Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon
Abstract
:1. Introduction
2. Motion Management
3. Paradigm Shift
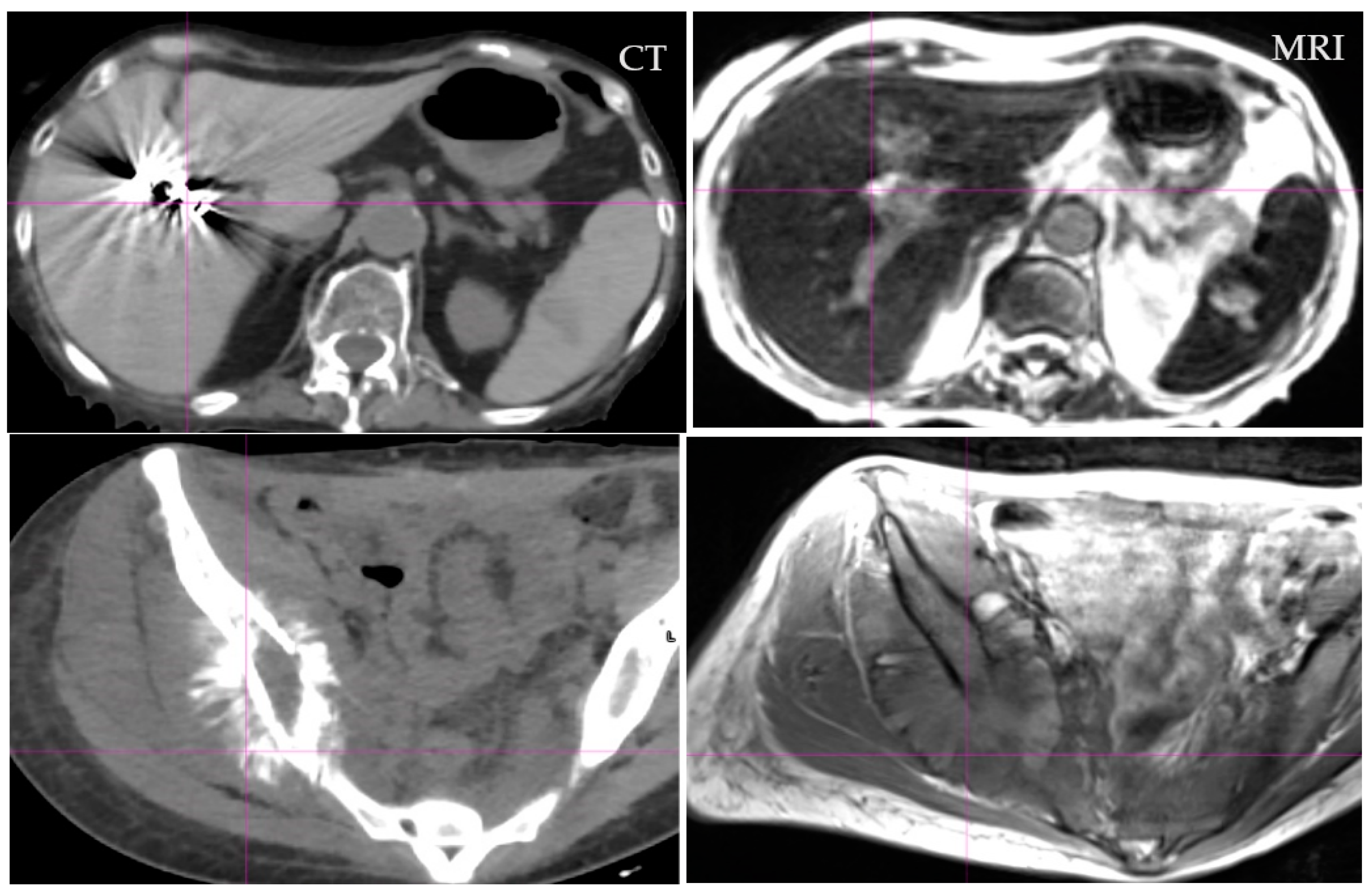
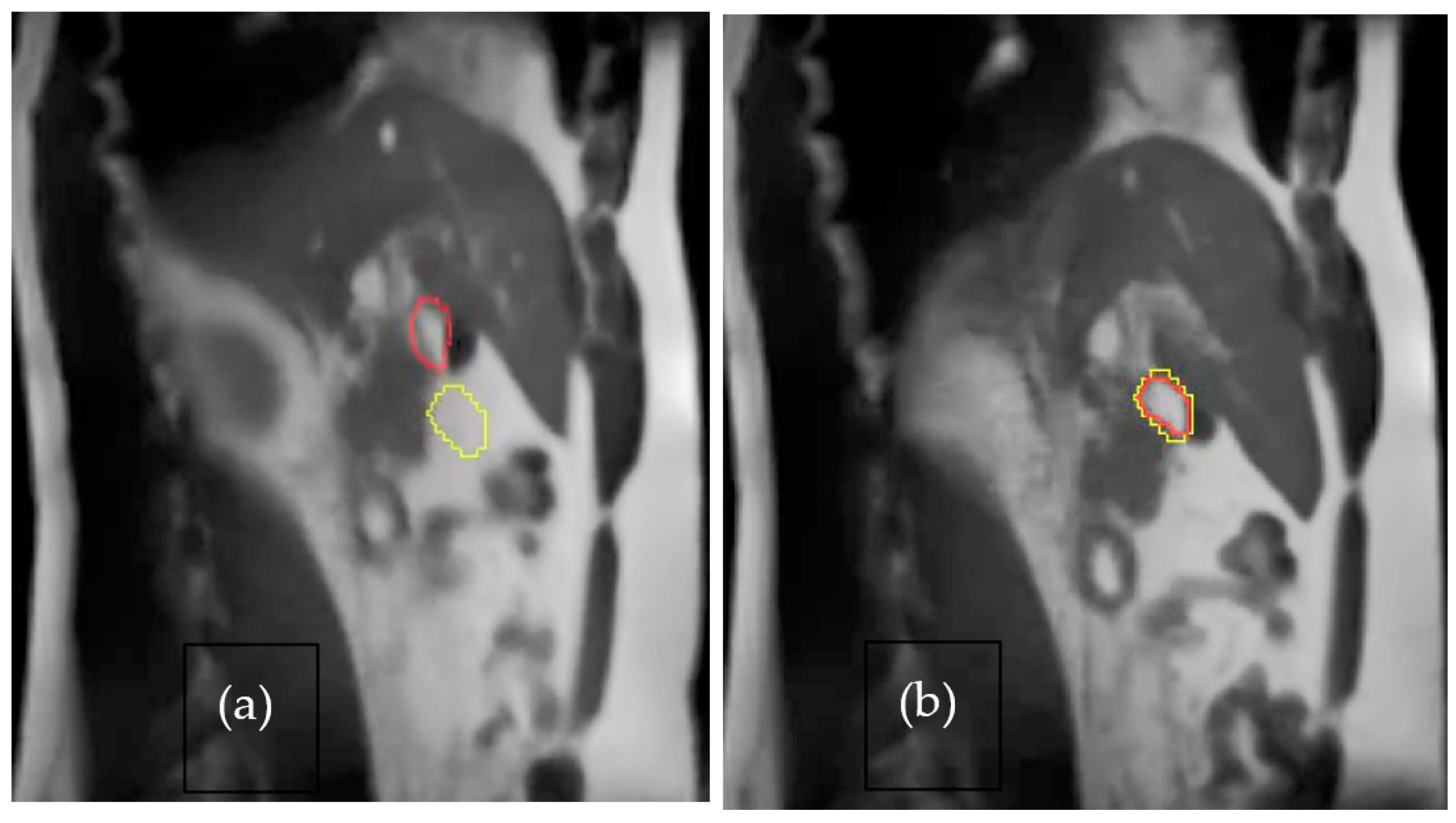
4. Success
5. Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Jaffray, D.A.; Siewerdsen, J.H. Cone-beam computed tomography with a flat-panel imager: Initial performance characterization. Med. Phys. 2000, 27, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, J.; McNeeley, S.; Pinover, W.; Horwitz, E.; Das, I.; Schultheiss, T.E.; Hanks, G.E. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int. J. Radiat. Oncol. 1999, 43, 719–725. [Google Scholar] [CrossRef]
- Kupelian, P.; Willoughby, T.; Mahadevan, A.; Djemil, T.; Weinstein, G.; Jani, S.; Enke, C.; Solberg, T.; Flores, N.; Liu, D.; et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int. J. Radiat. Oncol. 2007, 67, 1088–1098. [Google Scholar] [CrossRef]
- Willoughby, T.R.; Kupelian, P.A.; Pouliot, J.; Shinohara, K.; Aubin, M.; Roach, M.; Skrumeda, L.L.; Balter, J.M.; Litzenberg, D.W.; Hadley, S.W.; et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int. J. Radiat. Oncol. 2006, 65, 528–534. [Google Scholar] [CrossRef]
- Leunens, G.; Menten, J.; Weltens, C.; Verstraete, J.; Van der Schueren, E. Quality assessment of medical decision making in radiation oncology: Variability in target volume delineation for brain tumors. Radiother. Oncol. 1993, 28, 169–175. [Google Scholar] [CrossRef]
- Cazzaniga, L.F.; Marinoni, M.A.; Bossi, A.; Bianchi, E.; Cagna, E.; Cosentino, D.; Scandolaro, L.; Valli, M.; Frigerio, M. Interphysician variability in defining the planning target volume in the irradiation of prostate and seminal vesicles. Radiother. Oncol. 1998, 47, 293–296. [Google Scholar] [CrossRef]
- Dawson, L.; Mah, K.; Franssen, E.; Morton, G. Target position variability throughout prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 1155–1161. [Google Scholar] [CrossRef]
- Fiorino, C.; Reni, M.; Bolognesi, A.; Cattaneo, G.M.; Calandrino, R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: Implications for conformal treatment planning. Radiother. Oncol. 1998, 47, 285–292. [Google Scholar] [CrossRef]
- Caldwell, C.B.; Mah, K.; Ung, Y.C.; Danjoux, C.E.; Balogh, J.M.; Ganguli, S.; Ehrlich, L.E. Observer variation in contouring gross tumor volume in patients with poorly defined non-small-cell lung tumors on CT: The impact of 18 FDG-hybrid PET fusion. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 923–931. [Google Scholar] [CrossRef]
- Hurkmans, C.W.; Borger, J.H.; Pieters, B.R.; Russell, N.S.; Jansen, E.P.; Mijnheer, B.J. Variability in target volume delineation on CT scans of the breast. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1366–1372. [Google Scholar] [CrossRef]
- Geets, X.; Daisne, J.-F.; Arcangeli, S.; Coche, E.; De Poel, M.; Duprez, T.; Nardella, G.; Grégoire, V. Inter-observer variability in the delineation of pharyngo-laryngeal tumor, parotid glands and cervical spinal cord: Comparison between CT-scan and MRI. Radiother. Oncol. 2005, 77, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Louie, A.V.; Rodrigues, G.; Olsthoorn, J.; Palma, D.; Yu, E.; Yaremko, B.; Ahmad, B.; Aivas, I.; Gaede, S. Inter-observer and intra-observer reliability for lung cancer target volume delineation in the 4D-CT era. Radiother. Oncol. 2010, 95, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, J.; de Haas-Kock, D.F.; Beukema, J.C.; Neelis, K.J.; Woutersen, D.; Ceha, H.; Rozema, T.; Slot, A.; Vos-Westerman, H.; Intven, M.; et al. Target volume delineation variation in radiotherapy for early stage rectal cancer in the Netherlands. Radiother. Oncol. 2012, 102, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, S.; Belderbos, J.S.A.; van Herk, M. Challenges in the target volume definition of lung cancer radiotherapy. Transl. Lung Cancer Res. 2021, 10, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Das, I.J.; Compton, J.J.; Bajaj, A.; Johnstone, P.A. Intra- and inter-physician variability in target volume delineation in radiation therapy. J. Radiat. Res. 2021, 62, 1083–1089. [Google Scholar] [CrossRef]
- Bitar, R.; Leung, G.; Perng, R.; Tadros, S.; Moody, A.R.; Sarrazin, J.; McGregor, C.; Christakis, M.; Symons, S.; Nelson, A.; et al. MR Pulse Sequences: What Every Radiologist Wants to Know but Is Afraid to Ask. RadioGraphics 2006, 26, 513–537. [Google Scholar] [CrossRef]
- Yadav, P.; Chang, S.X.; Cheng, C.-W.; DesRosiers, C.M.; Mitra, R.K.; Das, I.J. Dosimetric evaluation of high-Z inhomogeneity used for hip prosthesis: A multi-institutional collaborative study. Phys. Med. 2022, 95, 148–155. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F.; Bosch, W.R.; Low, D.A.; Drzymala, R.E.; Chao, K.; Goddu, S.; Cutler, P.; Purdy, J.A. Multimodality image registration quality assurance for conformal three-dimensional treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 255–260. [Google Scholar] [CrossRef]
- Nenoff, L.; Ribeiro, C.O.; Matter, M.; Hafner, L.; Josipovic, M.; Langendijk, J.A.; Persson, G.F.; Walser, M.; Weber, D.C.; Lomax, A.J.; et al. Deformable image registration uncertainty for inter-fractional dose accumulation of lung cancer proton therapy. Radiother. Oncol. 2020, 147, 178–185. [Google Scholar] [CrossRef]
- Brock, K.R. Image Processing in Radiation Therapy; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Brock, K.K.; Dawson, L.A.; Sharpe, M.B.; Moseley, D.J.; Jaffray, D.A. Feasibility of a novel deformable image registration technique to facilitate classification, targeting, and monitoring of tumor and normal tissue. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1245–1254. [Google Scholar] [CrossRef]
- Jonsson, J.H.; Karlsson, M.G.; Karlsson, M.; Nyholm, T. Treatment planning using MRI data: An analysis of the dose calculation accuracy for different treatment regions. Radiat. Oncol. 2010, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Garbarino, K.; Schultz, L.; Levin, K.; Movsas, B.; Siddiqui, M.S.; Chetty, I.J.; Glide-Hurst, C. Dosimetric evaluation of synthetic CT relative to bulk density assignment-based magnetic resonance-only approaches for prostate radiotherapy. Radiat. Oncol. 2015, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chandarana, H.; Block, K.T.; Vahle, T.; Fenchel, M.; Das, I.J. Dosimetric evaluation of synthetic CT for magnetic resonance-only based radiotherapy planning of lung cancer. Radiat. Oncol. 2017, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, K.; Qu, J.; Chandarana, H.; Das, I.J. Dosimetric evaluation of magnetic resonance-generated synthetic CT for radiation treatment of rectal cancer. PLoS ONE 2018, 13, e0190883. [Google Scholar] [CrossRef] [PubMed]
- Farjam, R.; Tyagi, N.; Deasy, J.O.; Hunt, M.A. Dosimetric evaluation of an atlas-based synthetic CT generation approach for MR-only radiotherapy of pelvis anatomy. J. Appl. Clin. Med. Phys. 2019, 20, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.; Kapanen, M.; Tenhunen, M.; Keyriläinen, J.; Seppälä, T. A dual model HU conversion from MRI intensity values within and outside of bone segment for MRI-based radiotherapy treatment planning of prostate cancer. Med. Phys. 2014, 41, 011704. [Google Scholar] [CrossRef]
- Tyagi, N.; Fontenla, S.; Zhang, J.; Cloutier, M.; Kadbi, M.; Mechalakos, J.; Zelefsky, M.; Deasy, J.; Hunt, M. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys. Med. Biol. 2017, 62, 2961–2975. [Google Scholar] [CrossRef]
- Lerner, M.; Medin, J.; Jamtheim Gustafsson, C.; Alkner, S.; Siversson, C.; Olsson, L.E. Clinical validation of a commercially available deep learning software for synthetic CT generation for brain. Radiat. Oncol. 2021, 16, 66. [Google Scholar] [CrossRef]
- Spadea, M.F.; Maspero, M.; Zaffino, P.; Seco, J. Deep learning based synthetic-CT generation in radiotherapy and PET: A review. Med. Phys. 2021, 48, 6537–6566. [Google Scholar] [CrossRef]
- Tang, B.; Wu, F.; Fu, Y.; Wang, X.; Wang, P.; Orlandini, L.C.; Li, J.; Hou, Q. Dosimetric evaluation of synthetic CT image generated using a neural network for MR-only brain radiotherapy. J. Appl. Clin. Med. Phys. 2021, 22, 55–62. [Google Scholar] [CrossRef]
- Qi, M.; Li, Y.; Wu, A.; Jia, Q.; Li, B.; Sun, W.; Dai, Z.; Lu, X.; Zhou, L.; Deng, X.; et al. Multi-sequence MR image-based synthetic CT generation using a generative adversarial network for head and neck MRI-only radiotherapy. Med. Phys. 2020, 47, 1880–1894. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Lenkowicz, J.; Votta, C.; Boldrini, L.; Placidi, L.; Catucci, F.; Dinapoli, N.; Antonelli, M.V.; Romano, A.; De Luca, V.; et al. A deep learning approach to generate synthetic CT in low field MR-guided adaptive radiotherapy for abdominal and pelvic cases. Radiother. Oncol. 2020, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, E.; Wyatt, J.; Henry, A.M.; Short, S.C.; Sebag-Montefiore, D.; Murray, L.; Kelly, C.G.; McCallum, H.M.; Speight, R. Systematic Review of Synthetic Computed Tomography Generation Methodologies for Use in Magnetic Resonance Imaging–Only Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yadav, P.; McMillan, A.B. Synthetic Computed Tomography Generation from 0.35T Magnetic Resonance Images for Magnetic Resonance-Only Radiation Therapy Planning Using Perceptual Loss Models. Pract. Radiat. Oncol. 2022, 12, e40–e48. [Google Scholar] [CrossRef] [PubMed]
- Spadea, M.F.; Pileggi, G.; Zaffino, P.; Salome, P.; Catana, C.; Izquierdo-Garcia, D.; Amato, F.; Seco, J. Deep Convolution Neural Network (DCNN) Multiplane Approach to Synthetic CT Generation From MR images—Application in Brain Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 495–503. [Google Scholar] [CrossRef]
- Tie, X.; Lam, S.K.; Zhang, Y.; Lee, K.H.; Au, K.; Cai, J. Pseudo-CT generation from multi-parametric MRI using a novel multi-channel multi-path conditional generative adversarial network for nasopharyngeal carcinoma patients. Med. Phys. 2020, 47, 1750–1762. [Google Scholar] [CrossRef]
- Das, I.J.; McGee, K.P.; Tyagi, N.; Wang, H. Role and future of MRI in radiation oncology. Br. J. Radiol. 2019, 92, 20180505. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Fuller, C.D.; Verkooijen, H.M.; Verheij, M.; Choudhury, A.; Harrington, K.J.; Schultz, C.; Sahgal, A.; Frank, S.J.; Goldwein, J.; et al. The MRI-Linear Accelerator Consortium: Evidence-Based Clinical Introduction of an Innovation in Radiation Oncology Connecting Researchers, Methodology, Data Collection, Quality Assurance, and Technical Development. Front. Oncol. 2016, 6, 215. [Google Scholar] [CrossRef]
- Roberts, D.A.; Sandin, C.; Vesanen, P.T.; Lee, H.; Hanson, I.M.; Nill, S.; Perik, T.; Lim, S.B.; Vedam, S.; Yang, J.; et al. Machine QA for the Elekta Unity system: A Report from the Elekta MR-linac consortium. Med. Phys. 2021, 48, e67–e85. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Feng, L.; Delacoste, J.; Smith, D.; Weissbrot, J.; Flagg, E.; Moore, W.H.; Girvin, F.; Raad, R.; Bs, P.B.; Stoffel, D.; et al. Simultaneous Evaluation of Lung Anatomy and Ventilation Using 4D Respiratory-Motion-Resolved Ultrashort Echo Time Sparse MRI. J. Magn. Reson. Imaging 2018, 49, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Das, I.J.; Kase, K.R.; Tello, V.M. Dosimetric accuracy at low monitor unit settings. Br. J. Radiol. 1991, 64, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Barish, R.J.; Fleischman, R.C.; Pipman, Y.M. Teletherapy beam characteristics: The first second. Med. Phys. 1987, 14, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.W.; Rammohan, N.; Das, I.J.; Yadav, P. Towards Accurate and Precise Image-Guided Radiotherapy: Clinical Applications of the MR-Linac. J. Clin. Med. 2022, 11, 4044. [Google Scholar] [CrossRef] [PubMed]
- Huynh, E.; Boyle, S.; Campbell, J.; Penney, J.; Mak, R.H.; Schoenfeld, J.D.; Leeman, J.E.; Williams, C.L. Technical note: Toward implementation of MR-guided radiation therapy for laryngeal cancer with healthy volunteer imaging and a custom MR-CT larynx phantom. Med. Phys. 2022, 49, 1814–1821. [Google Scholar] [CrossRef]
- Lewis, B.C.; Gu, B.; Klett, R.; Lotey, R.; Green, O.L.; Kim, T. Characterization of radiotherapy component impact on MR imaging quality for an MRgRT system. J. Appl. Clin. Med. Phys. 2020, 21, 20–26. [Google Scholar] [CrossRef]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Roach, M.; Wan, L.; Portelance, L.; Mellon, E.A.; Bruynzeel, A.; Lagerwaard, F.; et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019, 8, 2123–2132. [Google Scholar] [CrossRef]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Hörner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Valentini, V.; Fuller, C.D.; Slotman, B.J. Editorial: Online Adaptive MR-Guided Radiotherapy. Front. Oncol. 2021, 11, 748685. [Google Scholar] [CrossRef]
- Hehakaya, C.; Vanneste, B.G.; Grutters, J.P.; Grobbee, D.E.; Verkooijen, H.M.; Frederix, G.W. Early health economic analysis of 1.5 T MRI-guided radiotherapy for localized prostate cancer: Decision analytic modelling. Radiother. Oncol. 2021, 161, 74–82. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Rigo, M.; Figlia, V.; Giaj-Levra, N.; Mazzola, R.; Nicosia, L.; Ricchetti, F.; Trapani, G.; De Simone, A.; Gurrera, D.; et al. 1.5T MR-Guided Daily Adaptive Stereotactic Body Radiotherapy for Prostate Re-Irradiation: A Preliminary Report of Toxicity and Clinical Outcomes. Front. Oncol. 2022, 12, 858740. [Google Scholar] [CrossRef] [PubMed]
- Weykamp, F.; Hoegen, P.; Klüter, S.; Spindeldreier, C.K.; König, L.; Seidensaal, K.; Regnery, S.; Liermann, J.; Rippke, C.; Koerber, S.A.; et al. Magnetic Resonance-Guided Stereotactic Body Radiotherapy of Liver Tumors: Initial Clinical Experience and Patient-Reported Outcomes. Front. Oncol. 2021, 11, 610637. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Cheung, P.; Louie, A.V.; Myrehaug, S.; Niglas, M.; Atenafu, E.G.; Chu, W.; Chung, H.T.; Poon, I.; Sahgal, A.; et al. Outcomes of extra-cranial stereotactic body radiotherapy for metastatic breast cancer: Treatment indication matters. Radiother. Oncol. 2021, 161, 159–165. [Google Scholar] [CrossRef]
- Chuong, M.D.; Herrera, R.; Kaiser, A.; Rubens, M.; Romaguera, T.; Alvarez, D.; Kotecha, R.; Hall, M.D.; McCulloch, J.; Ucar, A.; et al. Induction Chemotherapy and Ablative Stereotactic Magnetic Resonance Image-Guided Adaptive Radiation Therapy for Inoperable Pancreas Cancer. Front. Oncol. 2022, 12, 888462. [Google Scholar] [CrossRef]
- Pirzkall, A.; Carol, M.P.; Pickett, B.; Xia, P.; Roach, M.; Verhey, L.J. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 434–442. [Google Scholar] [CrossRef]
- Van Heijst, T.C.; Den Hartogh, M.D.; Lagendijk, J.J.; van den Bongard, H.D.; Van Asselen, B. MR-guided breast radiotherapy: Feasibility and magnetic-field impact on skin dose. Phys. Med. Biol. 2013, 58, 5917–5930. [Google Scholar] [CrossRef]
- Groot Koerkamp, M.L.; Vasmel, J.E.; Russell, N.S.; Shaitelman, S.F.; Anandadas, C.N.; Currey, A.; Vesprini, D.; Keller, B.M.; De-Colle, C.; Han, K.; et al. Optimizing MR-Guided Radiotherapy for Breast Cancer Patients. Front. Oncol. 2020, 10, 1107. [Google Scholar] [CrossRef]
- Nachbar, M.; Mönnich, D.; Boeke, S.; Gani, C.; Weidner, N.; Heinrich, V.; Russo, M.L.; Livi, L.; Winter, J.; Tsitsekidis, S.; et al. Partial breast irradiation with the 1.5 T MR-Linac: First patient treatment and analysis of electron return and stream effects. Radiother. Oncol. 2020, 145, 30–35. [Google Scholar] [CrossRef]
- Musunuru, H.B.; Yadav, P.; Olson, S.J.; Anderson, B.M. Improved Ipsilateral Breast and Chest Wall Sparing With MR-Guided 3-fraction Accelerated Partial Breast Irradiation: A Dosimetric Study Comparing MR-Linac and CT-Linac Plans. Adv. Radiat. Oncol. 2021, 6, 100654. [Google Scholar] [CrossRef]
- Bajaj, A.; Das, I.J. In Regard to Nichol et al. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1543. [Google Scholar] [CrossRef] [PubMed]
- Oborn, B.M.; Metcalfe, P.E.; Butson, M.J.; Rosenfeld, A.B. Monte Carlo characterization of skin doses in 6 MV transverse field MRI-linac systems: Effect of field size, surface orientation, magnetic field strength, and exit bolus. Med. Phys. 2010, 37, 5208–5217. [Google Scholar] [CrossRef] [PubMed]
- Shortall, J.; Vasquez Osorio, E.; Aitkenhead, A.; Berresford, J.; Agnew, J.; Budgell, G.; Chuter, R.; McWilliam, A.; Kirkby, K.; Mackay, R.; et al. Experimental verification the electron return effect around spherical air cavities for the MR-Linac using Monte Carlo calculation. Med. Phys. 2020, 47, 2506–2515. [Google Scholar] [CrossRef]
- Chandarana, H.; Wang, H.; Tijssen, R.H.N.; Das, I.J. Emerging Role of MRI in Radiation Therapy. J. Magn. Reson. Imaging 2018, 48, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Curcuru, A.N.; Lewis, B.C.; Kim, T.; Yang, D.; Michael Gach, H. Effects of B0 eddy currents on imaging isocenter shifts in 0.35-T MRI-guided radiotherapy (MR-IGRT) system. Med. Phys. 2021, 48, 2929–2938. [Google Scholar] [CrossRef]
- Das, I.J.; Sanfilippo, N.J.; Fogliata, A.; Luca Cozzi, L. Intensity Modulated Radiation Therapy: A Clinical Overview; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Fast, M.F.; Eiben, B.; Menten, M.J.; Wetscherek, A.; Hawkes, D.J.; McClelland, J.R.; Oelfke, U. Tumour auto-contouring on 2d cine MRI for locally advanced lung cancer: A comparative study. Radiother. Oncol. 2017, 125, 485–491. [Google Scholar] [CrossRef]
- Palm, R.F.; Eicher, K.G.; Sim, A.J.; Peneguy, S.; Rosenberg, S.A.; Wasserman, S.; Johnstone, P.A.S. Assessment of MRI-Linac Economics under the RO-APM. J. Clin. Med. 2021, 10, 4706. [Google Scholar] [CrossRef]
- Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 2008, 35, 310–317. [Google Scholar] [CrossRef]
- Das, I.J.; McGee, K.P.; Desobrey, G.E. The digitally reconstructed radiograph. In A Practical Guide to CT Simulation; Coia, L.R., Schultheiss, T.E., Hanks, G.E., Eds.; Advanced Medical Publishing: Madison, WI, USA, 1995; pp. 39–50. [Google Scholar]
- Das, I.J.; McGee, K.P.; Fein, D.A.; Milito, S.J.; Shammo, G.; Curran, W.J.; Coia, L.R. Use of multiplanar reformatted radiographic and digitally reconstructed radiographic images for planning conformal radiation therapy. RadioGraphics 1995, 15, 1483–1488. [Google Scholar] [CrossRef]
- Lee, S.L.; Mahler, P.; Olson, S.; Witt, J.S.; Musunuru, H.B.; Rajamanickam, V.; Bassetti, M.F.; Yadav, P. Reduction of cardiac dose using respiratory-gated MR-linac plans for gastro-esophageal junction cancer. Med. Dosim. 2021, 46, 152–156. [Google Scholar] [CrossRef]
- McGee, K.P.; Tyagi, N.; Bayouth, J.E.; Cao, M.; Fallone, B.G.; Glide-Hurst, C.K.; Goerner, F.L.; Green, O.L.; Kim, T.; Paulson, E.S.; et al. Findings of the AAPM Ad Hoc committee on magnetic resonance imaging in radiation therapy: Unmet needs, opportunities, and recommendations. Med. Phys. 2021, 48, 4523–4531. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, I.J.; Yadav, P.; Mittal, B.B. Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon. J. Clin. Med. 2022, 11, 5136. https://doi.org/10.3390/jcm11175136
Das IJ, Yadav P, Mittal BB. Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon. Journal of Clinical Medicine. 2022; 11(17):5136. https://doi.org/10.3390/jcm11175136
Chicago/Turabian StyleDas, Indra J., Poonam Yadav, and Bharat B. Mittal. 2022. "Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon" Journal of Clinical Medicine 11, no. 17: 5136. https://doi.org/10.3390/jcm11175136
APA StyleDas, I. J., Yadav, P., & Mittal, B. B. (2022). Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon. Journal of Clinical Medicine, 11(17), 5136. https://doi.org/10.3390/jcm11175136






