Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups and Clinical Definitions
- One hundred twenty-eight samples from controls who were health care workers. selected based on having no prior/present positive SARS-CoV-2 PCR and/or rapid antigen test, and/or having no detectable IgG or IgM plasma antibodies at the moment of inclusion. They were grouped according to SARS-CoV-2 vaccination status as follows: (a) unvaccinated individuals (n = 80), and (b) vaccinated individuals with Pfizer (n = 47) or Moderna (n = 1). Days between sampling and the first/second dose administration were recorded.
- Seventy-one samples from patients with asymptomatic (n = 6), mild (n = 3), moderate (n = 33), and severe (n = 29) COVID-19 disease enrolled during the acute phase of the disease. All patients had a reported positive SARS-CoV-2 PCR and/or rapid antigen test. Inside this group, 19 patients with moderate or severe COVID-19 were followed-up (having two or more consecutive samples, being the overall number of samples 48) during days 0, 2, 7, 28 or discharge after admission into semi-critical or ICU.
- Sixty samples from individuals recruited during the convalescence phase after mild (n = 22), moderate (n = 18), or severe disease (n = 20). All of them had a record of the number of days between sampling and COVID-19 diagnosis with a positive test.
2.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation and Cryopreservation
2.3. ELISPOT Assay for IFN-γ T-Cell Response Detection
2.4. ELISA for Humoral Response Detection
2.5. Statistical Analysis
3. Results
3.1. Positivity Rate between Patient Groups
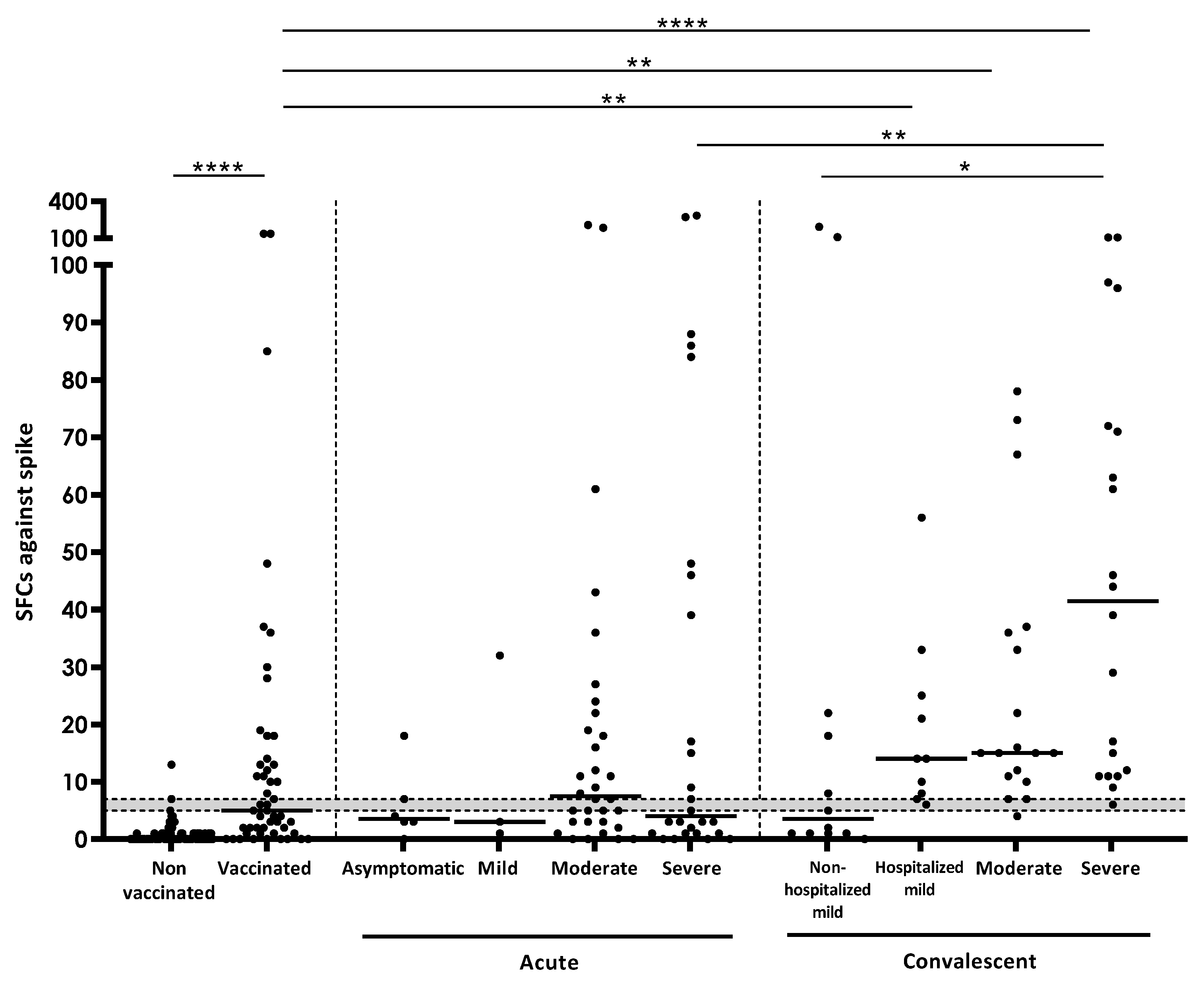
3.2. Quantitative IFN-γ Response against SARS-CoV-2 Antigens
3.3. IFN-γ Response According to Days after Vaccination and after COVID-19 Diagnosis
3.4. T-Cell IFN-γ Production and Humoral Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 12 July 2022).
- Comella-Del-Barrio, P.; De Souza-Galvão, M.L.; Prat-Aymerich, C.; Domínguez, J. Impact of COVID-19 on Tuberculosis Control. Arch. Bronconeumol. 2021, 57, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, Z.; Gonçalves-Carvalho, F.; Marín, A.; Capa, J.A.; Domínguez, J.; Latorre, I.; Lacoma, A.; Prat-Aymerich, C. Advances in diagnostic tools for respiratory tract infections. From tuberculosis to COVID19: Changing paradigms? ERJ Open Res. 2022. [Google Scholar] [CrossRef]
- Shariq, M.; Sheikh, J.A.; Quadir, N.; Sharma, N.; Hasnain, S.E.; Ehtesham, N.Z. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022, 31, 210264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. COVID-19: Joint Statement from ECDC and EMA on the Administration of a Fourth Dose of mRNA Vaccines. 2022. Available online: https://www.ecdc.europa.eu/en/news-events/ema-ecdc-statement-fourth-covid-vaccine-dose (accessed on 17 August 2022).
- Coronavirus Pandemic (COVID-19). Our World in Data. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 17 August 2022).
- Nielsen, S.S.; Vibholm, L.K.; Monrad, I.; Olesen, R.; Frattari, G.S.; Pahus, M.H.; Højen, J.F.; Gunst, J.D.; Erikstrup, C.; Holleufer, A.; et al. SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine 2021, 68, 103410. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, N.-C.; Chang, Y.-H.; Tian, X.-Y.; Na, D.-Y.; Zhang, L.-Y.; Zheng, L.; Lan, T.; Wang, L.-F.; Liang, G.-D. Duration of Antibody Responses after Severe Acute Respiratory Syndrome. Emerg. Infect. Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef]
- Jordan, S.C. Innate and adaptive immune responses to SARS-CoV-2 in humans: Relevance to acquired immunity and vaccine responses. Clin. Exp. Immunol. 2021, 204, 310–320. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell. Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- White Paper: T-Cell Testing in SARS-CoV-2. T-Spot Discovery. Available online: https://www.tspotdiscovery.com/wp-content/uploads/sites/4/2021/02/Oxford-Immunotec-White-Paper_P432-UK-WP-MPN475-0001-V3.pdf (accessed on 3 August 2022).
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lu, Z.; Zhang, L.; Fan, T.; Xiong, R.; Shen, X.; Feng, H.; Meng, H.; Lin, W.; Jiang, W.; et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020, 127, 104361. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Lin, C. T cell response in patients with COVID-19. Blood Sci. 2020, 2, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Schank, M.; Dang, X.; Lu, Z.; Cao, D.; Khanal, S.; Nguyen, L.N.; Nguyen, L.N.T.; Zhang, J.; et al. SARS-CoV-2 specific memory T cell epitopes identified in COVID-19-recovered subjects. Virus Res. 2021, 304, 198508. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Ye, B.; Zhao, M.; Zhan, J.; Dong, S.; Guo, Y.; Zhao, Y.; Li, M.; Liu, S.; et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin. Infect. Dis. 2021, ciab884. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Long, Q.X.; Jia, Y.J.; Wang, X.; Deng, H.-J.; Cao, X.-X.; Yuan, J.; Fang, L.; Cheng, X.-R.; Luo, C.; He, A.-R.; et al. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov. 2021, 7, 18. [Google Scholar] [CrossRef]
- Mak, W.A.; Koeleman, J.G.M.; Van der Vliet, M.; Keuren, F.; Ong, D.S.Y. SARS-CoV-2 antibody and T cell responses one year after COVID-19 and the booster effect of vaccination: A prospective cohort study. J. Infect. 2022, 84, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.A.; Lacy, J.; Stowe, J.; Seghezzo, G.; Sachdeva, R.; Simmons, R.; Bukasa, A.; O’Boyle, S.; Andrews, N.; Ramsay, M.; et al. Disease severity during SARS-COV-2 reinfection: A nationwide study. J. Infect. 2022, 84, 542–550. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; Van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Ministerio de Sanidad—Profesionales—Circulación de Variantes de SARS-CoV-2 de Interés para la Salud Pública en España. Evaluación Rápida de Riesgo. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/20211221-ERR.pdf (accessed on 3 August 2022).
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Cohen, D.; Muhsen, K.; Chodick, G.; Patalon, T. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: Reinfections versus breakthrough infections. medRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- Tan, A.T.; Lim, J.M.E.; Le Bert, N.; Kunasegaran, K.; Chia, A.; Qui, M.D.; Tan, N.; Ni Chia, W.; de Alwis, R.; Ying, D.; et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J. Clin. Investig. 2021, 131, e152379. [Google Scholar] [CrossRef]
- Almendro-Vázquez, P.; Laguna-Goya, R.; Ruiz-Ruigomez, M.; Utrero-Rico, A.; Lalueza, A.; de la Calle, G.M.; Delgado, P.; Perez-Ordoño, L.; Muro, E.; Vila, J.; et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021, 17, e1010211. [Google Scholar] [CrossRef]
- Thijsen, S.; Heron, M.; Gremmels, H.; van der Kieft, R.; Reusken, C.; Kremer, K.; Limonard, G.; Bossink, A. Elevated nucleoprotein-induced interferon-γ release in COVID-19 patients detected in a SARS-CoV-2 enzyme-linked immunosorbent spot assay. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Berger, M.M.; Brenner, T.; et al. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS-CoV-2 Proteins Is Not Associated with Recovery in Critical COVID-19 Patients. Cell Rep. Med. 2020, 1, 100092. [Google Scholar] [CrossRef]
- Jiang, X.-L.; Wang, G.-L.; Zhao, X.-N.; Yan, F.-H.; Yao, L.; Kou, Z.-Q.; Ji, S.-X.; Zhang, X.-L.; Li, C.-B.; Duan, L.-J.; et al. Lasting antibody and T cell responses to SARS-CoV-2 in COVID-19 patients three months after infection. Nat. Commun. 2021, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.Y.R.; Perlman, S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses—Are we our own worst enemy? Nat. Rev. Immunol. 2022, 22, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020. [Google Scholar] [CrossRef]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]


| Participants Variables | Controls (n = 128) | Acute (n = 42) | Convalescent (n = 60) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (n = 80) | Vaccinated (n = 48) | Asymptomatic (n = 6) | Mild (n = 3) | Moderate (n = 16) | Severe (n = 17) | Mild (n = 22) | Moderate (n = 18) | Severe (n = 20) | |||||||||
| Non-Hospitalized (n = 12) | Hospitalized (n = 10) | ||||||||||||||||
| Age (years ± SD) | 39 ± 13.3 | 42.9 ± 13.9 | 37.8 ± 18.1 | 25.3 ± 3.5 | 56.4 ± 16.1 | 65.8 ± 14.1 | 42.9 ± 12.7 | 63.8 ± 9.7 | 58.1 ± 14.6 | 56.9 ± 11.3 | |||||||
| Male N (%) | 24 (30) | 9 (18.8) | 4 (66.7) | 0 (0) | 13 (81.3) | 13 (76.5) | 3 (25) | 6 (60) | 10 (54.5) | 15 (75) | |||||||
| Pneumonia N (%) * | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (100) | 17 (100) | 0 (0) | 9 (90) | 18 (100) | 20 (100) | |||||||
| Unilobar | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 2 (20) | 2 (11.1) | 0 (0) | |||||||
| Multilobar | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 32 (93.7) | 17 (100) | 0 (0) | 7 (70) | 16 (88.9) | 20 (100) | |||||||
| ICU admission N (%) * | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (100) | 0 (0) | 0 (0) | 0 (0) | 20 (100) | |||||||
| Oxygen support N (%) * | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (100) | 17 (100) | 0 (0) | 5 (50) | 18 (100) | 20 (100) | |||||||
| Nasal prongs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (50) | 0 (0) | 0 (0) | |||||||
| Non-invasive mechanical vent. | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (100) | 0 (0) | 0 (0) | 0 (0) | 18 (100) | 0 (0) | |||||||
| Invasive mechanical vent. | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 17 (100) | 0 (0) | 0 (0) | 0 (0) | 20 (100) | |||||||
| Vaccinated with 1st dose N (%) | 0 (0) | 48 (100) | 2 (33.3) | 0 (0) | 1 (6.3) | 3 (17.6) | 6 (50) | 0 (0) | 0 (0) | 0 (0) | |||||||
| Vaccinated with 2nd dose N (%) | 0 (0) | 40 (83.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (33.3) | 0 (0) | 0 (0) | 0 (0) | |||||||
| Comorbidities N (%) | 2 (2.6) | 9 (18.8) | 0 (0) | 0 (0) | 14 (87.5) | 12 (70.6) | 3 (25) | 6 (60) | 12 (66.7) | 12 (60) | |||||||
| Respiratory disorders (asthma, OSAS, COPD) | 0 (0) | 2 (4.2) | 0 (0) | 0 (0) | 9 (56.3) | 1 (5.9) | 0 (0) | 4 (40) | 1 (5.9) | 0 (0) | |||||||
| Cardiovascular diseases (AHT, ictus, atrial fibrillation) | 1 (1.3) | 2 (4.2) | 0 (0) | 0 (0) | 7 (43.8) | 10 (58.8) | 1 (8.3) | 3 (30) | 8 (44.4) | 9 (45) | |||||||
| Autoimmune disorders (DM2, psoriasis, Jorgen, other) | 1 (1.3) | 4 (8.3) | 0 (0) | 0 (0) | 4 (25) | 6 (35.3) | 1 (8.3) | 1 (10) | 4 (22.2) | 5 (25) | |||||||
| Central nervous system disorders (dementia, epilepsy, Parkinson) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 2 (12.5) | 4 (23.5) | 0 (0) | 0 (0) | 1 (5.5) | 1 (5) | |||||||
| Malignant neoplasies | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | 3 (17.6) | 0 (0) | 1 (10) | 1 (5.5) | 2 (10) | |||||||
| Obesity | n/a | n/a | 0 (0) | 0 (0) | 3 (18.8) | 4 (23.5) | 1 (8.3) | 1 (10) | 6 (33.3) | 6 (30) | |||||||
| Immunosuppressive treatment N (%) | 2 (2.5) | 4 (6.3) | 0 (0) | 1 (16.7) | 4 (25) | 1 (5.9) | 1 (9) | 1 (10) | 3 (16.7) | 2 (10) | |||||||
| Oral (betamethasone, prednisone, NSAIDS) | 1 (1.3) | 1 (2.1) | 0 (0) | 1 (16.7) | 1 (6.3) | 1 (5.9) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | |||||||
| Inhaled | 1 (1.3) | 2 (4.2) | 0 (0) | 0 (0) | 3 (18.8) | 0 (0) | 0 (0) | 1 (10) | 3 (16.7) | 1 (5) | |||||||
| Topic | 0 (0) | 2 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | |||||||
| Deaths N (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (29.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||||
| Groups | Spike (n = 252) | Nucleocapsid (n = 249) | Membrane (n = 249) | Any SARS-CoV-2 Antigen (n = 252) |
|---|---|---|---|---|
| Overall (n = 259) | 118/252 (46.8) | 77/249 (30.9) | 73/249 (29.3) | 130/252 (51.6) |
| Controls (n = 128) | 27/125 (21.6) | 2/125 (1.6) | 3/125 (2.4) | 27/125 (21.6) |
| Vaccinated (n = 48) | 24/48 (50) | 1/48 (2.1) | 3/48 (6.3) | 24/48 (50) |
| Unvaccinated (n = 80) | 3/77 (3.9) | 1/77 (1.3) | 0/77 (0) | 3/77 (3.9) |
| Acute disease (n = 71) | 38/67 (56.7) | 31/64 (48.4) | 21/64 (32.8) | 48/67 (71.6) |
| Asymptomatic (n = 6) | 2/6 (33.3) | 2/6 (33.3) | 1/6 (16.7) | 3/6 (50) |
| Mild (n = 3) | 1/3 (33.3) | 1/3 (33.3) | 1/3 (33.3) | 1/3 (33.3) |
| Moderate (n = 33) | 22/32 (68.8) | 14/32 (43.8) | 8/32 (25) | 24/32 (75) |
| Severe (n = 29) | 13/26 (50) | 13/23 (56.5) | 10/23 (43.5) | 20/26 (76.9) |
| Convalescent (n = 60) | 53/60 (88.3) | 44/60 (73.3) | 49/60 (81.7) | 55/60 (91.7) |
| Mild * (n = 22) | 16/22 (72.7) | 13/22 (59.1) | 15/22 (69.6) | 17/22 (78.3) |
| Non-hospitalized (n = 12) | 6/12 (50) | 6/12 (50) | 5/12 (41.7) | 7/12 (58.3) |
| Hospitalized (n = 10) | 10/10 (100) | 7/10 (70) | 10/10 (100) | 10/10 (100) |
| Moderate * (n = 18) | 17/18 (94.5) | 13/18 (72.2) | 16/18 (88.9) | 18/18 (100) |
| Severe * (n = 20) | 20/20 (100) | 18/20 (90) | 18/20 (90) | 20/20 (100) |
| Groups | Antibody Response | T-Cell Response | ||
|---|---|---|---|---|
| IgG Spike (n = 155) | IgG NCP (n = 154) | Spike (n = 155) | NCP (n = 154) | |
| Overall (n = 159) | 86/155 (55.5) | 55/154 (35.7) | 63/155 (40.6) | 32/154 (20.8) |
| Controls (n = 94) | 33/92 (35.9) | 4/92 (4.3) | 17/92 (18.5) | 1/92 (1.1) |
| Vaccinated (n = 30) | 27/30 (90) | 0/30 (0) | 14/30 (46.7) | 0/30 (0) |
| Unvaccinated (n = 64) | 6/62 (9.7) | 4/62 (6.5) | 3/62 (4.8) | 1/62 (1.6) |
| Acute (n = 36) | 23/34 (67.6) | 23/33 (69.7) | 18/34 (52.9) | 12/33 (38) |
| Asymptomatic (n = 6) | 4/6 (66.7) | 3/6 (50) | 2/6 (33.3) | 2/6 (33.3) |
| Mild (n = 3) | 1/3 (33.3) | 1/3 (33.3) | 1/3 (33.3) | 1/3 (33.3) |
| Moderate (n = 12) | 8/12 (66.7) | 9/12 (75) | 9/12 (75) | 4/12 (33.3) |
| Severe (n = 15) | 10/13 (76.9) | 10/12 (83.3) | 6/13 (46.2) | 5/12 (41.7) |
| Convalescent (n = 29) | 29/29 (100) | 28/29 (95.6) | 28/29 (95.6) | 20/29 (69) |
| Mild (n = 8) | 8/8 (100) | 7/8 (87.5) | 8/8 (100) | 6/8 (75) |
| Moderate (n = 10) | 10/10 (100) | 10/10 (100) | 9/10 (90) | 5/10 (50) |
| Severe (n = 11) | 11/11 (100) | 11/11 (100) | 11/11 (100) | 9/11 (81.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safont, G.; Latorre, I.; Villar-Hernández, R.; Stojanovic, Z.; Marín, A.; Pérez-Cano, C.; Lacoma, A.; Molina-Moya, B.; Solis, A.J.; Arméstar, F.; et al. Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management. J. Clin. Med. 2022, 11, 5103. https://doi.org/10.3390/jcm11175103
Safont G, Latorre I, Villar-Hernández R, Stojanovic Z, Marín A, Pérez-Cano C, Lacoma A, Molina-Moya B, Solis AJ, Arméstar F, et al. Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management. Journal of Clinical Medicine. 2022; 11(17):5103. https://doi.org/10.3390/jcm11175103
Chicago/Turabian StyleSafont, Guillem, Irene Latorre, Raquel Villar-Hernández, Zoran Stojanovic, Alicia Marín, Cristina Pérez-Cano, Alicia Lacoma, Bárbara Molina-Moya, Alan Jhunior Solis, Fernando Arméstar, and et al. 2022. "Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management" Journal of Clinical Medicine 11, no. 17: 5103. https://doi.org/10.3390/jcm11175103
APA StyleSafont, G., Latorre, I., Villar-Hernández, R., Stojanovic, Z., Marín, A., Pérez-Cano, C., Lacoma, A., Molina-Moya, B., Solis, A. J., Arméstar, F., Matllo, J., Díaz-Fernández, S., Cendón, A., Sokalchuk, L., Tolosa, G., Casas, I., Rosell, A., & Domínguez, J. (2022). Measuring T-Cell Responses against SARS-CoV-2 Is of Utility for Disease and Vaccination Management. Journal of Clinical Medicine, 11(17), 5103. https://doi.org/10.3390/jcm11175103






