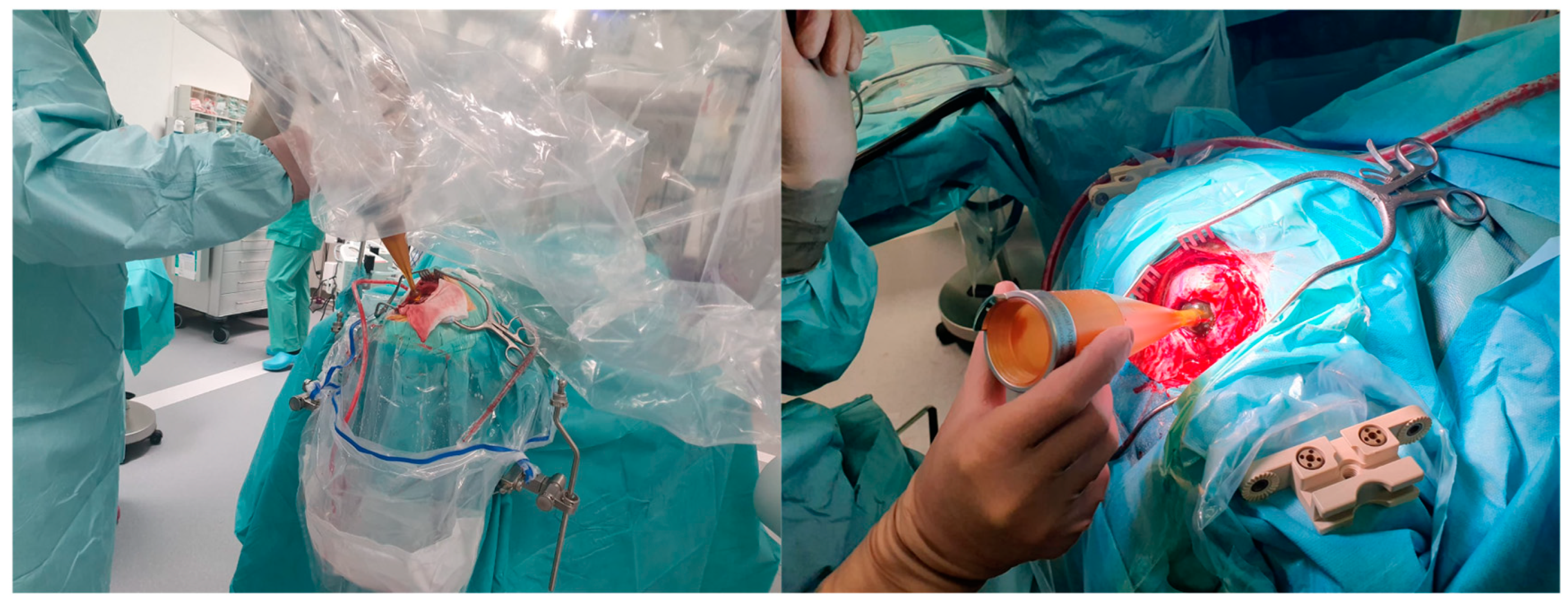
5. Intraoperative Radiotherapy: Targeting Infiltrative Margins
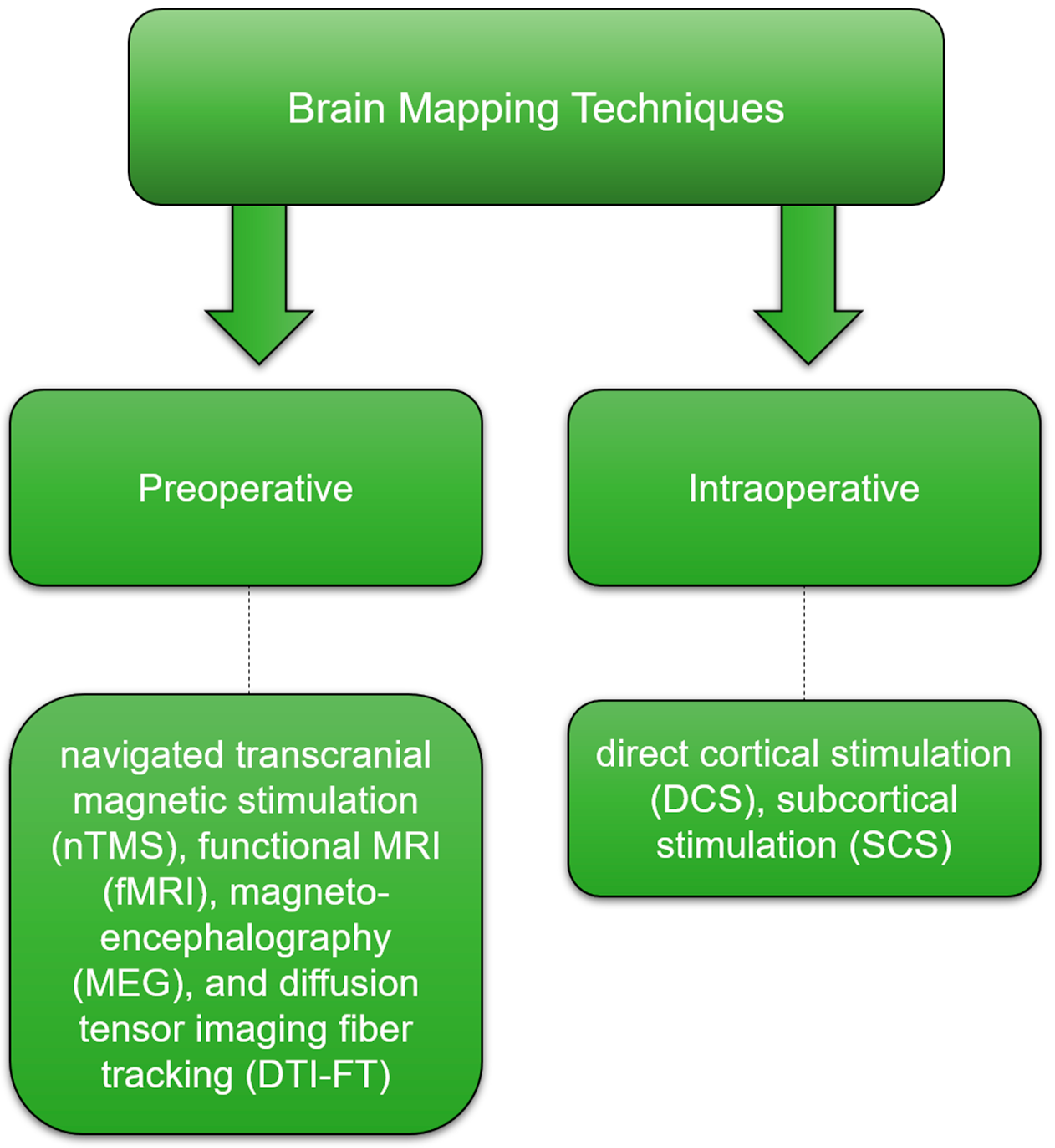
6. Brain Mapping, Monitoring Strategies, and Awake Surgery: Locating and Preserving Critical Functions
7. Confocal Intraoperative Microscope, Intraoperative Mass Spectrometry and Laser Interstitial Thermal Therapy: Current Trends
8. Raman Spectroscopy and Optical Coherence Tomography: The Future of Glioblastoma Surgery?
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Carlsson, S.K.; Brothers, S.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Harter, D.H.; Wilson, T.A.; Karajannis, M.A. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neuro-Oncol. 2011, 107, 359–364. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Bush, N.A.O.; Chang, S.M.; Berger, M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017, 40, 1–14. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; Demonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Orringer, D.; Lau, D.; Khatri, S.; Zamora-Berridi, G.J.; Zhang, K.; Wu, C.; Chaudhary, N.; Sagher, O. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012, 117, 851–859. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Oppenlander, M.E.; Wolf, A.B.; Snyder, L.A.; Bina, R.; Wilson, J.R.; Coons, S.W.; Ashby, L.S.; Brachman, D.; Nakaji, P.; Porter, R.W.; et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 2014, 120, 846–853. [Google Scholar] [CrossRef]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Goyal, A.; Graffeo, C.S.; Perry, A.; Burns, T.C.; Parney, I.F.; Quinones-Hinojosa, A.; Chaichana, K.L. Survival Benefit of Maximal Resection for Glioblastoma Reoperation in the Temozolomide Era: A Meta-Analysis. World Neurosurg. 2019, 127, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Cabrera-Aldana, E.E.; Jusue-Torres, I.; Wijesekera, O.; Olivi, A.; Rahman, M.; Quinones-Hinojosa, A. When Gross Total Resection of a Glioblastoma Is Possible, How Much Resection Should Be Achieved? World Neurosurg. 2014, 82, e257–e265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Li, Y.-B.; Cao, Y.; Li, P.-L.; Liang, B.; Sun, J.-D.; Feng, E.-S. Prognostic implications of resection extent for patients with glioblastoma multiforme: A meta-analysis. J. Neurosurg. Sci. 2017, 61, 631–639. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Bette, S.; Barz, M.; Wiestler, B.; Huber, T.; Gerhardt, J.; Buchmann, N.; Combs, S.E.; Schmidt-Graf, F.; Delbridge, C.; Zimmer, C.; et al. Prognostic Value of Tumor Volume in Glioblastoma Patients: Size Also Matters for Patients with Incomplete Resection. Ann. Surg. Oncol. 2017, 25, 558–564. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-oncology 2013, 16, 113–122. [Google Scholar] [CrossRef]
- Woo, P.Y.; Ho, J.M.; Tse, T.P.; Lam, S.W.; Mak, C.H.; Chan, D.T.; Lee, M.W.; Wong, S.-T.; Chan, K.-Y.; Poon, W.-S. Determining a cut-off residual tumor volume threshold for patients with newly diagnosed glioblastoma treated with temozolomide chemoradiotherapy: A multicenter cohort study. J. Clin. Neurosci. 2019, 63, 134–141. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, X. Which Parameter Is More Important for the Prognosis of New-Onset Adult Glioblastoma: Residual Tumor Volume or Extent of Resection? World Neurosurg. 2018, 116, e444–e451. [Google Scholar] [CrossRef]
- Pessina, F.; Navarria, P.; Cozzi, L.; Tomatis, S.; Riva, M.; Ascolese, A.M.; Santoro, A.; Simonelli, M.; Bello, L.; Scorsetti, M. Role of surgical resection in recurrent glioblastoma: Prognostic factors and outcome evaluation in an observational study. J. Neuro-Oncol. 2016, 131, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.H.A.; Bette, S.; Barz, M.; Huber, T.; Wiestler, B.; Ryang, Y.-M.; Schmidt-Graf, F.; Liesche, F.; Combs, S.E.; Meyer, B.; et al. Role of postoperative tumor volume in patients with MGMT-unmethylated glioblastoma. J. Neuro-Oncol. 2019, 142, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, Y.; Friedman, E.; Liu, Z.; Zhu, J.-J.; Hsu, S.; Tandon, N. The Survival Advantage of “Supratotal” Resection of Glioblastoma Using Selective Cortical Mapping and the Subpial Technique. Neurosurgery 2017, 81, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Kreth, F.W.; Thon, N.; Simon, M.; Westphal, M.; Schackert, G.; Nikkhah, G.; Hentschel, B.; Reifenberger, G.; Pietsch, T.; Weller, M.; et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann. Oncol. 2013, 24, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Mahavadi, A.; Di, L.; Sanjurjo, A.; Eichberg, D.G.; Borowy, V.; Figueroa, J.; Luther, E.; De La Fuente, M.I.; Semonche, A.; et al. Survival benefit of lobectomy for glioblastoma: Moving towards radical supramaximal resection. J. Neuro-Oncol. 2020, 148, 501–508. [Google Scholar] [CrossRef]
- Altieri, R.; Melcarne, A.; Soffietti, R.; Rudá, R.; Franchino, F.; Pellerino, A.; Rocca, G.L.; Sabatino, G.; Olivi, A.; Ducati, A.; et al. Supratotal Resection of Glioblastoma: Is Less More? Surg. Technol. Int. 2019, 35, 432–440. [Google Scholar]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Diez-Valle, R.; Tejada-Solis, S.; Idoate-Gastearena, M.; Garcia-De-Eulate, R.; Dominguez, P.D.; Mendiroz, J.A. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neuro-Oncol. 2010, 102, 105–113. [Google Scholar] [CrossRef]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef]
- Aldave, G.; Tejada, S.; Pay, E.; Marigil, M.; Bejarano, B.; Idoate, M.A.; Díez-Valle, R. Prognostic Value of Residual Fluorescent Tissue in Glioblastoma Patients After Gross Total Resection in 5-Aminolevulinic Acid-Guided Surgery. Neurosurgery 2013, 72, 915–921. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Panciani, P.P.; Fontanella, M.; Schatlo, B.; Garbossa, D.; Agnoletti, A.; Ducati, A.; Lanotte, M. Fluorescence and image guided resection in high grade glioma. Clin. Neurol. Neurosurg. 2012, 114, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.-C.; Mehdorn, H.M.; Nestler, U.; Franz, K.; Goetz, C.; Bink, A.; Pichlmeier, U. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J. Neurosurg. 2011, 114, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Shi, C.; Liu, Y.; Teng, L.; Han, D.; et al. Intraoperative Fluorescence-Guided Resection of High-Grade Malignant Gliomas Using 5-Aminolevulinic Acid–Induced Porphyrins: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA–enhanced intraoperative MRI at the border of contrast-enhancing lesions: A prospective study based on histopathological assessment. Neurosurg. Focus 2014, 36, E3. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for Glioblastoma: Impact of the Combined Use of 5-Aminolevulinic Acid and Intraoperative MRI on Extent of Resection and Survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef]
- Schucht, P.; Seidel, K.; Beck, J.; Murek, M.; Jilch, A.; Wiest, R.; Fung, C.; Raabe, A. Intraoperative monopolar mapping during 5-ALA–guided resections of glioblastomas adjacent to motor eloquent areas: Evaluation of resection rates and neurological outcome. Neurosurg. Focus 2014, 37, E16. [Google Scholar] [CrossRef]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Stockhammer, F.; Misch, M.; Horn, P.; Koch, A.; Fonyuy, N.; Plotkin, M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir. 2009, 151, 1377–1383. [Google Scholar] [CrossRef]
- Kamp, M.A.; Felsberg, J.; Sadat, H.; Kuzibaev, J.; Steiger, H.-J.; Rapp, M.; Reifenberger, G.; Dibué, M.; Sabel, M. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2014, 157, 207–214. [Google Scholar] [CrossRef]
- Yamada, S.; Muragaki, Y.; Maruyama, T.; Komori, T.; Okada, Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin. Neurol. Neurosurg. 2015, 130, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roder, C.; Bisdas, S.; Ebner, F.; Honegger, J.; Naegele, T.; Ernemann, U.; Tatagiba, M. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: High-field iMRI versus conventional and 5-ALA-assisted surgery. Eur. J. Surg. Oncol. 2014, 40, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2013, 156, 305–312. [Google Scholar] [CrossRef]
- Esteves, S.; Alves, M.; Castel-Branco, M.; Stummer, W. A Pilot Cost-Effectiveness Analysis of Treatments in Newly Diagnosed High-Grade Gliomas. Neurosurgery 2015, 76, 552–562. [Google Scholar] [CrossRef]
- Slof, J.; Valle, R.D.; Galván, J. Análisis coste-efectividad de la cirugía del glioma maligno guiada por fluorescencia con ácido 5-aminolevulínico. Neurología 2015, 30, 163–168. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cavallo, C.; Boffano, C.; Cordella, R.; Cuppini, L.; Pollo, B.; Schiariti, M.; et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg. Focus 2014, 36, E5. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Ohta, T. Comparison between operative findings on malignant glioma by a fluorescein surgical microscopy and histological findings. Neurol. Res. 1999, 21, 130–134. [Google Scholar] [CrossRef]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef]
- Miller, S.E.; Tummers, W.S.; Teraphongphom, N.; Berg, N.S.V.D.; Hasan, A.; Ertsey, R.D.; Nagpal, S.; Recht, L.D.; Plowey, E.D.; Vogel, H.; et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J. Neuro-Oncol. 2018, 139, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-S.; Gong, X.; Song, Y.-Y.; Zhuang, D.-X.; Yao, C.-J.; Qiu, T.-M.; Lu, J.-F.; Zhang, J.; Zhu, W.; Mao, Y.; et al. 3.0-T Intraoperative Magnetic Resonance Imaging-Guided Resection in Cerebral Glioma Surgery. Neurosurgery 2014, 61, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.G.; Lawrie, T.A.; Hart, M.G. Image guided surgery for the resection of brain tumours. In Cochrane Database of Systematic Reviews; Barone, D.G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Schatlo, B.; Fandino, J.; Smoll, N.; Wetzel, O.; Remonda, L.; Marbacher, S.; Perrig, W.; Landolt, H.; Fathi, A.-R. Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro-Oncology 2015, 17, 1560–1567. [Google Scholar] [CrossRef]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Wirtz, C.R.; Konig, R.W. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field iMRI. J. Neurosurg. Sci. 2017, 61, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kubben, P.; Scholtes, F.; Schijns, O.; ter Laak-Poort, M.; Teernstra, O.; Kessels, A.H.; van Overbeeke, J.; Martin, D.; van Santbrink, H. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: A randomized controlled trial. Surg. Neurol. Int. 2014, 5, 70. [Google Scholar] [CrossRef]
- Marongiu, A.; D’Andrea, G.; Raco, A. 1.5-T Field Intraoperative Magnetic Resonance Imaging Improves Extent of Resection and Survival in Glioblastoma Removal. World Neurosurg. 2016, 98, 578–586. [Google Scholar] [CrossRef]
- Li, P.; Qian, R.; Niu, C.; Fu, X. Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: A meta-analysis. Curr. Med. Res. Opin. 2017, 33, 621–630. [Google Scholar] [CrossRef]
- Hauser, S.B.; Kockro, R.A.; Actor, B.; Sarnthein, J.; Bernays, R.-L. Combining 5-Aminolevulinic Acid Fluorescence and Intraoperative Magnetic Resonance Imaging in Glioblastoma Surgery. Neurosurgery 2016, 78, 475–483. [Google Scholar] [CrossRef]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the Combination of 5-Aminolevulinic Acid–Induced Fluorescence with Intraoperative Magnetic Resonance Imaging–Guided Surgery for Glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Muvdi, T.; Kut, C.; Li, X.; Chaichana, K.L. Intraoperative imaging techniques for glioma surgery. Future Oncol. 2017, 13, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.D.; Berger, M.S.; Wang, K.; Mack, L.A.; Ojemann, G.A. Low grade gliomas: Comparison of intraoperative ultrasound characteristics with preoperative imaging studies. J. Neuro-Oncol. 1992, 13, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.D.; Winter, T.C.; Berger, M.S.; Mack, L.A.; Wang, K.; Elliott, J.P. A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J. Clin. Ultrasound 1994, 22, 29–36. [Google Scholar] [CrossRef]
- Solheim, O.; Selbekk, T.; Jakola, A.; Unsgård, G. Ultrasound-guided operations in unselected high-grade gliomas—overall results, impact of image quality and patient selection. Acta Neurochir. 2010, 152, 1873–1886. [Google Scholar] [CrossRef]
- Moiyadi, A.V.; Kannan, S.; Shetty, P. Navigated intraoperative ultrasound for resection of gliomas: Predictive value, influence on resection and survival. Neurol. India 2015, 63, 727–735. [Google Scholar] [CrossRef]
- Sæther, C.A.; Torsteinsen, M.; Torp, S.H.; Sundstrøm, S.; Unsgård, G.; Solheim, O. Did Survival Improve after the Implementation of Intraoperative Neuronavigation and 3D Ultrasound in Glioblastoma Surgery? A Retrospective Analysis of 192 Primary Operations. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2012, 73, 73–78. [Google Scholar] [CrossRef]
- Jakola, A.S.; Unsgård, G.; Solheim, O. Quality of life in patients with intracranial gliomas: The impact of modern image-guided surgery. J. Neurosurg. 2011, 114, 1622–1630. [Google Scholar] [CrossRef]
- Mahboob, S.; McPhillips, R.; Qiu, Z.; Jiang, Y.; Meggs, C.; Schiavone, G.; Button, T.; Desmulliez, M.; Demore, C.; Cochran, S.; et al. Intraoperative Ultrasound-Guided Resection of Gliomas: A Meta-Analysis and Review of the Literature. World Neurosurg. 2016, 92, 255–263. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moiraghi, A.; Prada, F.; Delaidelli, A.; Guatta, R.; May, A.; Bartoli, A.; Saini, M.; Perin, A.; Wälchli, T.; Momjian, S.; et al. Navigated Intraoperative 2-Dimensional Ultrasound in High-Grade Glioma Surgery: Impact on Extent of Resection and Patient Outcome. Oper. Neurosurg. 2019, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Moiyadi, A.V.; Shetty, P.M.; Mahajan, A.; Udare, A.; Sridhar, E. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir. 2013, 155, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Hickmann, A.-K.; Henkel, C.; Nadji-Ohl, M.; Hopf, N.J.; Renovanz, M. Navigated versus Non-Navigated Intraoperative Ultrasound: Is There Any Impact on the Extent of Resection of High-Grade Gliomas? A Retrospective Clinical Analysis. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2014, 75, 224–230. [Google Scholar] [CrossRef]
- Ganau, M.; Ligarotti, G.K.; Apostolopoulos, V. Real-time intraoperative ultrasound in brain surgery: Neuronavigation and use of contrast-enhanced image fusion. Quant. Imaging Med. Surg. 2019, 9, 350–358. [Google Scholar] [CrossRef]
- Giordano, F.A.; Abo-Madyan, Y.; Brehmer, S.; Herskind, C.; Sperk, E.; Schneider, F.; Clausen, S.; Welzel, G.; Schmiedek, P.; Wenz, F. Intraoperative radiotherapy (IORT)-a resurrected option for treating glioblastoma? Transl. Cancer Res. 2014, 3, 94–105. [Google Scholar] [CrossRef]
- Matsutani, M.; Nakamura, O.; Nagashima, T.; Asai, A.; Fujimaki, T.; Tanaka, H.; Nakamura, M.; Ueki, K.; Tanaka, Y.; Matsuda, T. Intra-operative radiation therapy for malignant brain tumors: Rationale, method, and treatment results of cerebral glioblastomas. Acta Neurochir. 1994, 131, 80–90. [Google Scholar] [CrossRef]
- Sakai, N.; Yamada, H.; Andoh, T.; Hirata, T.; Nishimura, Y.; Miwa, Y.; Shimizu, K.; Yanagawa, S. Intraoperative Therapy for Malignant glioma. Neurol. Med. Chir. 1991, 31, 702–707. [Google Scholar] [CrossRef]
- Fujiwara, T.; Honma, Y.; Ogawa, T.; Irie, K.; Kuyama, H.; Nagao, S.; Takashima, H.; Hosokawa, A.; Ohkawa, M.; Tanabe, M. Intraoperative radiotherapy for gliomas. J. Neuro-Oncol. 1995, 23, 81–86. [Google Scholar] [CrossRef]
- Yamada, S.; Takai, Y.; Nemoto, K.; Ogawa, Y.; Kakuto, T.; Hoshi, A.; Sakamoto, K.; Kayama, T.; Yoshimoto, T. Treatment results by uneven fractionated irradiation, low-dose rate telecobalt therapy as a boost, and intraoperative irradiation for malignant glioma. Tohoku J Exp Med. 1992, 167, 259–266. [Google Scholar] [CrossRef]
- Schueller, P.; Micke, O.; Palkovic, S.; Schroeder, J.; Moustakis, C.; Bruns, F.; Schuck, A.; Wassmann, H.; Willich, N. 12 Years’ Experience with Intraoperative Radiotherapy (IORT) of Malignant Gliomas. Strahlenther. Und Onkol. 2005, 181, 500–506. [Google Scholar] [CrossRef] [PubMed]
- De Urbina, D.O.; Santos, M.; Garcia-Berrocal, I.; Bustos, J.C.; Samblas, J.; Gutierrez-Diaz, J.A.; Delgado, J.M.; Donckaster, G.; Calvo, F.A. Intraoperative radiation therapy in malignant glioma: Early clinical results. Neurol. Res. 1995, 17, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Schüller, P.; Willich, N.; Schober, O.; Palkovic, S.; Morgenroth, C.; Bartenstein, P.; Prott, F.J.; Niewöhner, U. Intraoperative radiotherapy (IORT) in malignant brain tumors. Strahlenther. Onkol. 1995, 171, 154–164. [Google Scholar]
- Chung, Y.G.; Kim, C.Y.; Lee, H.K.; Lee, K.C.; Chu, J.W.; Choi, M.S. Preliminary experiences with intraoperative radiation therapy (IORT) for the treatment of brain tumors. J. Korean Med. Sci. 1995, 10, 449–452. [Google Scholar] [CrossRef]
- Giordano, F.A.; Brehmer, S.; Mürle, B.; Welzel, G.; Sperk, E.; Keller, A.; Abo-Madyan, Y.; Scherzinger, E.; Clausen, S.; Schneider, F.; et al. Intraoperative Radiotherapy in Newly Diagnosed Glioblastoma (INTRAGO): An Open-Label, Dose-Escalation Phase I/II Trial. Neurosurgery 2019, 84, 41–49. [Google Scholar] [CrossRef]
- Sarria, G.R.; Smalec, Z.; Muedder, T.; Holz, J.A.; Scafa, D.; Koch, D.; Garbe, S.; Schneider, M.; Hamed, M.; Vatter, H.; et al. Dosimetric Comparison of Upfront Boosting With Stereotactic Radiosurgery Versus Intraoperative Radiotherapy for Glioblastoma. Front. Oncol. 2021, 11, 759873. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Ogawa, Y.; Matsushita, H.; Takeda, K.; Takai, Y.; Yamada, S.; Kumabe, T. Intraoperative radiation therapy (IORT) for previously untreated malignant gliomas. BMC Cancer 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Sarria, G.R.; Sperk, E.; Han, X.; Wenz, F.; Brehmer, S.; Fu, B.; Min, S.; Zhang, H.; Qin, S.; Qiu, X.; et al. Intraoperative radiotherapy for glioblastoma: An international pooled analysis. Radiother. Oncol. 2020, 142, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Usychkin, S.; Calvo, F.; dos Santos, M.A.; Samblás, J.; de Urbina, D.O.; Bustos, J.C.; Diaz, J.A.G.; Sallabanda, K.; Sanz, A.; Yélamos, C.; et al. Intra-operative electron beam radiotherapy for newly diagnosed and recurrent malignant gliomas: Feasibility and long-term outcomes. Clin. Transl. Oncol. 2012, 15, 33–38. [Google Scholar] [CrossRef]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Ricciardi, G.K.; Longhi, M.; Nicolato, A. Radiosurgical Options in Neuro-oncology: A Review on Current Tenets and Future Opportunities. Part II: Adjuvant Radiobiological Tools. Tumori J. 2015, 101, 57–63. [Google Scholar] [CrossRef]
- Krieg, S.M.; Schnurbus, L.; Shiban, E.; Droese, D.; Obermueller, T.; Buchmann, N.; Gempt, J.; Meyer, B.; Ringel, F. Surgery of highly eloquent gliomas primarily assessed as non-resectable: Risks and benefits in a cohort study. BMC Cancer 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Krieg, S.M.; Meyer, B.; Ringel, F. Functional preoperative and intraoperative mapping and monitoring: Increasing safety and efficacy in glioma surgery. Neurosurg. Focus 2015, 38, E3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Gempt, J.; Förschler, A.; Meyer, B.; Ringel, F. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J. Neurosurg. 2012, 116, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Vajkoczy, P.; Picht, T. Navigated transcranial magnetic stimulation for mapping the motor cortex in patients with rolandic brain tumors. Neurosurg. Focus 2013, 34, E3. [Google Scholar] [CrossRef]
- Frey, D.; Schilt, S.; Strack, V.; Zdunczyk, A.; Rösler, J.; Niraula, B.; Vajkoczy, P.; Picht, T. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro-Oncology 2014, 16, 1365–1372. [Google Scholar] [CrossRef]
- Krieg, S.M.; Sabih, J.; Bulubas, L.; Obermueller, T.; Negwer, C.; Janssen, I.; Shiban, E.; Meyer, B.; Ringel, F. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro-Oncology 2014, 16, 1274–1282. [Google Scholar] [CrossRef]
- Krieg, S.M.; Tarapore, P.E.; Picht, T.; Tanigawa, N.; Houde, J.; Sollmann, N.; Meyer, B.; Vajkoczy, P.; Berger, M.S.; Ringel, F.; et al. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. NeuroImage 2014, 100, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Picht, T.; Krieg, S.M.; Sollmann, N.; Rösler, J.; Niraula, B.; Neuvonen, T.; Savolainen, P.; Lioumis, P.; Mäkelä, J.P.; Deletis, V.; et al. A Comparison of Language Mapping by Preoperative Navigated Transcranial Magnetic Stimulation and Direct Cortical Stimulation During Awake Surgery. Neurosurgery 2013, 72, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Meyer, B.; Ringel, F. Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci. 2014, 15, 20. [Google Scholar] [CrossRef]
- Forster, M.-T.; Senft, C.; Hattingen, E.; Lorei, M.; Seifert, V.; Szelényi, A. Motor cortex evaluation by nTMS after surgery of central region tumors: A feasibility study. Acta Neurochir. 2012, 154, 1351–1359. [Google Scholar] [CrossRef]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Förschler, A.; Meyer, B.; Ringel, F. Functional Language Shift to the Right Hemisphere in Patients with Language-Eloquent Brain Tumors. PLoS ONE 2013, 8, e75403. [Google Scholar] [CrossRef] [PubMed]
- Conway, N.; Wildschuetz, N.; Moser, T.; Bulubas, L.; Sollmann, N.; Tanigawa, N.; Meyer, B.; Krieg, S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J. Neurosurg. 2017, 127, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, R.J.; Wunderlich, A.P.; Wang, Y.; Braun, V.; Antoniadis, G.; Görich, J.; Richter, H.-P.; Brambs, H.-J. fMRI for Preoperative Neurosurgical Mapping of Motor Cortex and Language in a Clinical Setting. J. Comput. Assist. Tomogr. 2000, 24, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.-E.; Boulanouar, K.; Lotterie, J.-A.; Mejdoubi, M.; Lesage, J.P.; Berry, I. Language Functional Magnetic Resonance Imaging in Preoperative Assessment of Language Areas: Correlation with Direct Cortical Stimulation. Neurosurgery 2003, 52, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Yetkin, F.Z.; Mueller, W.M.; Morris, G.L.; McAuliffe, T.L.; Ulmer, J.L.; Cox, R.W.; Daniels, D.L.; Haughton, V.M. Functional MR activation correlated with intraoperative cortical mapping. Am. J. Neuroradiol. 1997, 18, 1311–1315. [Google Scholar]
- Krings, T.; Schreckenberger, M.; Rohde, V.; Spetzger, U.; Sabri, O.; Reinges, M.H.T.; Hans, F.J.; Meyer, P.T.; Möller-Hartmann, W.; Gilsbach, J.M.; et al. Functional MRI and 18F FDG-Positron Emission Tomography for Presurgical Planning: Comparison with Electrical Cortical Stimulation. Acta Neurochir. 2002, 144, 889–899. [Google Scholar] [CrossRef]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef]
- Fujiwara, N.; Sakatani, K.; Katayama, Y.; Murata, Y.; Hoshino, T.; Fukaya, C.; Yamamoto, T. Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors. NeuroImage 2004, 21, 1464–1471. [Google Scholar] [CrossRef]
- Stufflebeam, S.M. Clinical Magnetoencephalography for Neurosurgery. Neurosurg. Clin. N. Am. 2011, 22, 153–167. [Google Scholar] [CrossRef]
- Schiffbauer, H.; Berger, M.; Ferrari, P.; Freudenstein, D.; Rowley, H.A.; Roberts, T.P.L. Preoperative magnetic source imaging for brain tumor surgery: A quantitative comparison with intraoperative sensory and motor mapping. Neurosurg. Focus 2003, 15, E7. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Tate, M.C.; Findlay, A.M.; Honma, S.M.; Mizuiri, D.; Berger, M.S.; Nagarajan, S.S. Preoperative multimodal motor mapping: A comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J. Neurosurg. 2012, 117, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.E.; Martino, J.; Guggisberg, A.G.; Owen, J.; Honma, S.M.; Findlay, A.; Berger, M.S.; Kirsch, H.E.; Nagarajan, S.S. Magnetoencephalographic Imaging of Resting-State Functional Connectivity Predicts Postsurgical Neurological Outcome in Brain Gliomas. Neurosurgery 2012, 71, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.G.; Lubelski, D.; Nucifora, P.G.P.; Brem, S. Use of diffusion tensor imaging in glioma resection. Neurosurg. Focus 2013, 34, E1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, L.; Gambini, A.; Castellano, A.; Carrabba, G.; Acerbi, F.; Fava, E.; Giussani, C.; Cadioli, M.; Blasi, V.; Casarotti, A.; et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. NeuroImage 2008, 39, 369–382. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Nimsky, C.; Gruber, S.; Moser, E.; Hammen, T.; Engelhorn, T.; Buchfelder, M.; Ganslandta, O. Changes in Fiber Integrity, Diffusivity, and Metabolism of the Pyramidal Tract Adjacent to Gliomas: A Quantitative Diffusion Tensor Fiber Tracking and MR Spectroscopic Imaging Study. AJNR Am. J. Neuroradiol. 2007, 28, 462–469. [Google Scholar]
- Nimsky, C.; Ganslandt, O.; Merhof, D.; Sorensen, A.G.; Fahlbusch, R. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. NeuroImage 2006, 30, 1219–1229. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Nimsky, C.; Buslei, R.; Salomonowitz, E.; Hammen, T.; Buchfelder, M.; Moser, E.; Ernst-Stecken, A.; Ganslandt, O. Diffusion tensor imaging and optimized fiber tracking in glioma patients: Histopathologic evaluation of tumor-invaded white matter structures. NeuroImage 2007, 34, 949–956. [Google Scholar] [CrossRef]
- Raffa, G.; Bährend, I.; Schneider, H.; Faust, K.; Germanò, A.; Vajkoczy, P.; Picht, T. A Novel Technique for Region and Linguistic Specific nTMS-based DTI Fiber Tracking of Language Pathways in Brain Tumor Patients. Front. Neurosci. 2016, 10, 552. [Google Scholar] [CrossRef]
- Romano, A.; D’Andrea, G.; Minniti, G.; Mastronardi, L.; Ferrante, L.; Fantozzi, L.M.; Bozzao, A. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur. Radiol. 2009, 19, 2798–2808. [Google Scholar] [CrossRef]
- Sollmann, N.; Kelm, A.; Ille, S.; Schröder, A.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. Setup presentation and clinical outcome analysis of treating highly language-eloquent gliomas via preoperative navigated transcranial magnetic stimulation and tractography. Neurosurg. Focus 2018, 44, E2. [Google Scholar] [CrossRef]
- Kombos, T.; Picht, T.; Derdilopoulos, A.; Suess, O. Impact of Intraoperative Neurophysiological Monitoring on Surgery of High-Grade Gliomas. J. Clin. Neurophysiol. 2009, 26, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg. Focus 2010, 28, E1. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Schäffner, M.; Shiban, E.; Droese, D.; Obermüller, T.; Gempt, J.; Meyer, B.; Ringel, F. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions. J. Neurosurg. 2013, 118, 1269–1278. [Google Scholar] [CrossRef]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional Outcome after Language Mapping for Glioma Resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef]
- Suess, O.; Suess, S.; Brock, M.; Kombos, T. Intraoperative electrocortical stimulation of Brodman area 4: A 10-year analysis of 255 cases. Head Face Med. 2006, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.-P.; Van Effenterre, R.; Capelle, L. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef]
- Neuloh, G.C.; Bien, C.G.; Clusmann, H.; von Lehe, M.; Schramm, J. Continuous motor monitoring enhances functional preservation and seizure-free outcome in surgery for intractable focal epilepsy. Acta Neurochir. 2010, 152, 1307–1314. [Google Scholar] [CrossRef]
- Krieg, S.M.; Shiban, E.; Droese, D.; Gempt, J.; Buchmann, N.; Pape, H.; Ryang, Y.-M.; Meyer, B.; Ringel, F. Predictive Value and Safety of Intraoperative Neurophysiological Monitoring With Motor Evoked Potentials in Glioma Surgery. Neurosurgery 2012, 70, 1060–1071. [Google Scholar] [CrossRef]
- Neuloh, G.; Pechstein, U.; Schramm, J. Motor tract monitoring during insular glioma surgery. J. Neurosurg. 2007, 106, 582–592. [Google Scholar] [CrossRef]
- Cedzich, C.; Taniguchi, M.; Schäfer, S.; Schramm, J. Somatosensory Evoked Potential Phase Reversal and Direct Motor Cortex Stimulation during Surgery in and around the Central Region. Neurosurgery 1996, 38, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Talacchi, A.; Turazzi, S.; Locatelli, F.; Sala, F.; Beltramello, A.; Alessandrini, F.; Manganotti, P.; Lanteri, P.; Gambin, R.; Ganau, M.; et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: A 171-patient series. J. Neuro-Oncol. 2010, 100, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Schucht, P.; Beck, J.; Raabe, A. Continuous Dynamic Mapping to Identify the Corticospinal Tract in Motor Eloquent Brain Tumors: An Update. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2020, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Schucht, P.; Seidel, K.; Jilch, A.; Beck, J.; Raabe, A. A review of monopolar motor mapping and a comprehensive guide to continuous dynamic motor mapping for resection of motor eloquent brain tumors. Neurochirurgie 2017, 63, 175–180. [Google Scholar] [CrossRef]
- Bello, L.; Riva, M.; Fava, E.; Ferpozzi, V.; Castellano, A.; Raneri, F.; Pessina, F.; Bizzi, A.; Falini, A.; Cerri, G. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro-Oncology 2014, 16, 1110–1128. [Google Scholar] [CrossRef]
- Rossi, M.; Nibali, M.C.; Viganò, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2020, 133, 642–654. [Google Scholar] [CrossRef]
- Rossi, M.; Viganò, L.; Puglisi, G.; Nibali, M.C.; Leonetti, A.; Gay, L.; Sciortino, T.; Fornia, L.; Callipo, V.; Lamperti, M.; et al. Targeting Primary Motor Cortex (M1) Functional Components in M1 Gliomas Enhances Safe Resection and Reveals M1 Plasticity Potentials. Cancers 2021, 13, 3808. [Google Scholar] [CrossRef]
- Rossi, M.; Puglisi, G.; Nibali, M.C.; Viganò, L.; Sciortino, T.; Gay, L.; Leonetti, A.; Zito, P.; Riva, M.; Bello, L. Asleep or awake motor mapping for resection of perirolandic glioma in the nondominant hemisphere? Development and validation of a multimodal score to tailor the surgical strategy. J. Neurosurg. 2022, 136, 16–29. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Li, J.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef]
- Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Quiñones-Hinojosa, A. The Cost of Brain Surgery: Awake vs Asleep Craniotomy for Perirolandic Region Tumors. Neurosurgery 2017, 81, 307–314. [Google Scholar] [CrossRef]
- Bajunaid, K.; Ajlan, A.M. Awake craniotomy. Neurosciences 2015, 20, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.K.W.; Viëtor, C.L.; Rizopoulos, D.; Schouten, J.W.; Klimek, M.; Dirven, C.M.F.; Vincent, A.J.-P.E. Awake craniotomy versus craniotomy under general anesthesia without surgery adjuncts for supratentorial glioblastoma in eloquent areas: A retrospective matched case-control study. Acta Neurochir. 2019, 161, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; McCutcheon, I.E.; Suki, D.; Weinberg, J.S.; Sawaya, R.; Lang, F.F.; Ferson, D.; Heimberger, A.B.; Demonte, F.; Prabhu, S.S. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery 2009, 64, 836–845. [Google Scholar] [CrossRef]
- Groshev, A.; Padalia, D.; Patel, S.; Garcia-Getting, R.; Sahebjam, S.; Forsyth, P.A.; Vrionis, F.D.; Etame, A.B. Clinical outcomes from maximum-safe resection of primary and metastatic brain tumors using awake craniotomy. Clin. Neurol. Neurosurg. 2017, 157, 25–30. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Arends, L.; Klimek, M.; Dirven, C.M.F.; Vincent, A.J.-P.E. Impact of intraoperative stimulation mapping on high-grade glioma surgery outcome: A meta-analysis. Acta Neurochir. 2018, 161, 99–107. [Google Scholar] [CrossRef]
- Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Lee, B.Y.M.; Nangiana, J.; Vivas-Buitrago, T.; Quiñones-Hinojosa, A. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery 2017, 81, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Breuskin, D.; Szczygielski, J.; Urbschat, S.; Kim, Y.-J.; Oertel, J. Confocal Laser Endomicroscopy in Neurosurgery—An Alternative to Instantaneous Sections? World Neurosurg. 2017, 100, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Snuderl, M.; Wirth, D.; Sheth, S.A.; Bourne, S.K.; Kwon, C.-S.; Ancukiewicz, M.; Curry, W.T.; Frosch, M.P.; Yaroslavsky, A.N. Dye-Enhanced Multimodal Confocal Imaging as a Novel Approach to Intraoperative Diagnosis of Brain Tumors. Brain Pathol. 2012, 23, 73–81. [Google Scholar] [CrossRef]
- Sankar, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Coons, S.W.; Scheck, A.C.; Smith, K.A.; Spetzler, R.F.; et al. Miniaturized Handheld Confocal Microscopy for Neurosurgery. Neurosurgery 2010, 66, 410–418. [Google Scholar] [CrossRef]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative Confocal Microscopy for Brain Tumors: A Feasibility Analysis in Humans. Neurosurgery 2011, 68, ons282–ons290. [Google Scholar] [CrossRef]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In Vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Kamen, A.; Sun, S.; Wan, S.; Kluckner, S.; Chen, T.; Gigler, A.M.; Simon, E.; Fleischer, M.; Javed, M.; Daali, S.; et al. Automatic Tissue Differentiation Based on Confocal Endomicroscopic Images for Intraoperative Guidance in Neurosurgery. BioMed Res. Int. 2016, 2016, 6183218. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.Y.; Golby, A.J.; Ligon, K.L.; Norton, I.; Mohan, V.; Wiseman, J.M.; Tannenbaum, A.; Jolesz, F.A. Development of Stereotactic Mass Spectrometry for Brain Tumor Surgery. Neurosurgery 2011, 68, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef] [Green Version]
- Santagata, S.; Eberlin, L.S.; Norton, I.; Calligaris, D.; Feldman, D.R.; Ide, J.L.; Liu, X.; Wiley, J.S.; Vestal, M.L.; Ramkissoon, S.H.; et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc. Natl. Acad. Sci. USA 2014, 111, 11121–11126. [Google Scholar] [CrossRef]
- Schwarzmaier, H.-J.; Eickmeyer, F.; von Tempelhoff, W.; Fiedler, V.U.; Niehoff, H.; Ulrich, S.D.; Yang, Q.; Ulrich, F. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: Preliminary results in 16 patients. Eur. J. Radiol. 2006, 59, 208–215. [Google Scholar] [CrossRef]
- Carpentier, A.; Chauvet, D.; Reina, V.; Beccaria, K.; LeClerq, D.; McNichols, R.J.; Gowda, A.; Cornu, P.; Delattre, J.-Y. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg. Med. 2012, 44, 361–368. [Google Scholar] [CrossRef]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; De Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Kast, R.E.; Rosenblum, M.L.; Mikkelsen, T.; Yurgelevic, S.M.; Nelson, K.M.; Raghunathan, A.; Poisson, L.M.; Auner, G.W. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neuro Oncol. 2014, 116, 477–485. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.-C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra219. [Google Scholar] [CrossRef]
- Kast, R.E.; Auner, G.W.; Rosenblum, M.L.; Mikkelsen, T.; Yurgelevic, S.M.; Raghunathan, A.; Poisson, L.M.; Kalkanis, S.N. Raman molecular imaging of brain frozen tissue sections. J. Neuro Oncol. 2014, 120, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Neidert, N.; Straehle, J.; Erny, D.; Sacalean, V.; El Rahal, A.; Steybe, D.; Schmelzeisen, R.; Vlachos, A.; Reinacher, P.C.; Coenen, V.A.; et al. Stimulated Raman histology in the neurosurgical workflow of a major European neurosurgical center—part A. Neurosurg. Rev. 2022, 45, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Straehle, J.; Erny, D.; Neidert, N.; Heiland, D.H.; El Rahal, A.; Sacalean, V.; Steybe, D.; Schmelzeisen, R.; Vlachos, A.; Mizaikoff, B.; et al. Neuropathological interpretation of stimulated Raman histology images of brain and spine tumors: Part B. Neurosurg. Rev. 2021, 45, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; De Pasquale, D.; Fiaschi, P.; Ciofani, G. Discrimination of glioma patient-derived cells from healthy astrocytes by exploiting Raman spectroscopy. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2021, 269, 120773. [Google Scholar] [CrossRef]
- Fujimoto, J.G. Optical coherence tomography for ultrahigh resolution In Vivo imaging. Nat. Biotechnol. 2003, 21, 1361–1367. [Google Scholar] [CrossRef]
- Boppart, S.A.; Brezinski, M.E.; Pitris, C.; Fujimoto, J.G. Optical Coherence Tomography for Neurosurgical Imaging of Human Intracortical Melanoma. Neurosurgery 1998, 43, 834–841. [Google Scholar] [CrossRef]
- Böhringer, H.; Boller, D.; Leppert, J.; Knopp, U.; Lankenau, E.; Reusche, E.; Hüttmann, G.; Giese, A. Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers Surg. Med. 2006, 38, 588–597. [Google Scholar] [CrossRef]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quiñones-Hinojosa, A.; Li, X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 292ra100. [Google Scholar] [CrossRef] [Green Version]


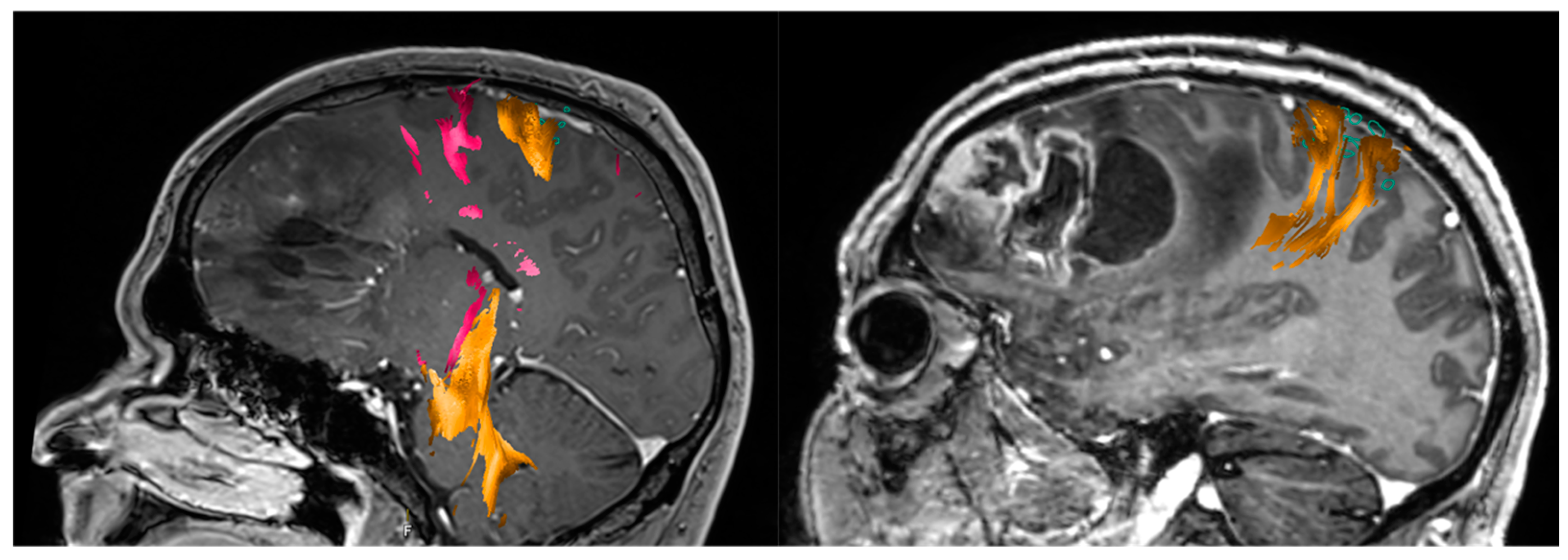

| Author, Year | Study Type | Patient Population | Conclusion |
|---|---|---|---|
| Lacroix et al., 2001 [8] | Retrospective | 416 patients with GBM | EOR ≥ 98% improved median survival |
| Sanai et al., 2011 [10] | Retrospective | 500 patients with newly diagnosed GBM | EOR ≥ 78% improved OS |
| Oppenlander et al., 2014 [11] | Retrospective | 170 patients with recurrent GBM | EOR ≥ 80% improved OS |
| Bloch et al., 2012 [12] | Retrospective | 107 patients with recurrent GBM | GTR improved OS regardless of initial EOR |
| Lu et al., 2019 [13] | Meta-analysis | 1507 patients with GBM | Maximal resection at reoperation improved OS |
| Chaichana et al., 2014 [14] | Retrospective | 84 patients with newly diagnosed GBM | RV < 2 cm3 and EOR > 95% presented the greatest reduction in the risk of death |
| Orringer et al., 2012 [9] | Retrospective | 46 patients with GBM | EOR ≥ 90% improved 1-year survival |
| Li et al., 2017 [15] | Meta-analysis | 1618 patients with GBM | GTR improved 1-year OS and PFS |
| Grabowski et al., 2014 [16] | Retrospective | 128 patients with newly diagnosed GBM | RV < 2 cm3 and EOR > 98% improved OS |
| Bette et al., 2018 [17] | Retrospective | 209 patients with newly diagnosed GBM | RV was significantly associated with survival |
| Chaichana et al., 2013 [18] | Retrospective | 259 patients with newly diagnosed GBM | RV < 5 cm3 and EOR > 70% improved OS and PFS |
| Woo et al., 2019 [19] | Retrospective (multicenter cohort) | 147 patients with newly diagnosed GBM | MGMT methylation and RV < 3.5 cc improved OS. (EOR was not an independent prognostic factor) |
| Xing et al., 2018 [20] | Retrospective | 292 patients with newly diagnosed GBM | RV, but not EOR, was associated with survival |
| Pessina et al., 2016 [21] | Retrospective | 64 patients with recurrent GBM | RV, but not EOR, was associated with OS and PFS in a multivariate analysis |
| Sales et al., 2019 [22] | Retrospective | 126 patients with newly diagnosed MGMT-unmethylated GBM | RV, but not GTR, improved OS |
| Esquenazi et al., 2017 [23] | Retrospective | 86 patients with newly diagnosed GBM | GTR and near-total resection improved OS |
| Kreth et al., 2013 [24] | Retrospective | 345 patients with newly diagnosed GBM | GTR improved OS; patients who received STR did not show a better OS than those who received biopsy only |
| Shah et al., 2020 [25] | Retrospective | 69 patients with non-eloquent GBM | Supramaximal resection improved OS and PFS compared to matched controls (propensity-matched analysis) |
| Author, Year | Study Type | Patient Population | Conclusion |
|---|---|---|---|
| Stummer et al., 2006 [27] | Multicenter, randomized, controlled trial | 322 patients with suspected malignant glioma | 5-ALA group: higher rate of GTR and higher 6-month PFS |
| Díez Valle et al., 2010 [28] | Prospective | 36 patients with GBM | GTR achieved in 83% of patients, EOR > 98% in 100% of cases, and mean EOR was 99.8% |
| Eljamel, 2015 [29] | Meta-analysis | 565 patients with GBM | GTR rate of 75.4% and mean OS gain of 6.2 months |
| Aldave et al., 2013 [30] | Retrospective | 118 patients with HGG | GTR + no residual fluorescence improved OS |
| Stummer et al., 2000 [31] | Prospective | 52 patients with GBM | GTR achieved in 63% of patients; residual fluorescence was a significant prognostic factor |
| Panciani et al., 2012 [32] | Multicenter, prospective, study | 23 patients with suspected HGG | 5-ALA-guided surgery showed a sensitivity of 91.1% and a specificity of 89.4% |
| Stummer et al., 2011 [33] | Randomized, controlled trial * | 349 patients with malignant glioma | 5-ALA improved OS and 6-month PFS * |
| Author, Year | Study Type | Patient Population | Conclusion |
|---|---|---|---|
| Senft et al., 2011 [54] | Randomized controlled trial | 58 patients with contrast-enhanced gliomas | GTR rate 96% in the iMRI group vs. 68% in the control group |
| Wu et al., 2014 [52] | Randomized, triple-blind, controlled trial | 87 patients with malignant gliomas | iMRI group: Trend toward improved 6-month PFS and higher rate of GTR |
| Schatlo et al., 2015 [55] | Retrospective | 200 patients with HGG | iMRI had no impact on OS |
| Kuhnt et al., 2011 [56] | Retrospective | 153 patients with GBM | iMRI contributed to optimal EOR with low postoperative morbidity |
| Corburger et al., 2017 [57] | Prospective | 170 patients with GBM | Surgery with iMRI presented higher OS and lower complication rates than previously published data |
| Kubben et al., 2014 [58] | Randomized, controlled trial (interim analysis) | 14 patients with suspected GBM | iMRI group: no advantage with respect to EOR, clinical performance, and survival |
| Marongiu et al., 2016 [59] | Retrospective | 114 patients with GBM | iMRI improved both EOR and 6-month PFS |
| Li et al., 2017 [60] | Meta-analysis | Patients with glioma | iMRI improved rate of GTR and 6-month PFS |
| Author, Year | Study Type | Patient Population | Conclusion |
|---|---|---|---|
| Jakola et al., 2011 [70] | Retrospective | 88 patients with glioma | IOUS improved QOL |
| Saether et al., 2012 [69] | Retrospective | 192 patients with GBM | IOUS improved survival since introduced in their department |
| Moiyadi et al., 2015 [68] | Prospective | 88 patients with glioma | Navigable US improved PFS and OS |
| Mahboob et al., 2016 [71] | Meta-analysis | 739 patients with glioma | IOUS improved EOR |
| Prada et al., 2016 [72] | Prospective | 10 patients with GBM | CEUS was extremely specific in identifying residual tumor |
| Moiraghi et al.,2020 [73] | Retrospective | 60 patients with supratentorial gliomas | N-ioUS improved EOR and neurological outcomes |
| Author, Year | Study Type | Patient Population | Conclusion |
|---|---|---|---|
| Sakai et al., 1991 [79] | Prospective | 73 patients with malignant glioma | IORT improved median survival |
| Fujiwara et al., 1995 [80] | Prospective | 36 patients with glioma | IORT improved median survival |
| Schueller et al., 2005 [82] | Retrospective | 71 patients with malignant glioma | IORT did not improve OS compared to a historical group |
| Nemoto et al., 2002 [88] | Retrospective | 32 patients with malignant glioma | IORT did not improve survival compared to matched control cases |
| Giordano et al., 2019 [86] | Clinical trial | 15 patients with newly diagnosed glioblastoma | IORT was associated with manageable toxicity |
| Sarria et al. 2020 [89] | Retrospective | 51 patients with glioblastoma | Improved efficacy and safety of IORT with low energy X-rays compared to historical data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, A.H.A.; Beck, J.; Schnell, O.; Fung, C.; Meyer, B.; Gempt, J. Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. J. Clin. Med. 2022, 11, 5354. https://doi.org/10.3390/jcm11185354
Sales AHA, Beck J, Schnell O, Fung C, Meyer B, Gempt J. Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. Journal of Clinical Medicine. 2022; 11(18):5354. https://doi.org/10.3390/jcm11185354
Chicago/Turabian StyleSales, Arthur H. A., Jürgen Beck, Oliver Schnell, Christian Fung, Bernhard Meyer, and Jens Gempt. 2022. "Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends" Journal of Clinical Medicine 11, no. 18: 5354. https://doi.org/10.3390/jcm11185354
APA StyleSales, A. H. A., Beck, J., Schnell, O., Fung, C., Meyer, B., & Gempt, J. (2022). Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. Journal of Clinical Medicine, 11(18), 5354. https://doi.org/10.3390/jcm11185354









