Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies
Abstract
1. Introduction
2. Detection of CMV
3. Is CMV Infection Associated with GBM?
4. CMV Infection Is Associated with the Prognosis of GBM
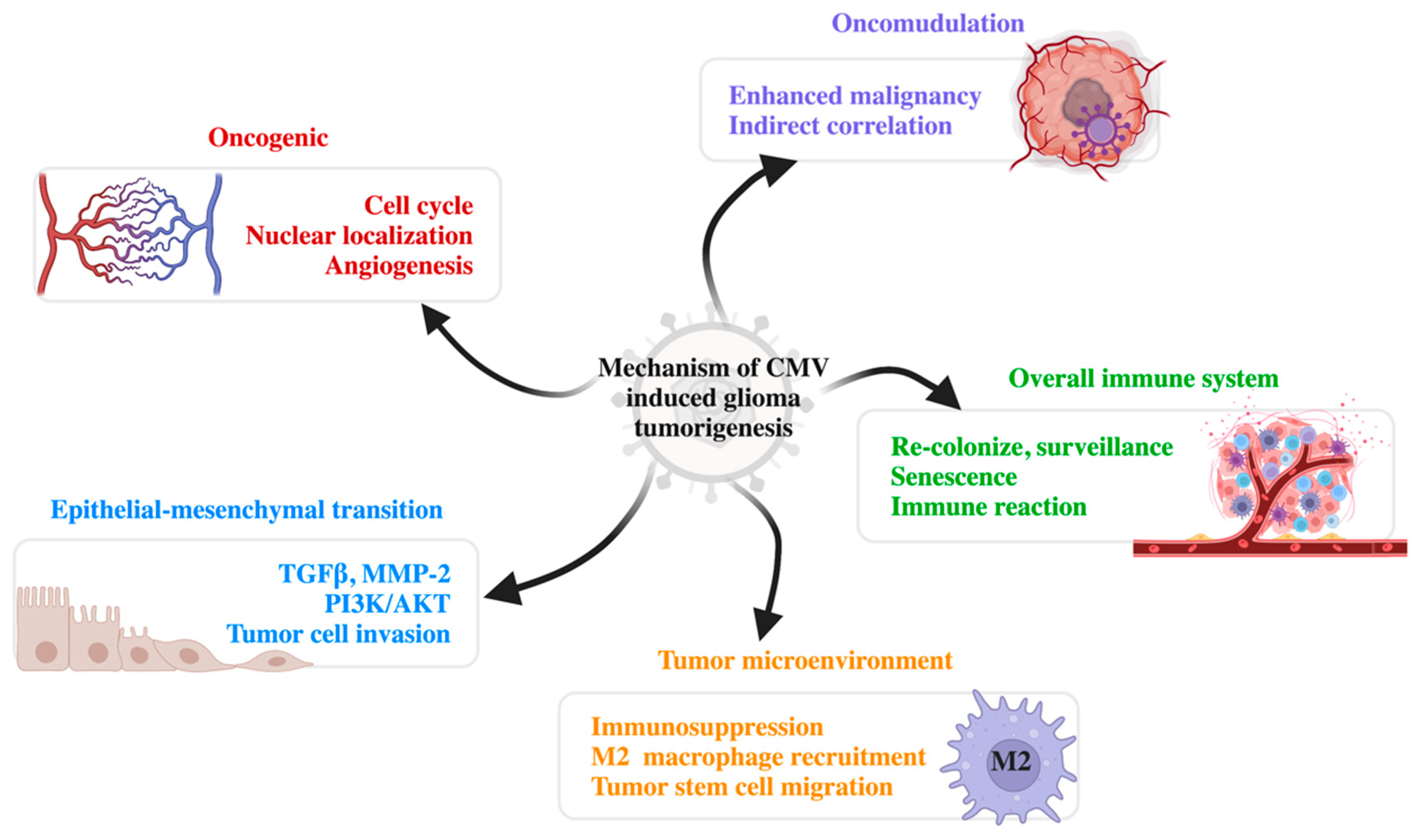
5. Mechanisms of CMV-Related Glioma Tumorigenesis
5.1. Oncomodulation
5.2. Oncogenic Features
5.3. Tumor Microenvironment
5.4. Epithelial–Mesenchymal Transition
5.5. Overall Immune System
6. CMV-Associated Encephalopathy in Standard Therapy
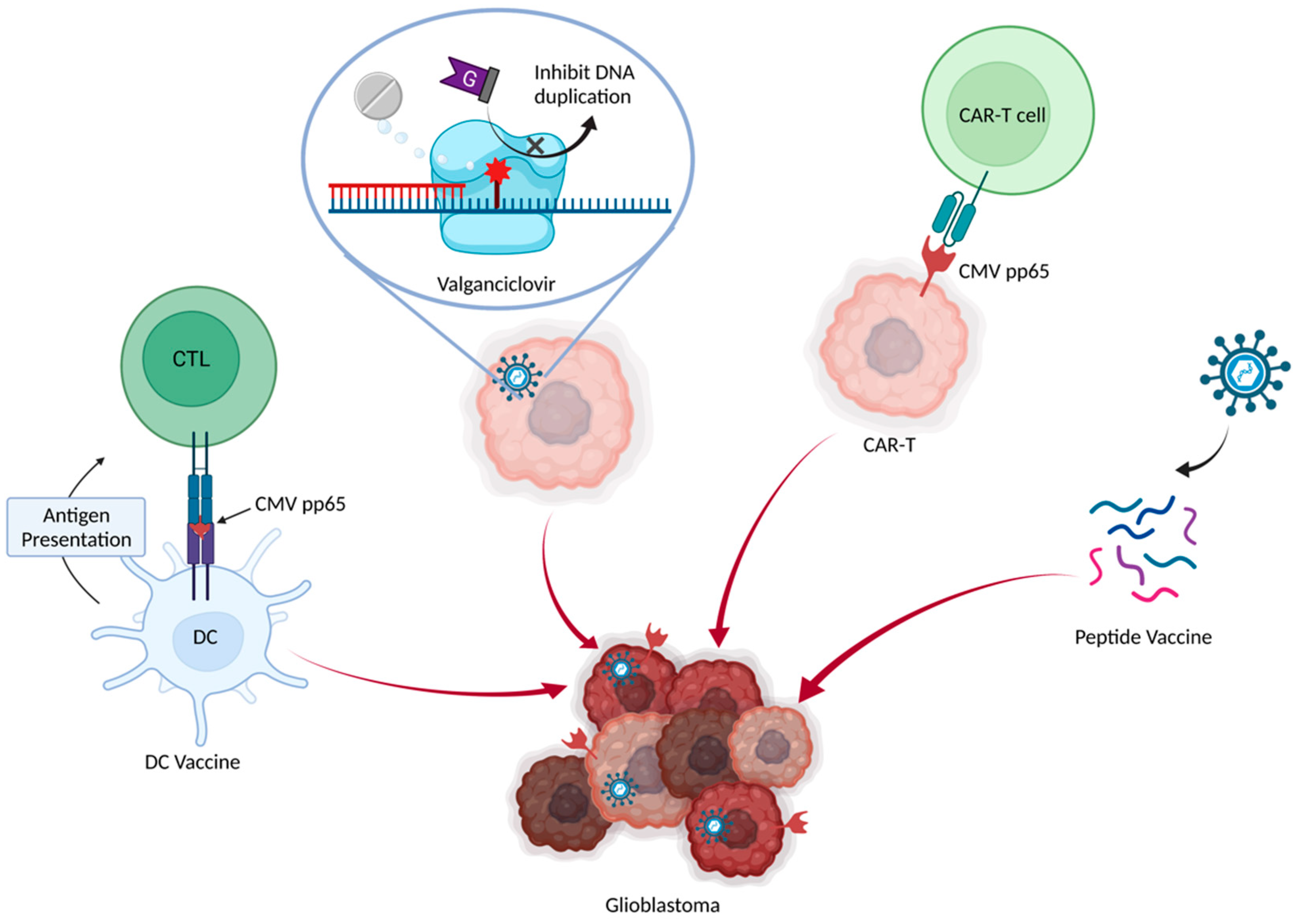
7. Anti-CMV Therapy in GBM
7.1. Valganciclovir
7.2. Dendritic Cell Vaccine
7.3. Adoptive CMV-Specific T Cells
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Nam, D.-H.; Ram, Z.; Poon, W.-S.; Wang, J.; Boldbaatar, D.; Mao, Y.; Ma, W.; Mao, Q.; You, Y.; et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2020, 499, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Landolfo, S.; Gariglio, M.; Gribaudo, G.; Lembo, D. The human cytomegalovirus. Pharmacol. Ther. 2003, 98, 269–297. [Google Scholar] [CrossRef]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef]
- Picarda, G.; Benedict, C.A. Cytomegalovirus: Shape-Shifting the Immune System. J. Immunol. 2018, 200, 3881–3889. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Halwachs-Baumann, G. Recent developments in human cytomegalovirus diagnosis. Expert. Rev. Anti. Infect. Ther. 2007, 5, 427–439. [Google Scholar] [CrossRef]
- Cobbs, C.S. Cytomegalovirus and brain tumor: Epidemiology, biology and therapeutic aspects. Curr. Opin. Oncol. 2013, 25, 682–688. [Google Scholar] [CrossRef]
- Charles, S.; Cobbs, L.H.; Minu Samanta, G.; Gillespie, Y.; Bharara, S.; Peter, H.; King, L.; Burt Nabors, C.; Cobbs, G.; William, J.B. Human Cytomegalovirus Infection and Expression in Human Malignant Glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Farias, K.; Moreli, M.L.; Floriano, V.G.; da Costa, V.G. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch. Virol. 2019, 164, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Kullberg-Lindh, C.; Olofsson, S.; Brune, M.; Lindh, M. Comparison of serum and whole blood levels of cytomegalovirus and Epstein-Barr virus DNA. Transpl. Infect. Dis. 2008, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.W.; Cinatl, J., Jr. Oncomodulation by human cytomegalovirus: Evidence becomes stronger. Med. Microbiol. Immunol. 2009, 198, 79–81. [Google Scholar] [CrossRef]
- Harkins, L.; Volk, A.L.; Samanta, M.; Mikolaenko, I.; Britt, W.J.; I Bland, K.; Cobbs, C.S. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Samanta, M.H.L.; Klemm, K.; Britt, W.J.; Cobbs, C.S. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Taher, C.; Frisk, G.; Fuentes, S.; Religa, P.; Costa, H.; Assinger, A.; Vetvik, K.K.; Bukholm, I.R.; Yaiw, K.-C.; Smedby, K.E.; et al. High Prevalence of Human Cytomegalovirus in Brain Metastases of Patients with Primary Breast and Colorectal Cancers. Transl. Oncol. 2014, 7, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Libard, S.; Popova, S.N.; Amini, R.-M.; Kärjä, V.; Pietiläinen, T.; Hämäläinen, K.M.; Sundström, C.; Hesselager, G.; Bergqvist, M.; Ekman, S.; et al. Human Cytomegalovirus Tegument Protein pp65 Is Detected in All Intra- and Extra-Axial Brain Tumours Independent of the Tumour Type or Grade. PLoS ONE 2014, 9, e108861. [Google Scholar] [CrossRef] [PubMed]
- Wolmer-Solberg, N.; Baryawno, N.; Rahbar, A.; Fuchs, D.; Odeberg, J.; Taher, C.; Wilhelmi, V.; Milosevic, J.; Mohammad, A.A.; Martinsson, T.; et al. Frequent detection of human cytomegalovirus in neuroblastoma: A novel therapeutic target? Int. J. Cancer 2013, 133, 2351–2361. [Google Scholar] [CrossRef]
- Wrensch, M.; Weinberg, A.; Wiencke, J.; Miike, R.; Barger, G.; Kelsey, K. Prevalence of Antibodies to Four Herpesviruses among Adults with Glioma and Controls. Am. J. Epidemiol. 2001, 154, 161–165. [Google Scholar] [CrossRef]
- Rahbar, A.; Peredo, I.; Solberg, N.W.; Taher, C.; Dzabic, M.; Xu, X.; Skarman, P.; Fornara, O.; Tammik, C.; Yaiw, K.; et al. Discordant humoral and cellular immune responses to Cytomegalovirus (CMV) in glioblastoma patients whose tumors are positive for CMV. Oncoimmunology 2015, 4, e982391. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Stragliotto, G. Valganciclovir in Patients with Glioblastoma. N. Engl. J. Med. 2013, 369, 2064–2066. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Clark, P.A.; Kuo, J.S.; Salamat, M.S.; Kalejta, R.F. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J. Virol. 2012, 86, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhao, F.; Qiu, Y.; Cheng, S.; Sun, J.Y.; Fang, W.; Rayner, S.; McVoy, M.A.; Jiang, X.J.; Tang, Q.; et al. Human cytomegalovirus DNA and immediate early protein 1/2 are highly associated with glioma and prognosis. Protein Cell 2020, 11, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Priel, E.; Wohl, A.; Teperberg, M.; Nass, D.; Cohen, Z.R. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. J. Clin. Neurosci. 2015, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.H.; Ramkissoon, S.H.; Milner, D.A., Jr.; Folkerth, R.D. Cytomegalovirus and Glioblastoma: A Review of Evidence for Their Association and Indications for Testing and Treatment. J. Neuropathol. Exp. Neurol. 2014, 73, 994–998. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Faranoush, M.; Naderi, A.; Kiani, S.J.; Sadeghipour, A.; Javanmard, D.; Farahmand, M.; Ghorbani, S.; Sedaghati, F.; et al. Molecular Investigation of Human Cytomegalovirus and Epstein-Barr virus in Glioblastoma Brain Tumor: A Case-Control Study in Iran. Iran. Biomed. J. 2021, 25, 426–433. [Google Scholar] [CrossRef]
- Foster, H.; Piper, K.; Depledge, L.; Li, H.-F.; Scanlan, J.; Jae-Guen, Y.; Boeckh, M.; Cobbs, C.; Yoon, J.-G. Human cytomegalovirus seropositivity is associated with decreased survival in glioblastoma patients. Neuro-Oncol. Adv. 2019, 1, vdz020. [Google Scholar] [CrossRef]
- Lisyany, N.I.; Klyuchnikova, A.A.; Belskaya, L.N.; Lisyany, A.A.; Gnedkova, I.A. Cytomegaloviruses and malignant brain tumors. Exp. Oncol. 2019, 41, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, S.; Li, X.; Chen, F.; Li, W. Viral infection and glioma: A meta-analysis of prognosis. BMC Cancer 2020, 20, 549. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Orrego, A.; Peredo, I.; Dzabic, M.; Wolmer-Solberg, N.; Strååt, K.; Stragliotto, G.; Söderberg-Nauclér, C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. 2013, 57, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Stragliotto, G.; Orrego, A.; Peredo, I.; Taher, C.; Willems, J.; Söderberg-Naucler, C. Low levels of Human Cytomegalovirus Infection in Glioblastoma multiforme associates with patient survival—A case-control study. Herpesviridae 2012, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Strååt, K.; Liu, C.; Rahbar, A.; Zhu, Q.; Liu, L.; Wolmer-Solberg, N.; Lou, F.; Liu, Z.; Shen, J.; Jia, J.; et al. Activation of Telomerase by Human Cytomegalovirus. J. Natl. Cancer Inst. 2009, 101, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J., Jr.; Nevels, M.; Paulus, C.; Michaelis, M. Activation of telomerase in glioma cells by human cytomegalovirus: Another piece of the puzzle. J. Natl. Cancer Inst. 2009, 101, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Maleki, F.; Sadigh, Z.A.; Sadeghi, F.; Muhammadnejad, A.; Farahmand, M.; Parvin, M.; Shirkoohi, R. Human cytomegalovirus infection in Iranian glioma patients correlates with aging and tumor aggressiveness. J. Med. Virol. 2020, 92, 1266–1276. [Google Scholar] [CrossRef]
- Geder, L.S.E.; Rohner, T.J.; Rapp, F. Cytomegalovirus and cancer of the prostate: In vitro transformation of human cells. Cancer Treat. Rep. 1977, 61, 139–146. [Google Scholar]
- Merchut-Maya, J.M.; Bartek, J., Jr.; Bartkova, J.; Galanos, P.; Pantalone, M.R.; Lee, M.; Cui, H.L.; Shilling, P.J.; Brøchner, C.B.; Broholm, H.; et al. Human cytomegalovirus hijacks host stress response fueling replication stress and genome instability. Cell Death Differ. 2022, 29, 1639–1653. [Google Scholar] [CrossRef]
- Joseph, G.P.; McDermott, R.; Baryshnikova, M.A.; Cobbs, C.S.; Ulasov, I.V. Cytomegalovirus as an oncomodulatory agent in the progression of glioma. Cancer Lett. 2017, 384, 79–85. [Google Scholar] [CrossRef]
- Soderberg-Naucler, C.; Johnsen, J.I. Cytomegalovirus in human brain tumors: Role in pathogenesis and potential treatment options. World J. Exp. Med. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, H.; Shenk, T. Human cytomagalovirus IE1 and IE2 proteins are mutagenic and mediate "hit-and-run" oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 3341–3345. [Google Scholar] [CrossRef] [PubMed]
- Lyon, S.M.Y.K.; Paulus, C.; Nevels, M.; Kalejta, R.F. Human Cytomegalovirus Genomes Survive Mitosis via the IE19 Chromatin-Tethering Domain. mBio 2020, 29, e02410–e02420. [Google Scholar] [CrossRef] [PubMed]
- Slinger, E.; Maussang, D.; Schreiber, A.; Siderius, M.; Rahbar, A.; Fraile-Ramos, A.; Lira, S.A.; Söderberg-Nauclér, C.; Smit, M.J. HCMV-Encoded Chemokine Receptor US28 Mediates Proliferative Signaling Through the IL-6–STAT3 Axis. Sci. Signal. 2010, 3, ra58. [Google Scholar] [CrossRef] [PubMed]
- Krenzlin, H.; Behera, P.; Lorenz, V.; Passaro, C.; Zdioruk, M.; Nowicki, M.O.; Grauwet, K.; Zhang, H.; Skubal, M.; Ito, H.; et al. Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. J. Clin. Investig. 2019, 129, 1671–1683. [Google Scholar] [CrossRef]
- Soroceanu, L.; Cobbs, C.S. Is HCMV a tumor promoter? Virus Res. 2011, 157, 193–203. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Kaverina, N.V.; Ghosh, D.; Baryshnikova, M.A.; Kadagidze, Z.G.; Karseladze, A.I.; Baryshnikov, A.Y.; Cobbs, C.S. CMV70-3P miRNA contributes to the CMV mediated glioma stemness and represents a target for glioma experimental therapy. Oncotarget 2017, 8, 25989–25999. [Google Scholar] [CrossRef] [PubMed]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; Dallas, S.R.M.; Smit, M.; Soroceanu, L.; Cobbs, C.S. The HCMV and Gliomas Symposium. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology 2012, 14, 246–255. [Google Scholar] [CrossRef]
- Maussang, D.; Langemeijer, E.; Fitzsimons, C.P.; Walsum, M.S.-V.; Dijkman, R.; Borg, M.K.; Slinger, E.; Schreiber, A.; Michel, D.; Tensen, C.P.; et al. The Human Cytomegalovirus–Encoded Chemokine Receptor US28 Promotes Angiogenesis and Tumor Formation via Cyclooxygenase-2. Cancer Res. 2009, 69, 2861–2869. [Google Scholar] [CrossRef]
- Dziurzynski, K.; Wei, J.; Qiao, W.; Hatiboglu, M.A.; Kong, L.-Y.; Wu, A.; Wang, Y.; Cahill, D.; Levine, N.; Prabhu, S.; et al. Glioma-Associated Cytomegalovirus Mediates Subversion of the Monocyte Lineage to a Tumor Propagating Phenotype. Clin. Cancer Res. 2011, 17, 4642–4649. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, B.; Hu, M.; Qian, D.; Wang, B. Human cytomegalovirus infection enhances invasiveness and migration of glioblastoma cells by epithelial-to-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2020, 13, 2637–2647. [Google Scholar]
- Shimamura, M.; Murphy-Ullrich, J.E.; Britt, W.J. Human cytomegalovirus induces TGF-beta1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog. 2010, 6, e1001170. [Google Scholar] [CrossRef]
- Cobbs, C. Cytomegalovirus is a tumor-associated virus: Armed and dangerous. Curr. Opin. Virol. 2019, 39, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-H.; Meng, Q.; Rao, M.; Liu, Z.; Paraschoudi, G.; Dodoo, E.; Maeurer, M. The impact of inflationary cytomegalovirus-specific memory T cells on anti-tumour immune responses in patients with cancer. Immunology 2018, 155, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Kallemeijn, M.J.; Boots, A.M.H.; Van Der Klift, M.Y.; Brouwer, E.; Abdulahad, W.H.; Verhaar, J.; Van Dongen, J.; Langerak, A.W. Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T-cell receptor gammadelta T-cells. Sci. Rep. 2017, 7, 5509. [Google Scholar] [CrossRef]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef]
- Goerig, N.; Semrau, S.; Frey, B.; Korn, K.; Fleckenstein, B.; Überla, K.; Dörfler, A.; Putz, F.; Gaipl, U.S.; Fietkau, R. Clinically significant CMV (re)activation during or after radiotherapy/chemotherapy of the brain: Correlation with neurological deterioration and improvement upon antiviral treatment. Strahlenther. Onkol. 2016, 192, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Überla, K.; Schmidt, M.A.; Dörfler, A.; Engelhorn, T.; Eyüpoglu, I.; Rühle, P.F.; et al. Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro-Oncology 2016, 18, 1664–1672. [Google Scholar] [CrossRef]
- Schneider, M.; Reitter, E.-M.; Kastner, M.-T.; Thannesberger, J.; Rieder, F.J.; Preusser, M.; Marosi, C.; Steininger, C. Absence of CMV viremia in high-grade glioma patients under low dosage glucocorticoid treatment. Neuro-Oncology 2017, 19, 1280–1282. [Google Scholar] [CrossRef]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Überla, K.; Schmidt, M.A.; Dörfler, A.; Engelhorn, T.; Eyüpoglu, I.; Rühle, P.F.; et al. Early Mortality of Brain Cancer Patients and its Connection to Cytomegalovirus Reactivation During Radiochemotherapy. Clin. Cancer Res. 2020, 26, 3259–3270. [Google Scholar] [CrossRef]
- Stragliotto, G.; Rahbar, A.; Solberg, N.W.; Lilja, A.; Taher, C.; Orrego, A.; Bjurman, B.; Tammik, C.; Skarman, P.; Peredo, I.; et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: A randomized, double-blind, hypothesis-generating study. Int. J. Cancer 2013, 133, 1204–1213. [Google Scholar] [CrossRef]
- Söderberg-Naucler, C.; Peredo, I.; Rahbar, A.; Hansson, F.; Nordlund, A.; Stragliotto, G. Use of Cox regression with treatment status as a time-dependent covariate to re-analyze survival benefit excludes immortal time bias effect in patients with glioblastoma who received prolonged adjuvant treatment with valganciclovir. Int. J. Cancer 2013, 135, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Bodhinayake, I.; Schaefer, P.M.; Boockvar, J.A. VIGAS and Beyond: The Impact of HCMVInfection and its Treatment in Glioblastoma. Neurosurgery 2014, 74, N23–N24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Söderberg-Nauclér, C.; Rahbar, A.; Stragliotto, G. Survival in Patients with Glioblastoma Receiving Valganciclovir. N. Engl. J. Med. 2013, 369, 985–986. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Bartek, J.; Söderberg-Naucler, C. Valganciclovir as Add-on to Standard Therapy in Glioblastoma Patients. Clin. Cancer Res. 2020, 26, 4031–4039. [Google Scholar] [CrossRef]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E., II; Healy, P.; et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.-N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Schuessler, A.; Smith, C.; Beagley, L.; Boyle, G.M.; Rehan, S.; Matthews, K.; Jones, L.; Crough, T.; Dasari, V.; Klein, K.; et al. Autologous T-cell Therapy for Cytomegalovirus as a Consolidative Treatment for Recurrent Glioblastoma. Cancer Res. 2014, 74, 3466–3476. [Google Scholar] [CrossRef]
- Smith, C.; Lineburg, K.E.; Martins, J.P.; Ambalathingal, G.R.; Neller, M.A.; Morrison, B.; Matthews, K.K.; Rehan, S.; Crooks, P.; Panikkar, A.; et al. Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J. Clin. Investig. 2020, 130, 6041–6053. [Google Scholar] [CrossRef]
- Weathers, S.-P.; Penas-Prado, M.; Pei, B.-L.; Ling, X.; Kassab, C.; Banerjee, P.; Bdiwi, M.; Shaim, H.; Alsuliman, A.; Shanley, M.; et al. Glioblastoma-mediated Immune Dysfunction Limits CMV-specific T Cells and Therapeutic Responses: Results from a Phase I/II Trial. Clin. Cancer Res. 2020, 26, 3565–3577. [Google Scholar] [CrossRef]
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E.; Healy, P.; Congdon, K.L.; et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res. 2018, 78, 256–264. [Google Scholar] [CrossRef]
- Åsberg, A.; Humar, A.; Rollag, H.; Jardine, A.G.; Mouas, H.; Pescovitz, M.D.; Sgarabotto, D.; Tuncer, M.; Noronha, I.L.; Hartmann, A.; et al. Oral Valganciclovir Is Noninferior to Intravenous Ganciclovir for the Treatment of Cytomegalovirus Disease in Solid Organ Transplant Recipients. Am. J. Transplant. 2007, 7, 2106–2113. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert Rev. Anti-Infect. Ther. 2013, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baryawno, N.; Rahbar, A.; Wolmer-Solberg, N.; Taher, C.; Odeberg, J.; Darabi, A.; Khan, Z.; Sveinbjörnsson, B.; Fuskevåg, O.-M.; Segerström, L.; et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J. Clin. Investig. 2011, 121, 4043–4055. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Wick, A.; Platten, M. Good maths is needed to understand CMV data in glioblastoma. Int. J. Cancer 2013, 134, 2991–2992. [Google Scholar] [CrossRef]
- Wick, W.; Wick, A.; Platten, M. Challenging cytomegalovirus data in glioblastoma. Neuro-Oncology 2014, 16, 165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weller, M.; Soffietti, R.; Brada, M. The legend of cytomegalovirus and glioblastoma lives on. Neuro-Oncology 2013, 16, 166. [Google Scholar] [CrossRef][Green Version]
- Liu, C.J.; Hu, Y.W. Immortal time bias in retrospective analysis: Is there a survival benefit in patients with glioblastoma who received prolonged treatment of adjuvant valganciclovir? Int. J. Cancer 2014, 135, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Soderberg-Naucler, C. Valganciclovir as Add-On to Standard Therapy in Secondary Glioblastoma. Microorganisms 2020, 8, 1471. [Google Scholar] [CrossRef]
- Pantalone, M.R.; Rahbar, A.; Söderberg-Naucler, C.; Stragliotto, G. Valganciclovir as Add-on to Second-Line Therapy in Patients with Recurrent Glioblastoma. Cancers 2022, 14, 1958. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, J.; Tanksley, J.P.; Mobley, B.C.; Ayers, G.D.; Moots, P.L.; Clark, S.W. Valganciclovir and bevacizumab for recurrent glioblastoma: A single-institution experience. Mol. Clin. Oncol. 2015, 4, 154–158. [Google Scholar] [CrossRef]
- Kim, J.W.; Kane, J.R.; Panek, W.K.; Young, J.S.; Rashidi, A.; Yu, D.; Kanojia, D.; Hasan, T.; Miska, J.; Gómez-Lim, M.A.; et al. A Dendritic Cell-Targeted Adenoviral Vector Facilitates Adaptive Immune Response Against Human Glioma Antigen (CMV-IE) and Prolongs Survival in a Human Glioma Tumor Model. Neurotherapeutics 2018, 15, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- A Hossain, J.; Latif, A.; Ystaas, L.A.R.; Ninzima, S.; Riecken, K.; Muller, A.; Azuaje, F.; Joseph, J.V.; Talasila, K.M.; Ghimire, J.; et al. Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro-Oncology 2019, 21, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Tetanus shot may improve glioblastoma treatment. Cancer Discov. 2015, 5, 571. [Google Scholar]
- Batich, K.A.; Mitchell, D.A.; Healy, P.; Herndon, J.E.; Sampson, J.H. Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in Glioblastoma. Clin. Cancer Res. 2020, 26, 5297–5303. [Google Scholar] [CrossRef]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef]
| Treatment | Year | Trial Registration | Trial Name | Interventions | Patients | N | Phase | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Valganciclovir | 2013 | NCT00400322 | VIGAS | Valganciclovir + CRT vs. CRT | ND-GBM | 42 | I/II | No difference in tumor volume, OS, and PFS. OS-24 27.3% in experiment arm vs. 25% in control. Treatment >6 m OS 24.1 m, <6 m OS 13.1 m. Treatment >6 m OS-2 year 67.6%, OS-4 year 27.3% | Soderberg-Naucler, C. [60] |
| 2013 | - | VIGAS re-analysis | Valganciclovir + CRT vs. CRT | ND-GBM | retrospective | Valganciclovir vs. control HR 2.44, treatment >6 m vs. control HR 0.441, treatment >6 m vs. <6 m HR 1.351 | Soderberg-Naucler, C. [61] | ||
| 2014 | - | VIGAS re-analysis | Valganciclovir + CRT vs. CRT | ND-GBM | retrospective | Patients with lower viral load have better prognosis | Malte Ottenhausen [62] | ||
| 2013 | - | VIGAS further study | Valganciclovir + CRT vs. CRT | ND-GBM | 50 | retrospective | OS-24 62% in experiment arm vs. 18% in control; OS 25.0 m vs. 13.5 m. Treatment >6 m OS-24 70%, OS 30.1 m | Soderberg-Naucler, C. [63] | |
| 2020 | - | Valganciclovir + CRT vs. CRT | R-GBM | 8 | retrospective | OS after relapse 19.1 m in experiment arm vs. 12.7 m in control; OS-24 37.5% vs. 2.8% | Soderberg-Naucler, C. [64] | ||
| DC vaccine | 2017 | NCT00639639 | ATTAC-GM | DI-TMZ + GM-CSF + CMV pp65 RNA-pulsed DC vs. CRT | ND-GBM | 11 | I | PFS 25.3 m, OS 41.1 m, 4 patients survived longer without progression (59–64 m) | John H. Sampson [65] |
| 2015 | NCT00639639 | ATTAC | CMV pp65 RNA-pulsed DC + Td+ CRT vs. unpulsed DC + Td+ CRT vs. CRT | ND-GBM | 12 | I | PFS, OS no worse than control, 3 patients survived >36.6 m. Td enhances DC vaccine because CCL3 enhances DC migration and inhibits tumor progression | John H. Sampson [66] | |
| CAR-T | 2014 | ACTRN12609000338268 | Autologous CMV pp65-specific T cells | R-GBM | 19 | I | PFS 246 d, OS 403 d, 4 of 10 patients remained progression-free during study | Rajiv Khanna [67] | |
| 2020 | ACTRN12615000656538 | Autologous CMV pp65-specific T cells | ND-GBM | 25 | I | PFS 25 m, PFS-12 20%, OS 21 m, OS-12 52%. Treatment before relapse was significantly longer OS than that after relapse | Rajiv Khanna [68] | ||
| 2020 | NCT02661282 | Autologous CMV pp65-specific T cells | ND + R-GBM | 65 | I/II | Increased circulating CMV-CD8 T cells, but did not improve survival | Amy B Heimberger [69] | ||
| 2017 | NCT00693095. | ATCT | CMV pp65-specific T cells + CMV pp65 RNA-pulsed DC vs. CMV pp65-specific T cells | ND-GBM | 22 | I | CMV DC vaccine enhanced polyfunctionality of adoptive CMV-specific T cells, correlated with OS. | John H. Sampson [70] |
| Treatment | Research Team | Trial Registration | Year | Trial Name | Study Title | Treatment Plan | Patients | N | Phase | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| Valganciclovir | Cecilia Soderberg-Naucler | NCT04116411 | September 2019–August 2024 | VIGAS2 | A Clinical Trial Evaluating the Efficacy of Valganciclovir in Glioblastoma Patients | Valganciclovir + CRT vs. placebo + CRT | ND-GBM | 220 | II, Randomized | recruiting |
| DC vaccine | Gary Archer | NCT03615404 | October 2018–July 2020 | ATTAC-P | Cytomegalovirus (CMV) RNA-Pulsed Dendritic Cells for Pediatric Patients and Young Adults With WHO Grade IV Glioma, Recurrent Malignant Glioma, or Recurrent Medulloblastoma | DI-TMZ + GM-CSF + Td + CMV pp65 RNA-pulsed DC | ND + R-GBM, recurrent medulloblastoma | 11 | I | completed |
| Gary Archer | NCT03927222 | September 2019–December 2023 | I-ATTAC | Immunotherapy Targeted Against Cytomegalovirus in Patients With Newly-Diagnosed WHO Grade IV Unmethylated Glioma | DI-TMZ + GM-CSF + Td+ CMV pp65 RNA-pulsed DC | ND-GBM | 48 | II | recruiting | |
| Duane Mitchell | NCT02465268 | August 2016–June 2024 | ATTAC-II | Vaccine Therapy for the Treatment of Newly Diagnosed Glioblastoma Multiforme | GM-CSF + Td + CMV pp65 RNA-pulsed DC vs. un-pulsed PBMC | ND-GBM | 120 | II, Randomized | recruiting | |
| Gary Archer | NCT02366728 | October 2015–August 2020 | ELEVATE | DC Migration Study for Newly-Diagnosed GBM | DC + CMV pp65 RNA-pulsed DC + TMZ vs. Td + CMV pp65 RNA-pulsed DC + TMZ vs. basiliximab + Td+ CMV pp65 RNA-pulsed DC + TMZ | ND-GBM | 100 | II, Randomized | Active, not recruiting | |
| Gary Archer | NCT03688178 | August 2020–March 2025 | DERIVe | DC Migration Study to Evaluate TReg Depletion In GBM Patients With and Without Varlilumab | DC pre-conditioning vaccine + TMZ vs. Td pre-conditioning + DC vaccine + TMZ vs. DC Vaccine + varlilumab (Td pre-conditioning) + TMZ | ND + R-GBM | 112 | II, Randomized | recruiting | |
| DC vaccine+ CAR-T | John Sampson | NCT00693095 | September 2008–April 2015 | ERaDICATe | Evaluation of Recovery From Drug-Induced Lymphopenia Using Cytomegalovirus-specific T-cell Adoptive Transfer | CMV-autologous lymphocyte transfer (CMV-ALT) vs. CMV-ALT + CMV pp65 RNA-pulsed DC | ND-GBM | 23 | I, Randomized | completed |
| CAR-T | Nabil Ahmed | NCT01109095 | October 2010–March 2018 | HERT-GBM | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM | HER2-CAR CMV-specific CTL | R-GBM | 16 | I | completed |
| Peptide Vaccine | Gary Archer | NCT02864368 | December 2016–September 2021 | PERFORMANCE | Peptide Targets for Glioblastoma Against Novel Cytomegalovirus Antigens | PEP CMV + Td + CRT vs. PEP CMV + Td+ TMZ | ND-GBM | 70 | I, Randomized | Active, not recruiting |
| Observational | Benjamin Frey | NCT02600065 | November 2015–February 2020 | GLIO-CMV-01 | Analysis of CMV Infections in Patients With Brain Tumors or Brain Metastases During and After Radio(Chemo)Therapy | CRT + TMZ | HGG, metastases | 250 | Observation | recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Liu, D.; Fang, S.; Ma, W.; Wang, Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. J. Clin. Med. 2022, 11, 5221. https://doi.org/10.3390/jcm11175221
Yang T, Liu D, Fang S, Ma W, Wang Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. Journal of Clinical Medicine. 2022; 11(17):5221. https://doi.org/10.3390/jcm11175221
Chicago/Turabian StyleYang, Tianrui, Delin Liu, Shiyuan Fang, Wenbin Ma, and Yu Wang. 2022. "Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies" Journal of Clinical Medicine 11, no. 17: 5221. https://doi.org/10.3390/jcm11175221
APA StyleYang, T., Liu, D., Fang, S., Ma, W., & Wang, Y. (2022). Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. Journal of Clinical Medicine, 11(17), 5221. https://doi.org/10.3390/jcm11175221