Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Examination
2.2. Variant Detection and Analysis
2.3. Test for CMV Infection
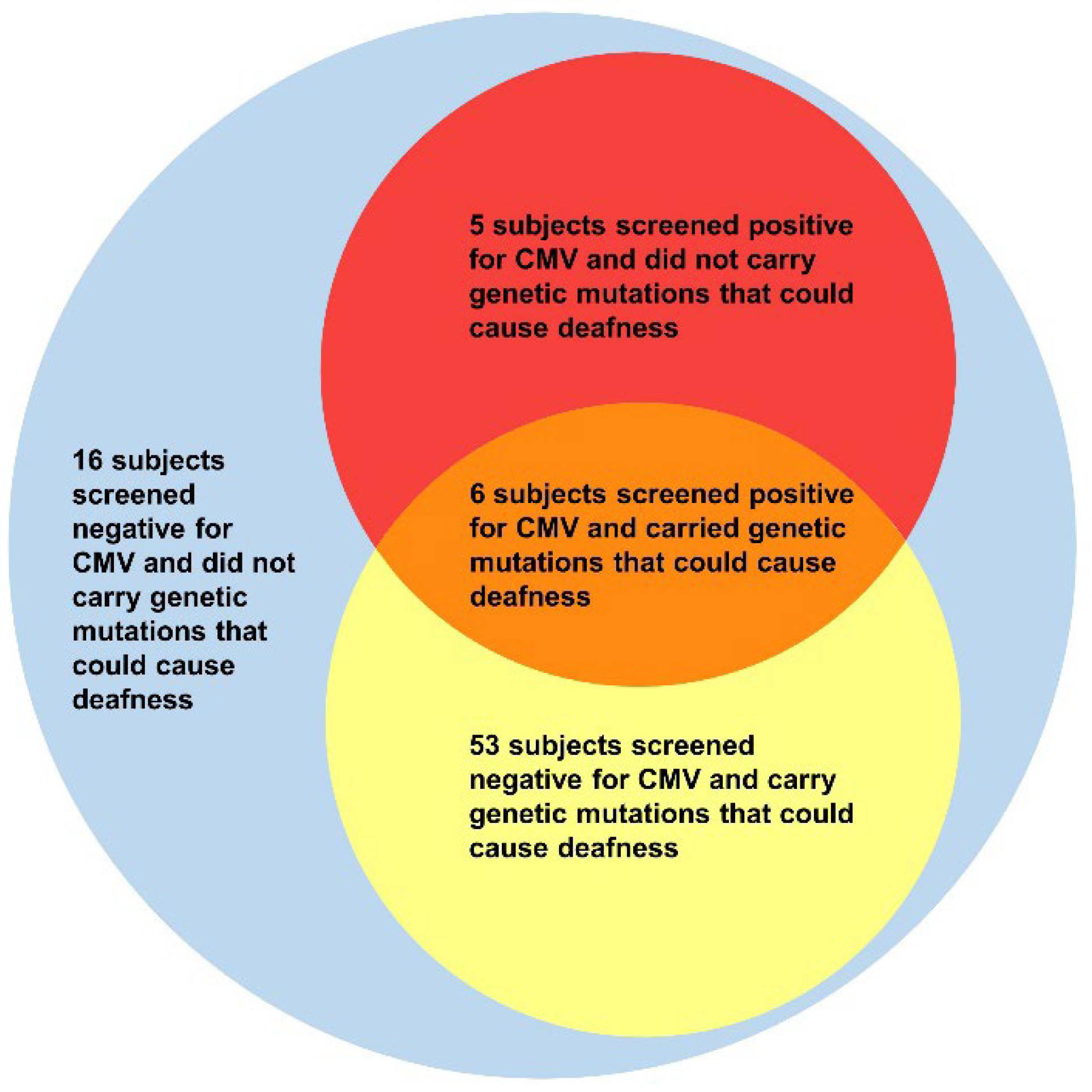
3. Results
3.1. Rate of CMV Infection
3.2. Clinical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilgert, N.; Smith, R.J.H.; van Camp, G. Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Mutat. Res. 2009, 681, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Bale, J.F., Jr.; White, K.R. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef]
- Available online: https://hereditaryhearingloss.org/ (accessed on 5 May 2022).
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Stagno, S.; Pass, R.F. Maternal age and congenital cytomegalovirus infection: Screening of two diverse newborn populations, 1980–1990. J. Infect. Dis. 1993, 168, 552–556. [Google Scholar] [CrossRef]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef]
- Dar, L.; Pati, S.K.; Patro, A.R.K.; Deorari, A.K.; Rai, S.; Kant, S.; Broor, S.; Fowler, K.B.; Britt, W.J.; Boppana, S.B. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr. Infect. Dis. J. 2008, 27, 841–843. [Google Scholar] [CrossRef]
- Kaye, S.; Miles, D.; Antoine, P.; Burny, W.; Ojuola, B.; Kaye, P.; Rowland-Jones, S.; Whittle, H.; Van Der Sande, M.; Marchant, A. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in the Gambia. J. Infect. Dis. 2008, 197, 1307–1314. [Google Scholar] [CrossRef]
- Van der Sande, M.A.B.; Kaye, S.; Miles, D.J.C.; Waight, P.; Jeffries, D.J.; Ojuola, O.O.; Palmero, M.; Pinder, M.; Ismaili, J.; Flanagan, K.L.; et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS ONE 2007, 2, e492. [Google Scholar] [CrossRef]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Stagno, S.; Whitley, R.J. Herpesvirus infections of pregnancy. Part II: Herpes simplex virus and varicella-zoster virus infections. N. Engl. J. Med. 1985, 313, 1327–1330. [Google Scholar] [CrossRef]
- Schleiss, M.R. Antiviral Therapy and Its Long-Term Impact on Hearing Loss Caused by Congenital Cytomegalovirus: Much Remains to Be Learned! J. Pediatric Infect. Dis. Soc. 2022, 11, 186–189. [Google Scholar] [CrossRef]
- Michaels, M.G.; Greenberg, D.P.; Sabo, D.L.; Wald, E.R. Treatment of children with congenital cytomegalovirus infection with ganciclovir. Pediatr. Infect. Dis. J. 2003, 22, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.J.D. Newborn Screening for Congenital Cytomegalovirus Infection... It Is Time. Clin. Infect. Dis. 2020, 70, 1385–1387. [Google Scholar]
- Liu, Y.F.; Wu, Y.; Wang, F.; Wang, S.; Zhao, W.; Chen, L.; Tu, S.; Qian, Y.; Liao, Y.; Huang, Y.; et al. The Association Between Previous TORCH Infections and Pregnancy and Neonatal Outcomes in IVF/ICSI-ET: A Retrospective Cohort Study. Front. Endocrinol. 2020, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Tanimura, K.; Morizane, M.; Fujioka, K.; Morioka, I.; Oohashi, M.; Minematsu, T.; Yamada, H. Clinical Factors Associated With Congenital Cytomegalovirus Infection: A Cohort Study of Pregnant Women and Newborns. Clin. Infect. Dis. 2020, 71, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R.; Permar, S.R.; Plotkin, S.A. Progress toward Development of a Vaccine against Congenital Cytomegalovirus Infection. Clin. Vaccine Immunol. 2017, 24, e00268-17. [Google Scholar] [CrossRef]
- Britt, W.J. Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection. Viruses 2018, 10, 405. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Hearing Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017, 139, e20162610. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Tsao, P.-N.; Ke, Y.-Y.; Lin, Y.-H.; Lin, Y.-H.; Hung, C.-C.; Su, Y.-N.; Hsu, W.-C.; Hsieh, W.-S.; Huang, L.-M.; et al. Concurrent Hearing, Genetic, and Cytomegalovirus Screening in Newborns, Taiwan. J. Pediatr. 2018, 199, 144–150.e1. [Google Scholar] [CrossRef]
- Vos, B.; Noll, D.; Whittingham, J.; Pigeon, M.; Bagatto, M.; Fitzpatrick, E.M. Cytomegalovirus-A Risk Factor for Childhood Hearing Loss: A Systematic Review. Ear Hear. 2021, 42, 1447–1461. [Google Scholar] [CrossRef]
- De Paschale, M.; Agrappi, C.; Manco, M.T.; Paganini, A.; Clerici, P. Incidence and risk of cytomegalovirus infection during pregnancy in an urban area of Northern Italy. Infect. Dis. Obstet. Gynecol. 2009, 2009, 206505. [Google Scholar] [CrossRef] [Green Version]
- Buxmann, H.; Hamprecht, K.; Meyer-Wittkopf, M.; Friese, K. Primary Human Cytomegalovirus (HCMV) Infection in Pregnancy. Dtsch. Arztebl. Int. 2017, 114, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hilditch, C.; Keir, A.K. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. Acta Paediatr. 2018, 107, 906. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, T.; Yu, H.; Tang, J.; Song, Q.; Guo, X.; Wang, H.; Li, C.; Wang, J.; Liang, C.; et al. Maternal CMV seroprevalence rate in early gestation and congenital cytomegalovirus infection in a Chinese population. Emerg. Microbes Infect. 2021, 10, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
| Subject | Age at the Time of Sample Collection (Months) | CMV DNA Quantification in Saliva Sample | CMV DNA Quantification in Urine Sample | Variants in Congenital Hearing Loss–Related Genes |
|---|---|---|---|---|
| 1 | 4 | 413,976.2 | 48,028.8 | GJB2 c. 560_605dup/c. 235del C |
| 2 | 7 | 114,985.0 | negative | - |
| 3 | 6 | 93,588.6 | negative | SLC26A4 c. 919-2 A > G/c. 589 G > A |
| 4 | 7 | 90,400.3 | 60,704.0 | GJB2 c. 263 C > T |
| 5 | 8 | 48,376.9 | 6928.1 | KCNQ1 c. 1684 A > GT/EXON 8-9 DEL |
| 6 | 7 | 37,786.3 | negative | - |
| 7 | 3 | 14,382.9 | negative | GJB2 c. 299_300 del AT/c. 235del C |
| 8 | 21 | negative | negative | - |
| 9 | 18 | negative | negative | - |
| 10 | 22 | negative | negative | GJB2 c. 235del C/c. 235del C |
| 11 | 12 | negative | negative | MYO7A c. 6320 G > A/c. 612 C > G |
| 12 | 22 | negative | negative | - |
| 13 | 20 | negative | negative | GJB2 c. 235del C/c. 176_191del |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Liu, X.; Chen, S.; Xiang, J.; Peng, Z.; Sun, Y. Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss. J. Clin. Med. 2022, 11, 5335. https://doi.org/10.3390/jcm11185335
Jin Y, Liu X, Chen S, Xiang J, Peng Z, Sun Y. Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss. Journal of Clinical Medicine. 2022; 11(18):5335. https://doi.org/10.3390/jcm11185335
Chicago/Turabian StyleJin, Yuan, Xiaozhou Liu, Sen Chen, Jiale Xiang, Zhiyu Peng, and Yu Sun. 2022. "Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss" Journal of Clinical Medicine 11, no. 18: 5335. https://doi.org/10.3390/jcm11185335
APA StyleJin, Y., Liu, X., Chen, S., Xiang, J., Peng, Z., & Sun, Y. (2022). Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss. Journal of Clinical Medicine, 11(18), 5335. https://doi.org/10.3390/jcm11185335







