Correlation between the Outcome of Vitrectomy for Proliferative Diabetic Retinopathy and Erythrocyte Hematocrit Level and Platelet Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Technique
2.3. Clinical Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakabayashi, Y.; Usui, Y.; Tsubota, K.; Ueda, S.; Umazume, K.; Muramatsu, D.; Goto, H. Persistent overproduction of intraocular vascular endothelial growth factor as a cause of late vitreous hemorrhage after vitrectomy for proliferative diabetic retinopathy. Retina 2017, 37, 2317–2325. [Google Scholar]
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984, 102, 527–532. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984, 102, 520–526. [Google Scholar]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995, 102, 7–16. [Google Scholar]
- Gordeuk, V.R.; Key, N.S.; Prchal, J.T. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica 2019, 104, 653–658. [Google Scholar]
- Wu, W.C.; Schifftner, T.L.; Henderson, W.G.; Eaton, C.B.; Poses, R.M.; Uttley, G.; Sharma, S.C.; Vezeridis, M.; Khuri, S.F.; Friedmann, P.D. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007, 297, 2481–2488. [Google Scholar] [PubMed]
- Romero, P.; Baget, M.; Mendez, I.; Fernández, J.; Salvat, M.; Martinez, I. Diabetic macular edema and its relationship to renal microangiopathy: A sample of type I diabetes mellitus patients in a 15-year follow-up study. J. Diabetes Complicat. 2007, 21, 172–180. [Google Scholar]
- Ji, S.; Zhang, J.; Fan, X.; Wang, X.; Ning, X.; Zhang, B.; Shi, H.; Yan, H. The relationship between mean platelet volume and diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 25. [Google Scholar]
- Ji, S.; Ning, X.; Zhang, B.; Shi, H.; Liu, Z.; Zhang, J. Platelet distribution width, platelet count, and plateletcrit in diabetic retinopathy: A systematic review and meta-analysis of PRISMA guidelines. Medicine 2019, 98, e16510. [Google Scholar] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar]
- Saga, T.; Aoyama, T.; Takekoshi, T. Changes in the number and volume of platelets in male elderly persons, and effects of various factors on them. Nihon Ronen Igakkai Zasshi. Jpn. J. Geriatr. 1995, 32, 270–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sivri, N.; Tekin, G.; Yalta, K.; Aksoy, Y.; Senen, K.; Yetkin, E. Statins decrease mean platelet volume irrespective of cholesterol lowering effect. Kardiol. Pol. 2013, 71, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Dolasık, I.; Sener, S.Y.; Celebı, K.; Aydın, Z.M.; Korkmaz, U.; Canturk, Z. The effect of metformin on mean platelet volume in diabetıc patients. Platelets 2013, 24, 118–121. [Google Scholar]
- Spaide, R.F. Retinal vascular cystoid macular edema: Review and new theory. Retina 2016, 36, 1823–1842. [Google Scholar]
- Natali, A.; Toschi, E.; Baldeweg, S.; Casolaro, A.; Baldi, S.; Sironi, A.M.; Yudkin, J.S.; Ferrannini, E. Haematocrit, type 2 diabetes, and endothelium-dependent vasodilatation of resistance vessels. Eur. Heart J. 2005, 26, 464–471. [Google Scholar]
- Coglianese, E.E.; Qureshi, M.M.; Vasan, R.S.; Wang, T.J.; Moore, L.L. Usefulness of the blood hematocrit level to predict development of heart failure in a community. Am. J. Cardiol. 2012, 109, 241–245. [Google Scholar]
- Sonmez, A.; Yilmaz, M.I.; Saglam, M.; Kilic, S.; Eyileten, T.; Uckaya, G.; Caglar, K.; Oguz, Y.; Vural, A.; Yenicesu, M.; et al. The relationship between hemoglobin levels and endothelial functions in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2010, 5, 45–50. [Google Scholar]
- Lipowsky, H.H. Microvascular rheology and hemodynamics. Microcirculation 2005, 12, 5–15. [Google Scholar]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, e0199911. [Google Scholar]
- Shiga, T.; Maeda, N.; Kon, K. Erythrocyte rheology. Crit. Rev. Oncol./Hematol. 1990, 10, 9–48. [Google Scholar]
- Warny, M.; Helby, J.; Birgens, H.S.; Bojesen, S.E.; Nordestgaard, B.G. Arterial and venous thrombosis by high platelet count and high hematocrit: 108 521 individuals from the Copenhagen General Population Study. J. Thromb. Haemost. 2019, 17, 1898–1911. [Google Scholar] [PubMed]
- Dintenfass, L.; Julian, D.G.; Miller, G.E. Viscosity of blood in normal subjects and in patients suffering from coronary occlusion and arterial thrombosis. An in vitro study in the absence of anticoagulants, by means of a rotational cone-in-cone trolley viscometer. Am. Heart J. 1966, 71, 587–600. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F. Systemic effects of smoking. Chest 2007, 131, 1557–1566. [Google Scholar]
- Kramer, C.K.; de Azevedo, M.J.; da Costa Rodrigues, T.; Canani, L.H.; Esteves, J. Smoking habit is associated with diabetic macular edema in type 1 diabetes mellitus patients. J. Diabetes Complicat. 2008, 22, 430. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nicotine promotes blood-retinal barrier damage in a model of human diabetic macular edema. Toxicol. In Vitro 2017, 44, 182–189. [Google Scholar]
- Al-Rubeaan, K.; Abu El-Asrar, A.M.; Youssef, A.M.; Subhani, S.N.; Ahmad, N.A.; Al-Sharqawi, A.H.; Alguwaihes, A.; Alotaibi, M.S.; Al-Ghamdi, A.; Ibrahim, H.M. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: A Saudi National Diabetes Registry-based study. Acta Ophthalmol. 2015, 93, e140–e147. [Google Scholar]
- Esteves, J.F.; Kramer, C.K.; Azevedo, M.J.D.; Stolz, A.P.; Roggia, M.F.; Larangeira, A.; Miozzo, S.A.; Rosa, C.; Lambert, J.H.; Pecis, M.; et al. Prevalence of diabetic retinopathy in patients with type 1 diabetes mellitus. Rev. Assoc. Med. Bras. 2009, 55, 268–273. [Google Scholar]
- Acan, D.; Calan, M.; Er, D.; Arkan, T.; Kocak, N.; Bayraktar, F.; Kaynak, S. The prevalence and systemic risk factors of diabetic macular edema: A cross-sectional study from Turkey. BMC Ophthalmol. 2018, 18, 91. [Google Scholar]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in diabetic retinopathy. Rev. Diabet. Stud. RDS 2015, 12, 159–195. [Google Scholar] [CrossRef]
- Jingi, A.M.; Noubiap, J.J.N.; Essouma, M.; Bigna, J.J.R.; Nansseu, J.R.N.; Ellong, A.; Mvogo, C.E. Association of insulin treatment versus oral hypoglycaemic agents with diabetic retinopathy and its severity in type 2 diabetes patients in Cameroon, sub-Saharan Africa. Ann. Transl. Med. 2016, 4, 395. [Google Scholar]
- Kang, H.; Ma, X.; Liu, J.; Fan, Y.; Deng, X. High glucose-induced endothelial progenitor cell dysfunction. Diabetes Vasc. Dis. Res. 2017, 14, 381–394. [Google Scholar]
- Vehkavaara, S.; Yki-Jarvinen, H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Ling, L.H.; Cheung, C.M.G.; Ong, P.G.; Gong, L.; Tai, E.S.; Mathur, R.; Wong, D.; Foulds, W.; Wong, T.Y. Relationship of systemic endothelial function and peripheral arterial stiffness with diabetic retinopathy. Br. J. Ophthalmol. 2015, 99, 837–841. [Google Scholar] [PubMed]
- Busch, C.; Katzmann, J.L.; Jochmann, C.; Unterlauft, J.D.; Vollhardt, D.; Wiedemann, P.; Laufs, U.; Rehak, M. General health of patients with diabetic macular edema—The LIPSIA study. PLoS ONE 2021, 16, e0252321. [Google Scholar]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: The twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology 2009, 116, 497–503. [Google Scholar]
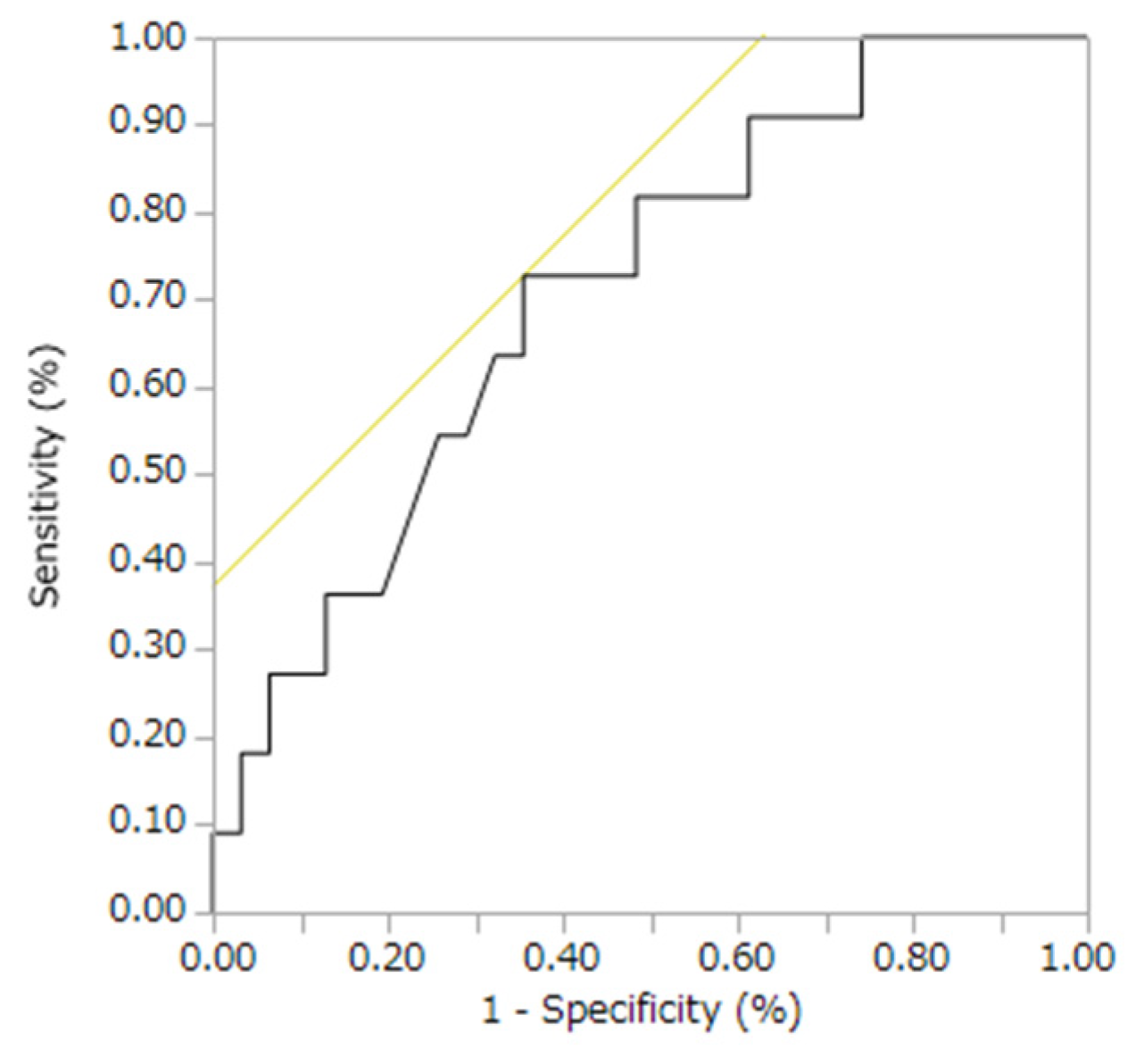
| Characteristic | Group T | Group N | p Value |
|---|---|---|---|
| No. of patients | 11 | 31 | |
| No. of eyes | 11 | 31 | |
| Sex (male/female) | 9/2 | 24/7 | 1.0 |
| Age (years; mean ± SD) | 52.0 ± 3.1 | 60.0 ± 11.6 | 0.05 |
| Hypertension, eyes (%) | 6 (54.6) | 26 (83.9) | 0.09 |
| Hyperlipidemia, eyes (%) | 4 (36.4) | 12 (38.7) | 1.0 |
| Oral hypoglycemic, eyes (%) | 6 (54.6) | 24 (77.4) | 0.24 |
| Oral metformin, eyes (%) | 4 (36.4) | 7 (22.6) | 0.44 |
| Insulin use, eyes (%) | 7 (63.6) | 8 (25.8) | 0.03 |
| Oral anticoagulant, eyes (%) | 1 (9.1) | 5 (16.1) | 1.0 |
| Oral antiplatelet, eyes (%) | 4 (36.4) | 7 (22.6) | 0.44 |
| Oral statin, eyes (%) | 5 (45.5) | 10 (32.3) | 0.48 |
| Oral diuretic, eyes (%) | 5 (45.5) | 16 (51.6) | 1.0 |
| Preoperative IOP (mmHg; mean ± SD) | 16.1 ± 3.1 | 13.6 ± 3.8 | 0.05 |
| Preoperative logMAR BCVA (mean ± SD) | 1.1 ± 0.7 | 1.1 ± 0.8 | 0.87 |
| Axial length (mm; mean ± SD) | 24.3 ± 1.5 | 24.1 ± 2.0 | 0.40 |
| Lens status; phakia/pseudophakia (eyes) | 8/3 | 22/9 | 1.0 |
| Surgical purpose, eyes | 11 | 31 | 0.41 |
| VH, eyes (%) | 6 (5) | 23 (74.2) | |
| RD, eyes (%) | 3 (27.3) | 3 (9.7) | |
| DME, eyes (%) | 1 (9.1) | 1 (3.2) | |
| Fibrovascular proliferation, eyes (%) | 1 (9.1) | 3 (9.7) | |
| Neovascularization elsewhere, eyes (%) | 0 (0) | 1 (3.2) | |
| Smoking, eyes (%) | 6 (54.6) | 20 (64.5) | 0.72 |
| Duration of smoking (years; mean ± SD) | 12.8 ± 9.6 | 14.6 ± 10.4 | 0.54 |
| BMI (kg/m2; mean ± SD) | 24.3 ± 2.9 | 27.0 ± 6.5 | 0.38 |
| Systolic blood pressure (mmHg; mean ± SD) | 131.6 ± 20.9 | 133.1 ± 17.8 | 0.82 |
| Diastolic blood pressure (mmHg; mean ± SD) | 69.4 ± 10.3 | 78.1 ± 10.9 | 0.03 |
| Pulse pressure (mmHg; ± SD) | 62.3 ± 20.0 | 55.0 ± 12.4 | 0.46 |
| Mean blood pressure (mmHg; mean ± SD) | 90.1 ± 10.5 | 96.4 ± 12.3 | 0.14 |
| Preoperative anti-VEGF vitreous injection, eyes (%) | 4 (36.4.2) | 6 (19.4) | 0.41 |
| Previous PRP, eyes (%) | 6 (54.6) | 21 (67.7) | 0.48 |
| Dialysis, eyes (%) | 3 (27.3) | 10 (65.7) | 1.0 |
| Preoperative DME, eyes (%) | 6 (54.6) | 7 (22.6) | 0.14 |
| Characteristic | Group T | Group N | p Value |
|---|---|---|---|
| HbA1c (%; mean ± SD) | 8.1 ± 2.2 | 6.8 ± 1.1 | 0.09 |
| Fasting blood glucose (mg/dL; mean ± SD) | 160.4 ± 89.3 | 136.4 ± 44.0 | 0.52 |
| PLT (103/µL; mean ± SD) | 235.9 ± 36.4 | 237.9 ± 65.4 | 0.92 |
| MPV (fl; mean ± SD) | 8.5 ± 1.1 | 8.2 ± 0.9 | 0.51 |
| PDW (fl; mean ± SD) | 16.9 ± 0.5 | 17.1 ± 0.5 | 0.43 |
| Hct (%; mean ± SD) | 42.0 ± 5.6 | 37.6 ± 5.6 | 0.04 |
| LDL (mg/dL; mean ± SD) | 134.8 ± 52.7 | 101.1 ± 42.3 | 0.06 |
| HDL (mg/dL; mean ± SD) | 54.0 ± 9.3 | 51.1 ± 18.4 | 0.26 |
| TG (mg/dL; mean ± SD) | 148.9± 111.0 | 150.7 ± 129.4 | 0.89 |
| eGFR (mL/min/1.73 m2; mean ± SD) | 42.8 ± 21.7 | 46.1 ± 57.7 | 0.68 |
| Hb (g/dL; mean ± SD) | 13.7 ± 2.0 | 12.6 ± 2.2 | 0.12 |
| Cr (mg/dL; mean ± SD) | 2.6 ± 3.2 | 3.0 ± 2.7 | 0.51 |
| BUN/Cr ratio (mean ± SD) | 14.5 ± 6.9 | 16.1 ± 8.7 | 0.71 |
| BUN (mg/dL; mean ± SD) | 26.7 ± 22.2 | 31.4 ± 16.1 | 0.06 |
| Characteristic | Group T | Group N | p Value |
|---|---|---|---|
| Cataract surgery, eyes (%) | 7 (63.6) | 18 (58.1) | 1.0 |
| ILM peeling, eyes (%) | 8 (72.7) | 22 (71.0) | 1.0 |
| Gas tamponade, eyes (%) | 2 (18.2) | 8 (25.8) | 0.84 |
| Air, eyes (%) | 1 (9.1) | 3 (9.7) | |
| SF6, eyes (%) | 1 (9.1) | 5 (16.1) | |
| C3F8, eyes (%) | 0 (0) | 0 (0) | |
| Postoperative logMAR BCVA (mean ± SD) | |||
| 1 month | 0.49 ± 0.43 | 0.51 ± 0.53 | 0.90 |
| 3 months | 0.38 ± 0.35 | 0.36 ± 0.36 | 0.80 |
| 6 months | 0.18 ± 0.18 | 0.31 ± 0.38 | 0.76 |
| 12 months | 0.02 ± 0.07 | 0.18 ± 0.29 | 0.43 |
| Postoperative IOP (mmHg; mean ± SD) | |||
| 1 month | 15.2 ± 4.7 | 14.9 ± 5.1 | 0.78 |
| 3 months | 14.3 ± 4.3 | 14.7 ± 4.9 | 0.81 |
| 6 months | 17.7 ± 5.3 | 13.8 ± 3.7 | 0.03 |
| 12 months | 16.4 ± 3.8 | 13.8 ± 3.0 | 0.11 |
| Follow-up period (months; mean ± SD) | 7.6 ± 4.3 | 9.3 ± 3.6 | 0.21 |
| Analysis (n = 42) | Factor | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Multivariate logistic regression | PLT (103/µL) | 1.00 (0.99–1.02) | 0.66 |
| MPV (fl) | 0.38 (0.13–1.16) | 0.09 | |
| PDW (fl) | 7.41 (0.80–68.6) | 0.08 | |
| Oral statin, eyes (%) | 1.20 (0.25–5.73) | 0.82 | |
| Oral metformin, eyes (%) | 2.34 (0.46–11.8) | 0.30 |
| Analysis (n = 42) | Factor | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Multivariate logistic regression | Hct (%) | 0.84 (0.72–0.98) | 0.02 |
| BUN/Cr ratio | 1.08 (0.97–1.21) | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Jujo, T.; Sekine, R.; Uchiyama, N.; Kakehashi, K.; Kogo, J. Correlation between the Outcome of Vitrectomy for Proliferative Diabetic Retinopathy and Erythrocyte Hematocrit Level and Platelet Function. J. Clin. Med. 2022, 11, 5055. https://doi.org/10.3390/jcm11175055
Sato K, Jujo T, Sekine R, Uchiyama N, Kakehashi K, Kogo J. Correlation between the Outcome of Vitrectomy for Proliferative Diabetic Retinopathy and Erythrocyte Hematocrit Level and Platelet Function. Journal of Clinical Medicine. 2022; 11(17):5055. https://doi.org/10.3390/jcm11175055
Chicago/Turabian StyleSato, Keiji, Tatsuya Jujo, Reio Sekine, Naoto Uchiyama, Kota Kakehashi, and Jiro Kogo. 2022. "Correlation between the Outcome of Vitrectomy for Proliferative Diabetic Retinopathy and Erythrocyte Hematocrit Level and Platelet Function" Journal of Clinical Medicine 11, no. 17: 5055. https://doi.org/10.3390/jcm11175055
APA StyleSato, K., Jujo, T., Sekine, R., Uchiyama, N., Kakehashi, K., & Kogo, J. (2022). Correlation between the Outcome of Vitrectomy for Proliferative Diabetic Retinopathy and Erythrocyte Hematocrit Level and Platelet Function. Journal of Clinical Medicine, 11(17), 5055. https://doi.org/10.3390/jcm11175055






