Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
Abstract
:1. Introduction
2. Materials and Methods
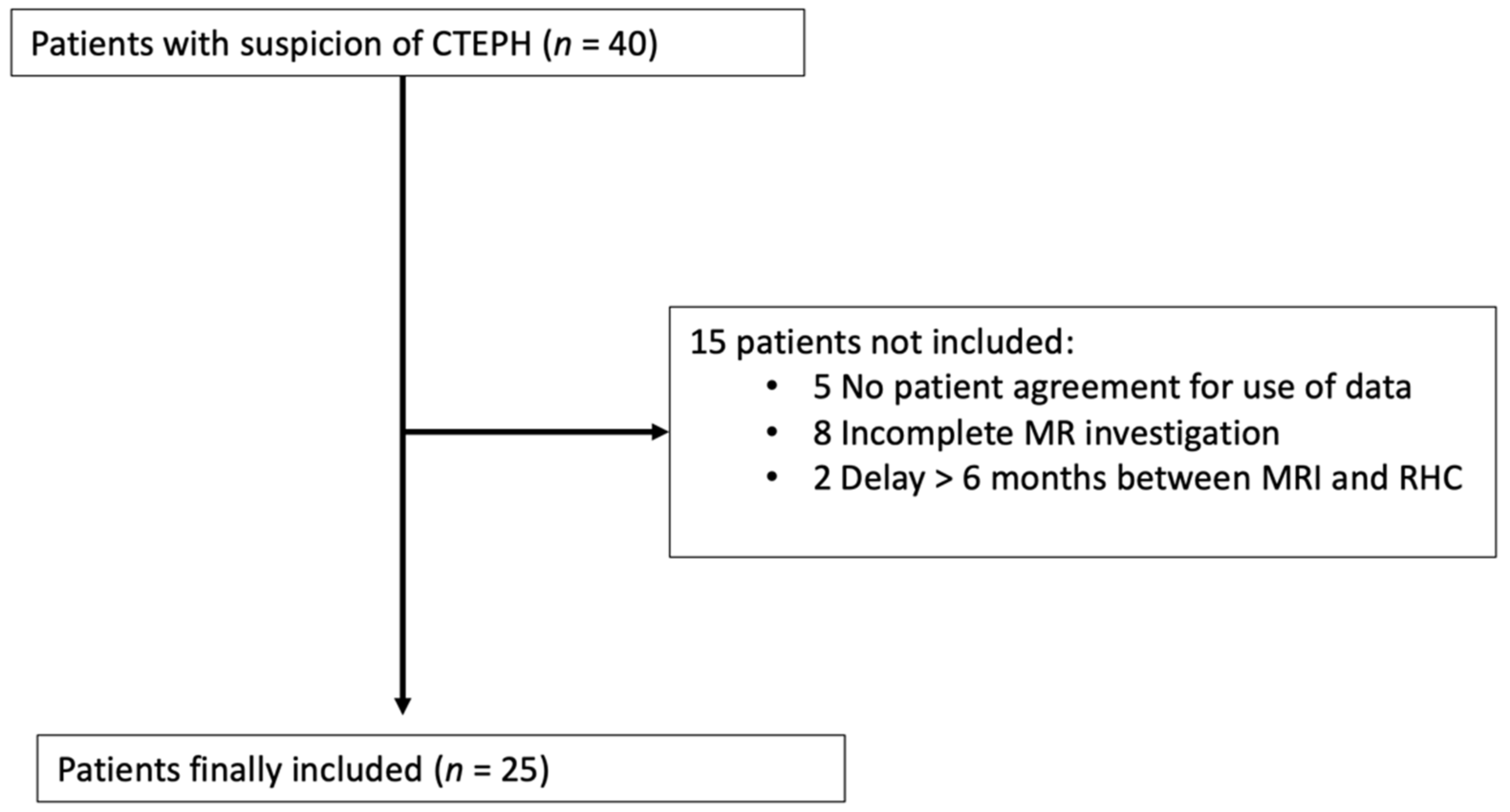
2.1. Study Population
2.2. Cardiac MRI
2.3. Right Heart Catheterisation (RHC)
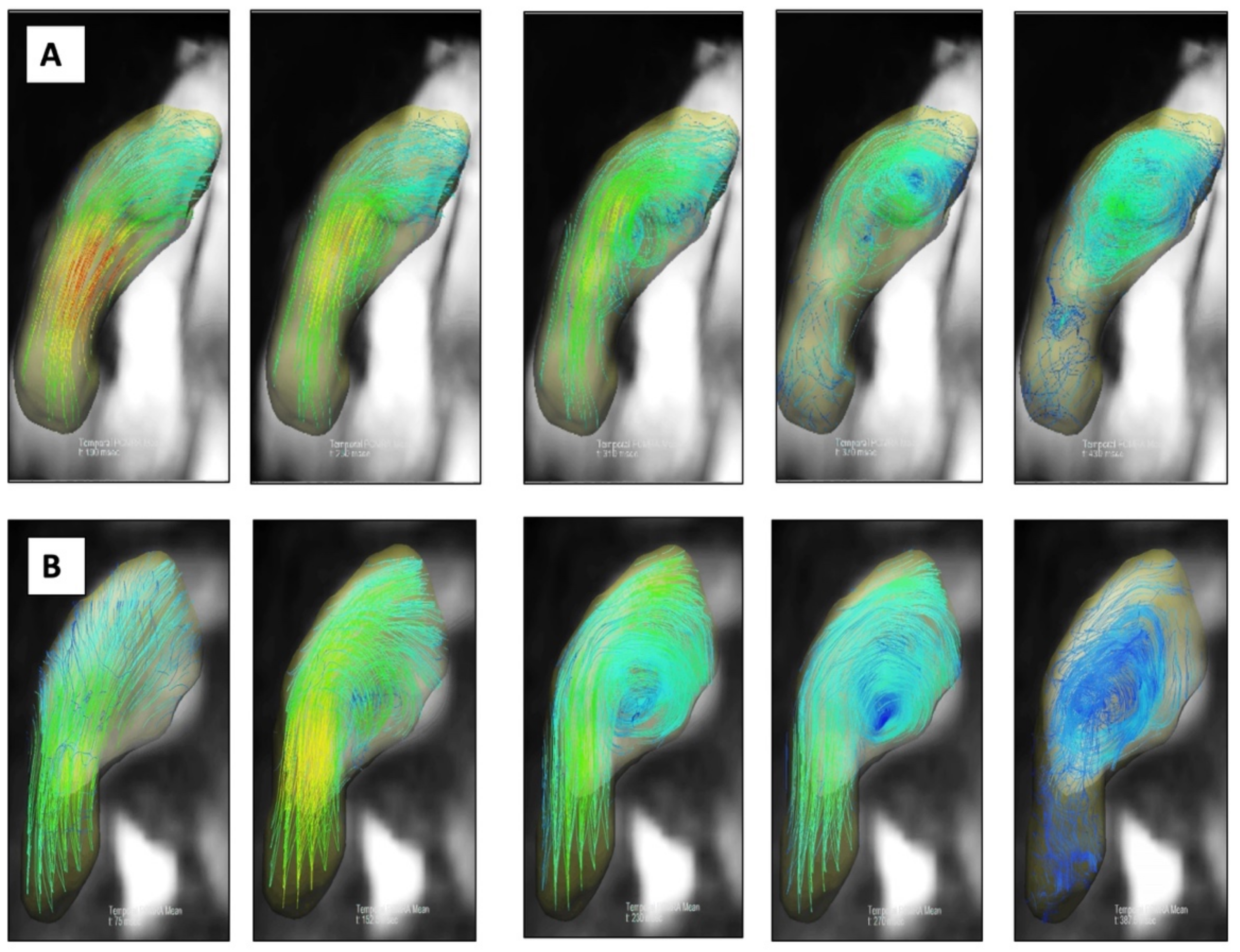
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Cardiac MRI Parameters
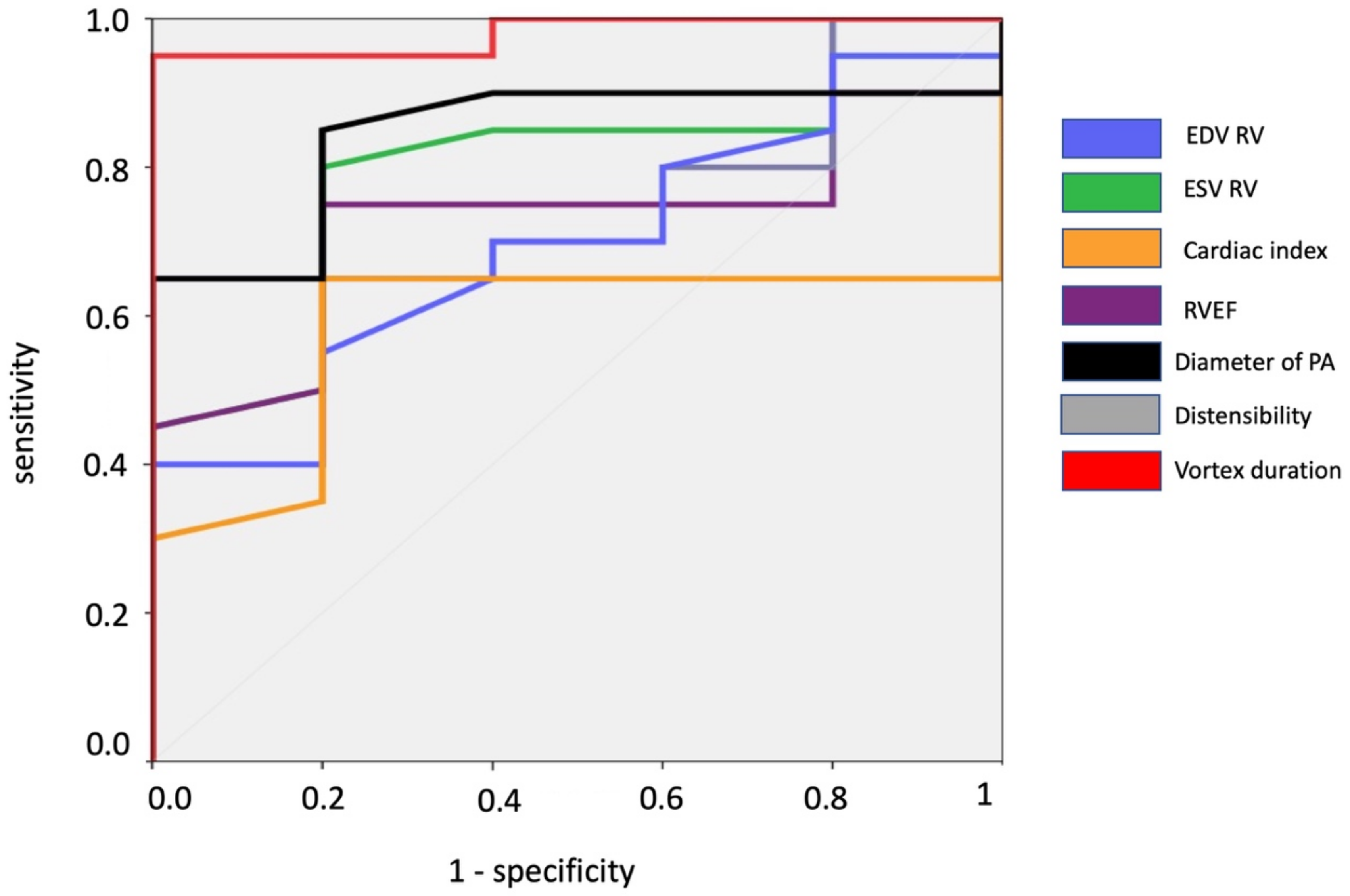
3.3. Receiver Operating Characteristic Curve Analysis
3.4. Relationships between Vortex Duration, mPAP and Other RHC and MRI Parameters
3.5. Reproducibility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.E.; Jenkins, D.P.; Hoole, S.P. Chronic thromboembolic pulmonary hypertension: A review of risk factors, management and current challenges. Expert Rev. Cardiovasc. Ther. 2022, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ulah, W.; Minalyan, A.; Saleem, S.; Nadeem, N.; Abdullah, H.M.; Abdalla, A.; Chan, V.; Saeed, R.; Khan, M.; Collins, S.; et al. Comparative accuracy of non-invasive imaging versus right heart catheterization for the diagnosis of pulmonary hypertension: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2020, 29, 100568. [Google Scholar]
- Topyla-Putowska, W.; Tomaszewski, M.; Wysokinski, A.; Tomaszewski, A. Echocardiography in Pulmonary Arterial Hypertension: Comprehensive Evaluation and Technical Considerations. J. Clin. Med. 2021, 10, 3229. [Google Scholar] [CrossRef] [PubMed]
- Johns, C.S.; Kiely, D.G.; Rajaram, S.; Hill, C.; Thomas, S.; Karunasaagarar, K.; Garg, P.; Hamilton, N.; Solanki, R.; Capener, D.A.; et al. Diagnosis of Pulmonary Hypertension with Cardiac MRI: Derivation and Validation of Regression Models. Radiology 2019, 290, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Ota, H.; Nakamura, M.; Sun, W.; Aoki, T.; Sato, H.; Sugimura, K.; Takase, K. Quantification of vortex flow in pulmonary arteries of patients with chronic thromboembolic pulmonary hypertension. Eur. J. Radiol. 2022, 148, 110142. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.J.; Roldan-Alzate, A.; Entezari, P.; Shah, S.J.; Chesler, N.C.; Wieben, O.; Markl, M.; Francois, C.J. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: Results from two institutions. Magn. Reson. Med. 2015, 73, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Odari, K.; Inui, N.; Hakamata, A.; Inoue, Y.; Suda, T.; Takehara, Y.; Sakahara, H.; Sugiyama, M.; Alley, M.T.; Wakayama, T.; et al. Non-invasive evaluation of pulmonary arterial blood flow and wall shear stress in pulmonary arterial hypertension with 3D phase contrast magnetic resonance imaging. Springerplus 2016, 5, 1071. [Google Scholar]
- Ota, H.; Kamada, H.; Higuchi, S.; Takase, K. Clinical Application of 4D Flow MR Imaging to Pulmonary Hypertension. Magn. Reson. Med. Sci. 2022, 21, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Browning, J.; Schroeder, J.D.; Shandas, R.; Kheyfets, V.O.; Buckner, J.K.; Hunter, K.S.; Hertzberg, J.R.; Fenster, B.E. Vorticity is a marker of diastolic ventricular interdependency in pulmonary hypertension. Pulm. Circ. 2016, 6, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin, S.; Maurac, A.; Mandry, D.; Selton-Suty, C.; Huttin, O.; Cherifi, A.; Guillaumot, A.; Gomez, E.; Chabot, F.; Chaouat, A. The role of cardiac magnetic resonance imaging in pulmonary arterial hypertension and chronic thrombo-embolic pulmonary hypertension. Rev. Mal. Respir. 2022, 39, 486–497. [Google Scholar] [CrossRef]
- Ota, H.; Sugimura, K.; Miura, M.; Shimokawa, H. Four-dimensional flow magnetic resonance imaging visualizes drastic change in vortex flow in the main pulmonary artery after percutaneous transluminal pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur. Heart J. 2015, 36, 1630. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.G.; Fyrdahl, A.; Wieslander, B.; Reiter, G.; Reiter, U.; Jin, N.; Maret, E.; Eriksson, M.; Caidahl, K.; Sorensson, P.; et al. Cardiovascular magnetic resonance 4D flow analysis has a higher diagnostic yield than Doppler echocardiography for detecting increased pulmonary artery pressure. BMC Med. Imaging 2020, 20, 28. [Google Scholar] [CrossRef]
- Reiter, G.; Reiter, U.; Kovacs, G.; Kainz, B.; Schmidt, K.; Maier, R.; Olschewski, H.; Rienmueller, R. Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure. Circ. Cardiovasc. Imaging. 2008, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.; Reiter, U.; Kovacs, G.; Olschewski, H.; Fuchsjager, M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology 2015, 275, 71–79. [Google Scholar] [CrossRef]
- Reiter, U.; Kovacs, G.; Reiter, C.; Krauter, C.; Nizhnikava, V.; Fuchsjager, M.; Olschewski, H.; Reiter, G. MR 4D flow-based mean pulmonary arterial pressure tracking in pulmonary hypertension. Eur. Radiol. 2021, 31, 1883–1893. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Krauter, C.; Reiter, U.; Kovacs, G.; Reiter, C.; Masana, M.; Olschewski, H.; Fuchsjager, M.; Stollberger, R.; Reiter, G. Automated vortical blood flow-based estimation of mean pulmonary arterial pressure from 4D flow MRI. Magn. Reson. Imaging 2022, 88, 132–141. [Google Scholar] [CrossRef] [PubMed]
- von Spiczak, J.; Crelier, G.; Giese, D.; Kozerke, S.; Maintz, D.; Bunck, A.C. Quantitative Analysis of Vortical Blood Flow in the Thoracic Aorta Using 4D Phase Contrast MRI. PLoS ONE 2015, 10, e0139025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheyfets, V.O.; Schafer, M.; Podgorski, C.A.; Schroeder, J.D.; Browning, J.; Hertzberg, J.; Buckner, J.K.; Hunter, K.S.; Shandas, R.; Fenster, B.E. 4D magnetic resonance flow imaging for estimating pulmonary vascular resistance in pulmonary hypertension. J. Magn. Reson. Imaging 2016, 44, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Barker, A.J.; Kheyfets, V.; Stenmark, K.R.; Crapo, J.; Yeager, M.E.; Truong, U.; Buckner, J.K.; Fenster, B.E.; Hunter, K.S. Helicity and Vorticity of Pulmonary Arterial Flow in Patients with Pulmonary Hypertension: Quantitative Analysis of Flow Formations. J. Am. Heart Assoc. 2017, 6, e007010. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Frank, B.S.; Ivy, D.D.; Abman, S.H.; Stenmark, K.R.; Mitchell, M.B.; Browne, L.P.; Barker, A.J.; Hunter, K.S.; Kheyfets, V.; et al. Short-Term Effects of Inhaled Nitric Oxide on Right Ventricular Flow Hemodynamics by 4-Dimensional-Flow Magnetic Resonance Imaging in Children with Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2021, 10, e020548. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Khuder, S.; Tinkel, J.; Khouri, S.J. The diagnostic accuracy of Doppler echocardiography in assessment of pulmonary artery systolic pressure: A meta-analysis. Echocardiography 2013, 30, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.; Badesch, D.; Gibbs, J.S.R.; Gopalan, D.; Khanna, D.; Manes, A.; Oudiz, R.; Satoh, T.; Torres, F.; Torbicki, A. Diagnosis of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801904. [Google Scholar] [CrossRef]
- Lammers, S.R.; Kao, P.H.; Qi, H.J.; Hunter, K.; Lanning, C.; Albietz, J.; Hofmeister, S.; Mecham, R.; Stenmark, K.R.; Shandas, R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1451-9. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Lodziewski, S.; Bollmann, T.; Opitz, C.F.; Ewert, R. Impaired peripheral endothelial function in severe idiopathic pulmonary hypertension correlates with the pulmonary vascular response to inhaled iloprost. Am. Heart J. 2007, 153, 1088.e1–1088.e7. [Google Scholar] [CrossRef]
- Xu, L.J.; Yin, L.K.; Liu, Y.J.; Liang, F.Y. A computational study on the influence of aortic valve disease on hemodynamics in dilated aorta. Math. Biosci. Eng. 2019, 17, 606–626. [Google Scholar] [CrossRef]
- Wehrum, T.; Hagenlocher, P.; Lodemann, T.; Vach, W.; Dragonu, I.; Hennemuth, A.; von Zur Muhlen, C.; Stuplich, J.; Ngo, B.T.; Harloff, A. Age dependence of pulmonary artery blood flow measured by 4D flow cardiovascular magnetic resonance: Results of a population-based study. J. Cardiovasc. Magn. Reson. 2016, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Pathrose, A.; Ma, L.; Berhane, H.; Scott, M.B.; Chow, K.; Forman, C.; Jin, N.; Serhal, A.; Avery, R.; Carr, J.; et al. Highly accelerated aortic 4D flow MRI using compressed sensing: Performance at different acceleration factors in patients with aortic disease. Magn. Reson. Med. 2021, 85, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, A.; Rich, A.; Liu, Y.; Jin, N.; Potter, L.; Tong, M.; Rajpal, S.; Simonetti, O.; Ahmad, R. Fully self-gated whole-heart 4D flow imaging from a 5-minute scan. Magn. Reson. Med. 2021, 85, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, J.R.; Stackl, M.; Weiss, K.; Baessler, B.; Gerhardt, F.; Rosenkranz, S.; Maintz, D.; Giese, D.; Bunck, A.C. k-t accelerated multi-VENC 4D flow MRI improves vortex assessment in pulmonary hypertension. Eur. J. Radiol. 2021, 145, 110035. [Google Scholar] [CrossRef]

| A. Clinical Parameters | |
|---|---|
| Age, years | 63 ± 16 |
| Men, n (%) | 10 (40) |
| Overweight, n (%) | 5 (20) |
| HBP, n (%) | 7 (28) |
| Diabetes, n (%) | 4 (16) |
| WHO functional class, n (IQR) | 2 (2–3) |
| B. Right Heart Catheterization | |
| Heart rythm, bpm | 83 ± 13 |
| Mean PAP, mm Hg | 33 ± 15 |
| PCWP, mm Hg | 7.4 ± 3.9 |
| LVEDP, mm Hg | 10.2 ± 4.7 |
| PVR, Wood unit | 5.8 ± 3.9 |
| Cardiac output, L/min | 4.4 ± 1.2 |
| Cardiac index, L/min/m2 | 2.4 ± 0.8 |
| All (n = 25) | PH (n = 19) | No PH (n = 6) | p | |
|---|---|---|---|---|
| Mean heart rate, bpm | 79 ± 17 | 82 ± 18 | 72 ± 8 | 0.08 |
| RV EDV, mL/m2 | 95 ± 43 | 101 ± 48 | 75 ± 14 | 0.1 |
| RV EDS, mL/m2 | 52 ± 35 | 58 ± 38 | 34 ± 10 | 0.02 |
| RV EF, % | 48 ± 12 | 46 ± 13 | 55 ± 6 | 0.2 |
| RV cardiac index, L/min/m2 | 2.9 ± 0.2 | 3.0 ± 0.9 | 2.5 ± 0.6 | 0.2 |
| Mean PA diameter, mm | 32 ± 6 | 33 ± 7 | 29 ± 2 | 0.01 |
| PA distensibility, % | 8.6 ± 6.6 | 7.3 ± 5.3 | 12.9 ± 9.1 | 0.07 |
| Mean peak velocity in PA, cm/s | 63 ± 20 | 61 ± 23 | 70 ± 7 | 0.1 |
| Vortex duration, % | 23 ± 16 | 27 ± 14 | 8.3 ± 13 | 0.007 |
| All (n = 25) | PH (n = 16) | No PH (n = 9) | p | |
|---|---|---|---|---|
| Mean heart rate, bpm | 79 ± 17 | 84 ± 19 | 73 ± 6 | 0.07 |
| RV EDV, mL/m2 | 95 ± 43 | 107 ± 50 | 73 ± 16 | 0.03 |
| RV EDS, mL/m2 | 52 ± 35 | 63 ± 40 | 33 ± 11 | 0.004 |
| RV EF, % | 48 ± 12 | 44 ± 13 | 56 ± 7 | 0.03 |
| RV cardiac index, L/min/m2 | 2.9 ± 0.2 | 3.0 ± 0.9 | 2.7 ± 0.6 | 0.6 |
| Mean PA diameter, mm | 32 ± 6 | 35 ± 4 | 28 ± 7 | 0.008 |
| PA distensibility, % | 8.6 ± 6.6 | 6.6 ± 5.3 | 12.2 ± 7.5 | 0.02 |
| Mean peak velocity in PA, cm/s | 63 ± 20 | 61 ± 24 | 63 ± 20 | 0.2 |
| Vortex duration, % | 23 ± 16 | 30 ± 13 | 9 ± 11 | 0.001 |
| AUC | 95% CI | 95% CI | |
|---|---|---|---|
| RV EDV (mL/m2) | 0.715 | 0.509 | 0.921 |
| RV ESV (mL/m2) | 0.803 | 0.619 | 0.988 |
| RV EF (%) | 0.697 | 0.495 | 0.900 |
| RV cardiac index (L/min/m2) | 0.680 | 0.455 | 0.905 |
| Mean diameter of PA (mm) | 0.842 | 0.687 | 0.998 |
| PA distensibility (%) | 0.754 | 0.563 | 0.946 |
| Vortex duration | 0.860 | 0.637 | 1 |
| AUC | 95% CI | 95% CI | |
|---|---|---|---|
| RV EDV (mL/m2) | 0.767 | 0.582 | 0.952 |
| RV ESV (mL/m2) | 0.844 | 0.690 | 0.998 |
| RV EF (%) | 0.760 | 0.571 | 0.995 |
| RV cardiac index (L/min/m2) | 0.563 | 0.331 | 0.794 |
| Mean diameter of PA (mm) | 0.819 | 0.643 | 0.996 |
| PA distensibility (%) | 0.785 | 0.403 | 0.972 |
| Vortex duration | 0.896 | 0.597 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deux, J.-F.; Crowe, L.A.; Genecand, L.; Hachulla, A.-L.; Glessgen, C.G.; Noble, S.; Beghetti, M.; Ning, J.; Giese, D.; Lador, F.; et al. Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH). J. Clin. Med. 2022, 11, 5237. https://doi.org/10.3390/jcm11175237
Deux J-F, Crowe LA, Genecand L, Hachulla A-L, Glessgen CG, Noble S, Beghetti M, Ning J, Giese D, Lador F, et al. Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Journal of Clinical Medicine. 2022; 11(17):5237. https://doi.org/10.3390/jcm11175237
Chicago/Turabian StyleDeux, Jean-François, Lindsey A. Crowe, Léon Genecand, Anne-Lise Hachulla, Carl G. Glessgen, Stéphane Noble, Maurice Beghetti, Jin Ning, Daniel Giese, Frédéric Lador, and et al. 2022. "Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH)" Journal of Clinical Medicine 11, no. 17: 5237. https://doi.org/10.3390/jcm11175237
APA StyleDeux, J.-F., Crowe, L. A., Genecand, L., Hachulla, A.-L., Glessgen, C. G., Noble, S., Beghetti, M., Ning, J., Giese, D., Lador, F., & Vallée, J.-P. (2022). Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Journal of Clinical Medicine, 11(17), 5237. https://doi.org/10.3390/jcm11175237







