Blood Based Biomarkers as Predictive Factors for Hyperprogressive Disease
Abstract
:1. Introduction
2. Method
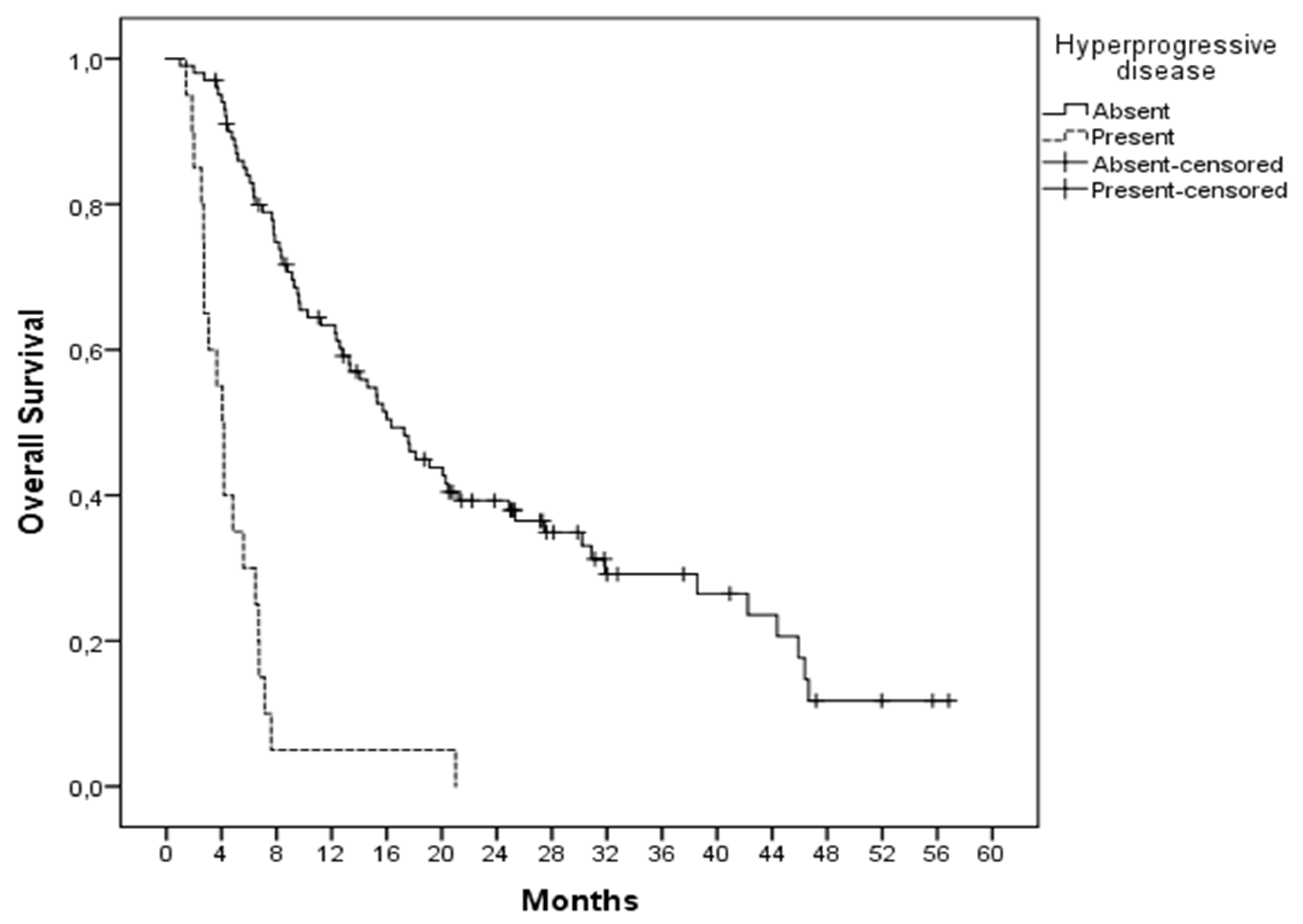
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larkin, J.; Minor, D.; D’Angelo, S.; Neyns, B.; Smylie, M.; Miller, W.H., Jr.; Gutzmer, R.; Linette, G.; Chmielowski, B.; Lao, C.D. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J. Clin. Oncol. 2018, 36, 383. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembroli-zumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Brower, V. Hyperprogressive disease with anti-PD-1 and anti-PD-L1. Lancet Oncol. 2016, 17, e527. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Liu, D.; Li, W. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: A meta-analysis. BMJ Open 2020, 10, e035031. [Google Scholar] [CrossRef]
- Russo, G.L.; Moro, M.; Sommariva, M.; Cancila, V.; Boeri, M.; Centonze, G.; Ferro, S.; Ganzinelli, M.; Gasparini, P.; Huber, V. Antibody–Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non–small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin. Cancer Res. 2019, 25, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Park, I.; Yoon, S.-K.; Lee, J.-L. Hyperprogressive disease (HPD) in genitourinary (GU) cancer patients treated with PD-1/PD-L1 inhibitors. J. Clin. Oncol. 2019, 37, 369. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Kanjanapan, Y.; Day, D.; Wang, L.; Al-Sawaihey, H.; Abbas, E.; Namini, A.; Siu, L.L.; Hansen, A.; Razak, A.A.; Spreafico, A. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer 2019, 125, 1341–1349. [Google Scholar] [CrossRef]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.; Pyo, K.-H.; Xin, C.-F.; Hong, M.; Ahn, B.-C.; Kim, Y.; Choi, S.; Yoon, H.; Lee, J. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1104–1113. [Google Scholar] [CrossRef]
- Sasaki, A.; Nakamura, Y.; Mishima, S.; Kawazoe, A.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Ohtsu, A.; Yoshino, T. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer 2019, 22, 793–802. [Google Scholar] [CrossRef]
- Petrova, M.; Donev, I.; Radanova, M.; Eneva, M.; Dimitrova, E.; Valchev, G.; Minchev, V.; Taushanova, M.; Boneva, M.; Karanikolova, T. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin. Exp. Immunol. 2020, 202, 353–362. [Google Scholar] [CrossRef]
- Yildirim, H.C.; Guven, D.C.; Aktepe, O.H.; Taban, H.; Yilmaz, F.; Yasar, S.; Aktas, B.Y.; Guner, G.; Dizdar, O.; Aksoy, S. Differences between hyperprogressive disease and progressive disease in patients receiving immunotherapy. EJMO 2022, 6, 59–63. [Google Scholar] [CrossRef]
| No | % | ||
|---|---|---|---|
| Sex | Female | 36 | 29.7 |
| Male | 85 | 70.2 | |
| Age | >65 | 39 | 32.2 |
| <65 | 82 | 67.7 | |
| Lactate dehydrogenase | Normal | 72 | 59.5 |
| >Upper limit of normal | 49 | 40.4 | |
| ECOG score | 0 | 77 | 63.6 |
| 1–4 | 36 | 31.8 | |
| >2 metastatic sites | Present | 16 | 13.2 |
| Absent | 105 | 86.7 | |
| Immunotherapy plus chemotherapy | Present | 28 | 23.1 |
| Absent | 93 | 76.8 | |
| Diagnosis | Melanoma | 41 | 33.8 |
| RCC | 40 | 33.0 | |
| NSCLC | 21 | 17.3 | |
| Other | 19 | 15.7 | |
| Line of treatment | 1 | 15 | 12.4 |
| 2 | 46 | 38.0 | |
| 3 | 27 | 22.3 | |
| 4 | 26 | 21.4 | |
| 5 | 7 | 5.7 |
| HPD Present | HPD Absent | p Score | ||
|---|---|---|---|---|
| Median age | 61.6 (47.2–67.0) | 62.28 (55.3–67.6) | ||
| Sex | Female | 3 (15%) | 33 (32.7%) | 0.091 |
| Male | 17 (85%) | 68 (67.3%) | ||
| Age | >65 | 7 (35%) | 32 (31.7%) | 0.772 |
| <65 | 13 (65%) | 69 (68.3%) | ||
| Lactate dehydrogenase | Normal | 5 (25%) | 67 (66.3%) | 0.001 |
| >ULN | 15 (75%) | 34 (33.7%) | ||
| Albumin (g/dL) | >4 | 3 (15%) | 43 (42.6%) | 0.016 |
| <4 | 17 (85%) | 58 (57.4%) | ||
| ECOG score | 0 | 11 (61.1%) | 66 (69.5%) | 0.485 |
| 1–4 | 7 (38.9%) | 29 (30.5%) | ||
| >2 metastatic sites | Present | 3 (15%) | 13 (12.9%) | 0.517 |
| Absent | 17 (85%) | 88 (87.1%) | ||
| Immunotherapy plus chemotherapy | Present | 6 (30%) | 22 (21.8%) | 0.426 |
| Absent | 14 (70%) | 79 (78.2%) | ||
| Neutrophil-to-lymphocyte ratio | >5 | 11 (55%) | 25 (24.7%) | 0.007 |
| <5 | 9 (45%) | 76 (75.7%) | ||
| Diagnosis | Melanoma | 9 (45%) | 32 (31.6%) | 0.026 |
| RCC | 3 (15%) | 37 (36.6%) | ||
| NSCLC | 7 (35%) | 14 (13.8%) | ||
| Other | 1 (5%) | 18 (17.8%) | ||
| Line of treatment | 1 | 2 (10%) | 13 (12.9%) | 0.112 |
| 2 | 12 (60%) | 34 (33.7%) | ||
| 3 | 3 (15%) | 24 (23.8%) | ||
| 4 | 1 (5%) | 25 (24.7%) | ||
| 5 | 2 (10%) | 5 (4.9%) |
| Clinical Factor | Risk of Hyperprogression | |
|---|---|---|
| Hazard Ratio (95%) | p Score | |
| Neutrophil-to-lymphocyte ratio (>5 vs. <5) | 1.972 (0.654–5.943) | 0.228 |
| Tumor type (NSCLC vs. other) | 2.514 (0.752–8.411) | 0.135 |
| Albumin (low vs. normal) | 3.743 (0.992–14.118) | 0.051 |
| Lactate dehydrogenase (high vs. normal) | 5.491 (1.809–16.672) | 0.003 |
| Diagnosis | p Score | |||
|---|---|---|---|---|
| NSCLC | Other | |||
| Lactate dehydrogenase | Normal > ULN | 11 10 | 61 39 | 0.474 |
| Neutrophil-to-lymphocyte | >5 <5 | 11 10 | 25 75 | 0.018 |
| Albumin (g/dL) | >4 <4 | 3 18 | 43 57 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, H.C.; Guven, D.C.; Aktepe, O.H.; Taban, H.; Yilmaz, F.; Yasar, S.; Aksoy, S.; Erman, M.; Kilickap, S.; Yalcin, S. Blood Based Biomarkers as Predictive Factors for Hyperprogressive Disease. J. Clin. Med. 2022, 11, 5171. https://doi.org/10.3390/jcm11175171
Yildirim HC, Guven DC, Aktepe OH, Taban H, Yilmaz F, Yasar S, Aksoy S, Erman M, Kilickap S, Yalcin S. Blood Based Biomarkers as Predictive Factors for Hyperprogressive Disease. Journal of Clinical Medicine. 2022; 11(17):5171. https://doi.org/10.3390/jcm11175171
Chicago/Turabian StyleYildirim, Hasan Cagri, Deniz Can Guven, Oktay Halit Aktepe, Hakan Taban, Feride Yilmaz, Serkan Yasar, Sercan Aksoy, Mustafa Erman, Saadettin Kilickap, and Suayib Yalcin. 2022. "Blood Based Biomarkers as Predictive Factors for Hyperprogressive Disease" Journal of Clinical Medicine 11, no. 17: 5171. https://doi.org/10.3390/jcm11175171
APA StyleYildirim, H. C., Guven, D. C., Aktepe, O. H., Taban, H., Yilmaz, F., Yasar, S., Aksoy, S., Erman, M., Kilickap, S., & Yalcin, S. (2022). Blood Based Biomarkers as Predictive Factors for Hyperprogressive Disease. Journal of Clinical Medicine, 11(17), 5171. https://doi.org/10.3390/jcm11175171







