Silent Hypoxemia in the Emergency Department: A Retrospective Cohort of Two Clinical Phenotypes in Critical COVID-19
Abstract
:1. Introduction
2. Methods
2.1. Study Settings
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
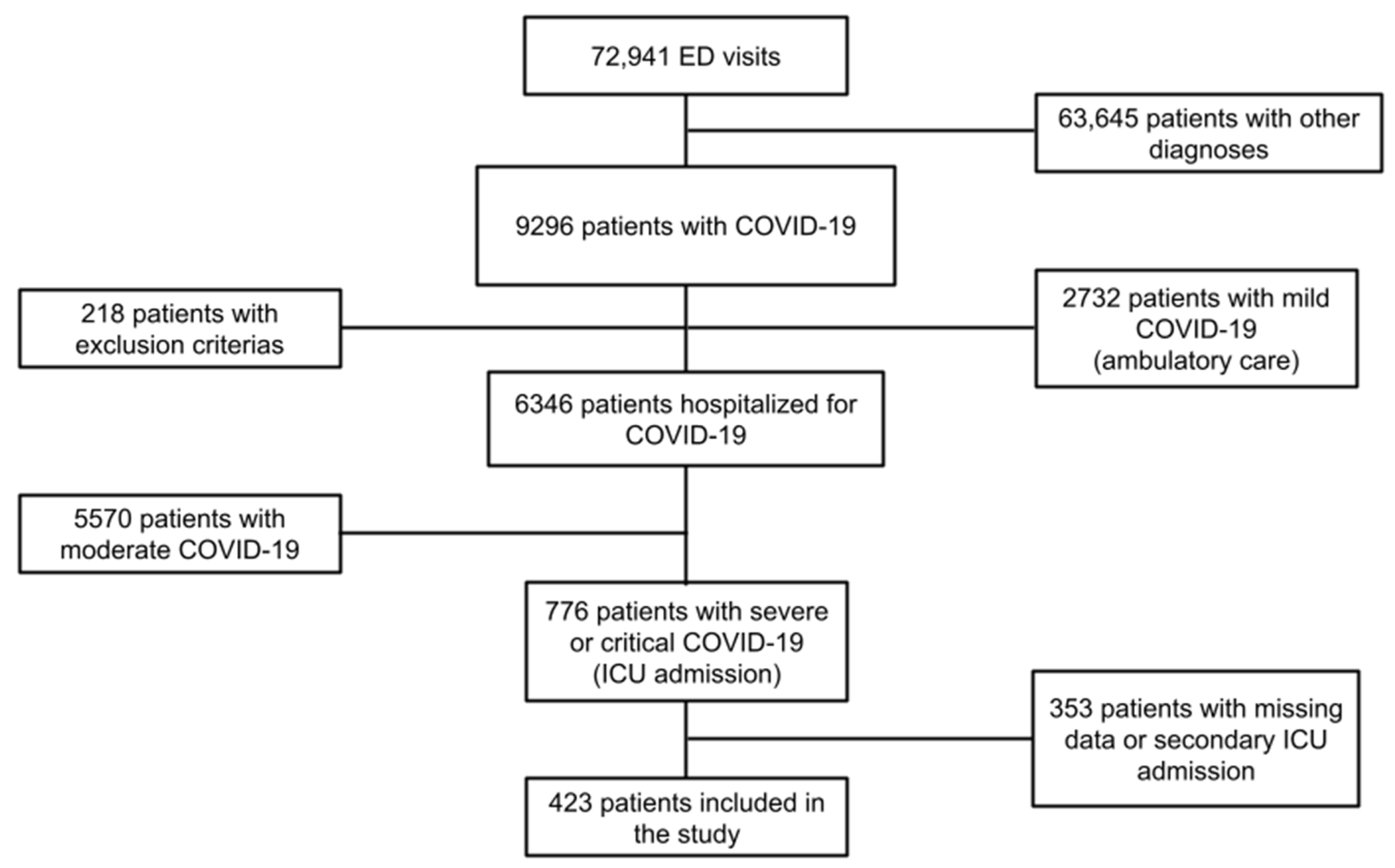
3.1. Study Population
3.2. Demographics, Clinical Characteristics and Management
3.3. Survival Status
3.4. Phenotype I versus Phenotype II: Characteristics and Management
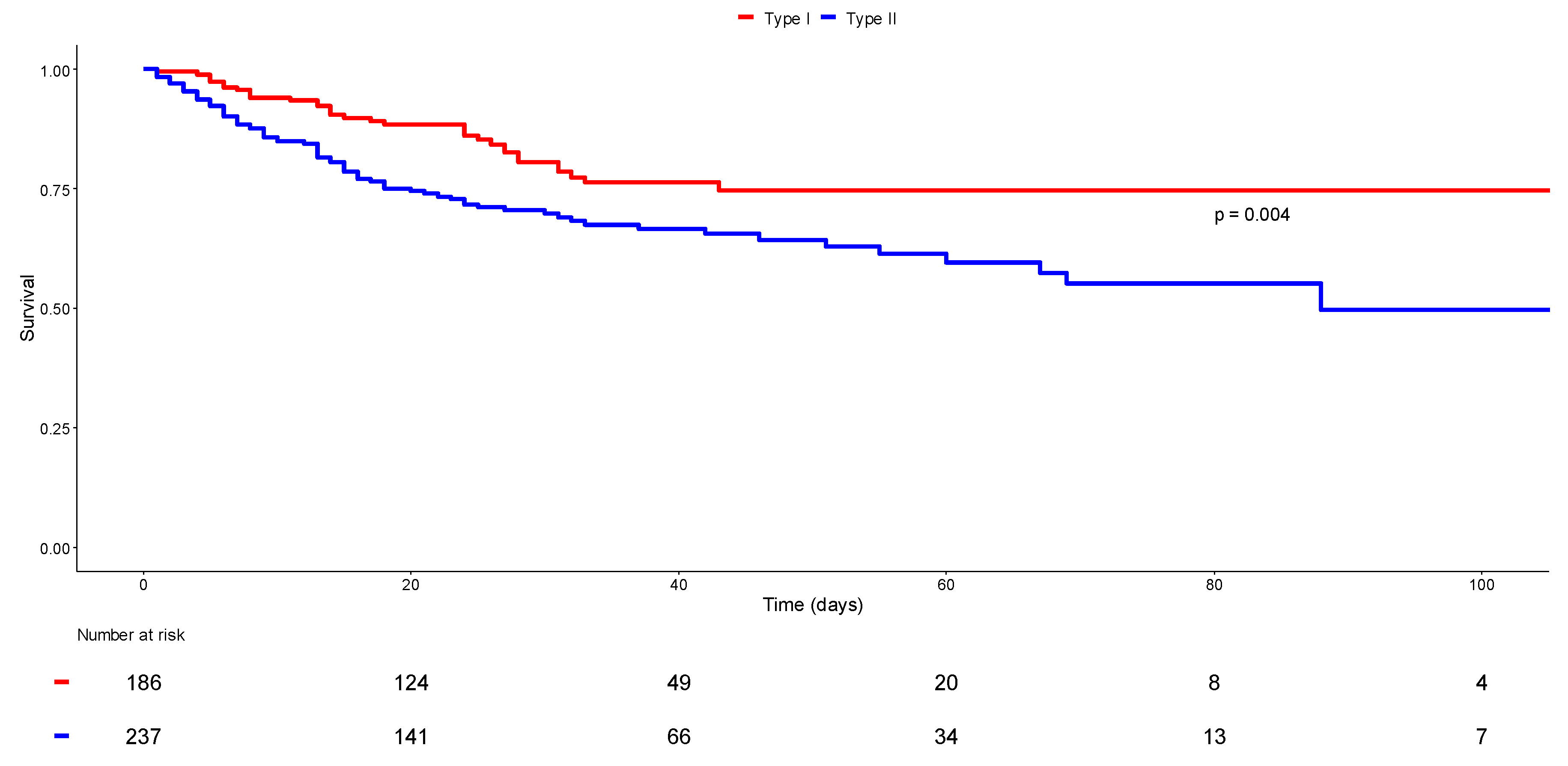
3.5. Phenotype I versus Phenotype II: Survival Status
3.6. Phenotype I versus Phenotype II: Multivariate Analysis
4. Discussion
4.1. Two Clinical Phenotypes in Critical COVID-19
4.2. In the Times of Pandemic Crisis
4.3. Beyond Silent Hypoxemia
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 16 August 2022).
- Cousin, V.L.; Giraud, R.; Bendjelid, K. Pathophysiology of COVID-19: Everywhere You Look You Will See ACE2! Front. Med. 2021, 8, 694029. [Google Scholar]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [PubMed]
- Hsu, R.J.; Yu, W.C.; Peng, G.R.; Ye, C.H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.-C.; Yu, S.-H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar]
- Milross, L.; Majo, J.; Cooper, N.; Kaye, P.M.; Bayraktar, O.; Filby, A.; Fisher, A.J. Post-mortem lung tissue: The fossil record of the pathophysiology and immunopathology of severe COVID-19. Lancet Respir. Med. 2022, 10, 95–106. [Google Scholar]
- Camporota, L.; Cronin, J.N.; Busana, M.; Gattinoni, L.; Formenti, F. Pathophysiology of coronavirus-19 disease acute lung injury. Curr. Opin. Crit. Care 2022, 28, 9. [Google Scholar]
- Peltan, I.D.; Caldwell, E.; Admon, A.J.; Attia, E.F.; Gundel, S.J.; Mathews, K.S.; Nagrebetsky, A.; Sahetya, S.K.; Ulysse, C.; Brown, S.M.; et al. Characteristics and Outcomes of US Patients Hospitalized With COVID-19. Am. J. Crit. Care 2022, 31, 146. [Google Scholar]
- Azoulay, E.; Zafrani, L.; Mirouse, A.; Lengliné, E.; Darmon, M.; Chevret, S. Clinical phenotypes of critically ill COVID-19 patients. Intensive Care Med. 2020, 46, 1651–1652. [Google Scholar]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar]
- Simonson, T.S.; Baker, T.L.; Banzett, R.B.; Bishop, T.; Dempsey, J.A.; Feldman, J.L.; Guyenet, P.G.; Hodson, E.J.; Mitchell, G.S.; Moya, E.A.; et al. Silent hypoxaemia in COVID-19 patients. J. Physiol. 2021, 599, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Ranieri, V.M.; Slutsky, A.S. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021, 47, 83–85. [Google Scholar] [CrossRef]
- Tavčar, P.; Potokar, M.; Kolenc, M.; Korva, M.; Avšič-Županc, T.; Zorec, R.; Jorgačevski, J. Neurotropic Viruses, Astrocytes, and COVID-19. Front. Cell. Neurosci. 2021, 15, 662578. [Google Scholar] [CrossRef]
- Ottestad, W.; Søvik, S. COVID-19 patients with respiratory failure: What can we learn from aviation medicine? Br. J. Anaesth. 2020, 125, e280–e281. [Google Scholar] [CrossRef] [PubMed]
- Kallet, R.; Branson, R.D.; Lipnick, M.S. Respiratory Drive, Dyspnea and Silent Hypoxemia: A Physiological Review in the Context of Coronavirus Disease 2019. Respir. Care 2022, 67, 10075. [Google Scholar] [CrossRef]
- Busana, M.; Gasperetti, A.; Giosa, L.; Forleo, G.B.; Schiavone, M.; Mitacchione, G.; Bonino, C.; Villa, P.; Galli, M.; Tondo, C.; et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021, 87, 9. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Clinical Spectrum. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 16 August 2022).
- Le Gall, J.-R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Pan, C.; Chen, L.; Lu, C.; Zhang, W.; Xia, J.A.; Sklar, M.C.; Du, B.; Brochard, L.; Qiu, H. Lung Recruitability in COVID-19-associated Acute Respiratory Distress Syndrome: A Single-Center Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1294–1297. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- Riviello, E.D.; Dechen, T.; O’Donoghue, A.L.; Cocchi, M.N.; Hayes, M.M.; Molina, R.L.; Moraco, N.H.; Mosenthal, A.; Rosenblatt, M.; Talmor, N.; et al. Assessment of a Crisis Standards of Care Scoring System for Resource Prioritization and Estimated Excess Mortality by Race, Ethnicity, and Socially Vulnerable Area During a Regional Surge in COVID-19. JAMA Netw. Open 2022, 5, e221744. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.A.; Sacha, G.L.; Welch, S.C.; Bass, S.N.; Kane-Gill, S.L.; Duggal, A.; Ammar, A.A. Sedation, Analgesia, and Paralysis in COVID-19 Patients in the Setting of Drug Shortages. J. Intensive Care Med. 2021, 36, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Y.; Dai, B.; Tan, W.; Zhao, H.W.; Wang, W.; Kang, J. Effectiveness of the use of a high-flow nasal cannula to treat COVID-19 patients and risk factors for failure: A meta-analysis. Ther. Adv. Respir. Dis. 2022, 16, 17534666221091932. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; de Gonzalo-Calvo, D.; Torres, G.; de Batlle, J.; Gómez, S.; Moncusi-Moix, A.; Carmona, P.; Santisteve, S.; Monge, A.; et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: A prospective cohort study. Crit Care 2022, 26, 18. [Google Scholar] [CrossRef]
- Le Terrier, C.; Suh, N.; Wozniak, H.; Boroli, F.; Giudicelli-Bailly, A.; Sangla, F.; Legouis, D.; Bendjelid, K.; Quintard, H.; Pugin, J. Delayed intubation is associated with mortality in patients with severe COVID-19: A single-centre observational study in Switzerland. Anaesth. Crit. Care Pain Med. 2022, 41, 101092. [Google Scholar] [CrossRef]
- Bravata, D.M.; Perkins, A.J.; Myers, L.J.; Arling, G.; Zhang, Y.; Zillich, A.J.; Reese, L.; Dysangco, A.; Agarwal, R.; Myers, J.; et al. Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw. Open. 2021, 4, e2034266. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Busana, M.; Giosa, L.; Cressoni, M.; Gasperetti, A.; Di Girolamo, L.; Martinelli, A.; Sonzogni, A.; Lorini, L.; Palumbo, M.M.; Romitti, F.; et al. The impact of ventilation–perfusion inequality in COVID-19: A computational model. J. Appl. Physiol. 2021, 130, 865–876. [Google Scholar] [CrossRef]
- Panwar, R.; Madotto, F.; Laffey, J.G.; van Haren, F.M.P. Compliance Phenotypes in Early Acute Respiratory Distress Syndrome before the COVID-19 Pandemic. Am. J. Respir. Crit. Care Med. 2020, 202, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
| Demographics | All Patients n = 423 | Survivors n = 311 | Non-Survivors n = 112 | p-Value |
|---|---|---|---|---|
| Age (years) | 66 [58–72] | 64.0 [56–71] | 69 [64–74.3] | <0.001 * |
| Male | 311 (73.5) | 222 (71.4) | 89 (79.5) | 0.096 |
| Comorbidities | ||||
| Hypertension | 235 (55.6) | 164 (52.7) | 71 (63.4) | 0.052 |
| BMI > 30 (kg/m2) | 174 (42.1) | 131 (43.0) | 43 (39.8) | 0.571 |
| Cardiovascular diseases | 135 (31.9) | 89 (28.6) | 46 (41.1) | 0.015 * |
| Diabetes mellitus | 118 (27.9) | 82 (26.4) | 36 (32.1) | 0.243 |
| Pre-existing renal failure | 69 (16.5) | 45 (14.6) | 24 (22.0) | 0.071 |
| Malignancies or ID | 52 (12.3) | 35 (11.3) | 17 (15.2) | 0.278 |
| Respiratory diseases | 95 (22.5) | 69 (22.2) | 26 (23.2) | 0.823 |
| ED management | ||||
| Respiratory rate (/min) | 30 [24–35] | 29 [24–35] | 30 [24.5–35.5] | 0.218 |
| First O2 saturation (%) | 90 [84–94] | 91 [85–95] | 88 [82–93] | 0.005 * |
| O2 requirement (L/min) | 15 [6–15] | 15 [6–15] | 15 [9–15] | 0.085 |
| Heart rate (/min) | 93 [82–107] | 93 [83–106.3] | 94 [79–107] | 0.845 |
| Systolic BP (mmHg) | 130 [115–142] | 130 [115.8–142] | 129 [113–145] | 0.364 |
| Glasgow score scale | 15 [15–15] | 15 [15–15] | 15 [15–15] | 0.213 |
| Temperature (°C) | 37.8 [37–38.6] | 37.8 [37–38.6] | 37.9 [36.9–38.5] | 0.719 |
| Duration since onset sympt (days) | 7 [5–10] | 7 [5–10] | 7.0 [4–8] | 0.007 * |
| Intubation in the ED | 159 (37.6) | 103 (33.1) | 56 (50.0) | 0.002 * |
| Phenotype I | 186 (44.0) | 151 (48.6) | 35 (31.3) | 0.002 * |
| Phenotype II | 237 (56.0) | 160 (51.4) | 77 (68.7) | 0.002 * |
| Laboratory findings | ||||
| Creatinine (μmol/L) | 84 [67–105] | 80 [66–101.9] | 93.0 [72–119] | 0.001 * |
| Lymphocytes (/μL) | 780 [580–1110] | 790 [600–1127.5] | 725 [500–1063] | 0.144 |
| CRP (mg/L) | 148.2 [83–223] | 147.2 [85.4–222.3] | 153 [79–223] | 0.766 |
| pH | 7.46 [7.42–7.49] | 7.46 [7.42–7.49] | 7.46 [7.41–7.49] | 0.456 |
| PaO2 (mmHg) | 67 [55–81] | 68 [58–82] | 63 [53–78] | 0.037 * |
| PaCO2 (mmHg) | 34 [30–38] | 34 [30–38] | 33 [28–37] | 0.116 |
| HCO3- (mmol/L) | 23.5 [21.4–25.9] | 23.8 [22.0–26.0] | 23.0 [19.8–25.0] | 0.009 |
| Lactate (mmol/L) | 1.4 [1.1–2] | 1.4 [1–1.9] | 1.6 [1.2–2.4] | 0.002 * |
| Radiological findings | ||||
| Typical CT-scan | 223 (53.5) | 170 (55.6) | 53 (47.8) | 0.158 |
| Extension > 50% | 140 (45.9) | 107 (46.1) | 33 (45.2) | 0.891 |
| ICU management | ||||
| SAPS II | 42 [32–54] | 40 [30.5–51] | 47 [39–58] | <0.001 * |
| ARDS | 372 (91.0) | 268 (89.0) | 104 (96.3) | 0.024 * |
| 200 < PaO2/FiO2 ≤ 300 | 35 (8.6) | 31 (10.3) | 4 (3.7) | 1.000 |
| 100 < PaO2/FiO2 ≤ 200 | 180 (44.0) | 148 (49.2) | 32 (29.6) | 0.466 |
| PaO2/FiO2 ≤ 100 | 157 (38.4) | 89 (29.6) | 68 (63.0) | <0.001 * |
| Mechanical ventilation (days) | 14 [7–24] | 15 [8–25] | 12 [5–19.5] | 0.014 * |
| Prone position | 263 (62.5) | 183 (59.2) | 80 (71.4) | 0.022 * |
| Continuous muscle blockers | 295 (77.4) | 210 (75.3) | 85 (83.3) | 0.095 |
| Tracheotomy | 76 (23.3) | 64 (27.1) | 12 (13.3) | 0.008 * |
| Catecholamines | 326 (78.2) | 230 (75.2) | 96 (86.5) | 0.013 * |
| ECMO | 16 (3.8) | 11 (3.5) | 5 (4.5) | 0.847 |
| Renal replacement therapy | 59 (14.0) | 31 (10.0) | 28 (25.0) | <0.001* |
| Thromboembolic events | 68 (16.2) | 46 (14.8) | 22 (19.8) | 0.221 |
| Outcome | ||||
| ICU LOS (days) | 17 [8–30] | 19 [10–31] | 13 [6–24] | <0.001 * |
| In-hospital LOS (days) | 26 [13–43] | 30.0 [19–48] | 13.5 [6–24.3] | <0.001 * |
| Demographics | Phenotype I n = 186 | Phenotype II n = 237 | p Value |
|---|---|---|---|
| Age (years) | 65 [57–71.8] | 66 [59–72] | 0.375 |
| Male | 134 (72.0) | 177 (74.7) | 0.541 |
| Comorbidities n (%) | |||
| Hypertension | 95 (51.1) | 140 (59.1) | 0.100 |
| BMI > 30 (kg/m2) | 71 (38.6) | 103 (45.0) | 0.191 |
| Cardiovascular diseases | 49 (26.3) | 86 (36.3) | 0.029 * |
| Diabetes mellitus | 47 (25.3) | 71 (30.0) | 0.286 |
| Pre-existing renal failure | 28 (15.1) | 41 (17.6) | 0.501 |
| Malignancies or ID | 16 (8.6) | 36 (15.2) | 0.041 * |
| Respiratory diseases | 42 (22.6) | 53 (22.4) | 0.958 |
| ED management | |||
| Respiratory rate (/min) | 26 [21–30] | 30.0 [26–37] | <0.001 * |
| First O2 saturation (%) | 91.2 [87–95] | 89 [82–93] | <0.001 * |
| O2 requirement (L/min) | 12 [4–15] | 15 [9–15] | <0.001 * |
| Heart rate (/min) | 93 [83–107] | 94.5 [81–106.8] | 0.788 |
| Systolic BP (mmHg) | 129 [116–140] | 130 [114–144] | 0.993 |
| Glasgow score scale | 15 [15–15] | 15 [15–15] | 0.448 |
| Temperature (°C) | 37.9 [37.1–38.6] | 37.8 [37–38.6] | 0.612 |
| Shock | 7 (3.8) | 30 (12.7) | 0.001 * |
| Confusion | 6 (3.2) | 3 (1.3) | 0.296 |
| Duration since onset symptom (days) | 7 [5–10] | 7 [4–10] | 0.822 |
| Intubation in the ED | 50 (26.9) | 109 (46.0) | <0.001 * |
| Laboratory findings | |||
| Creatinine (μmol/L) | 84 [67–105] | 84 [67–105] | 0.966 |
| CRP (mg/L) | 133.8 [78.7–224.8] | 154.4 [92.7–221.5] | 0.393 |
| Lymphocytes (/μL) | 851.4 ± 434.8 | 1029.5 ± 840.0 | 0.006 * |
| pH | 7.47 [7.44–7.50] | 7.46 [7.41–7.49] | <0.001 * |
| PaO2 (mmHg) | 67 [56–81] | 67 [55–80] | 0.648 |
| PaCO2 (mmHg) | 34 [30–37] | 34 [30–39] | 0.481 |
| HCO3- (mmol/L) | 24 [22–26] | 23 [20.9–25.4] | 0.013 * |
| Lactate (mmol/L) | 1.3 [1.0–1.9] | 1.5 [1.1–2.1] | 0.071 |
| Radiological findings | |||
| Typical CT-scan | 101 (54.9) | 122 (52.4) | 0.607 |
| Extension >50% | 45 (34.6) | 95 (54.3) | <0.001 * |
| ICU management | |||
| SAPS II | 41 [32–53] | 42 [32–55] | 0.422 |
| Intubation in the ICU | 105 (56.5) | 87 (36.9) | <0.001 * |
| ARDS | 161 (90.5) | 211 (91.3) | 0.755 |
| 200 < PaO2/FiO2 ≤ 300 | 17 (9.6) | 18 (7.8) | 1.000 |
| 100 < PaO2/FiO2 ≤ 200 | 72 (40.5) | 108 (46.8) | 0.583 |
| PaO2/FiO2 ≤ 100 | 72 (40.5) | 85 (36.8) | 1.000 |
| Mechanical ventilation (days) | 14 [8–22] | 13 [6–25] | 0.750 |
| Prone position | 118 (63.8) | 145 (61.4) | 0.622 |
| Continuous muscle blockers | 117 (74.5) | 178 (79.5) | 0.256 |
| Tracheotomy | 29 (20.3) | 47 (25.7) | 0.252 |
| Catecholamines | 144 (78.3) | 182 (78.1) | 0.971 |
| Renal replacement therapy | 27 (14.5) | 32 (13.5) | 0.765 |
| ECMO | 5 (2.7) | 11 (4.6) | 0.296 |
| Thromboembolic events | 27 (14.5) | 41 (17.5) | 0.417 |
| Bacterial coinfection | 114 (61.3) | 125 (52.7) | 0.078 |
| Outcome | |||
| ICU LOS (days) | 17.5 [9.0–29.8] | 17.0 [7.0–30.0] | 0.829 |
| In-hospital LOS (days) | 26.5 [16.0–42.5] | 25.0 [12.0–43.0] | 0.267 |
| ICU mortality | 35 (18.8) | 69 (29.2) | 0.014 * |
| In-hospital mortality | 35 (18.8) | 77 (32.5) | 0.002 * |
| Demographics | aOR | 95%CI | p Value |
|---|---|---|---|
| Age (years) | 1.001 | [0.966–1.036] | 0.975 |
| Male | 0.619 | [0.251–1.528] | 0.298 |
| Comorbidities | |||
| Hypertension | 1.876 | [0.776–4.537] | 0.162 |
| Cardiovascular diseases | 2.302 | [0.856–6.189] | 0.098 |
| Diabetes | 0.400 | [0.148–1.083] | 0.071 |
| Pre-existing renal failure | 0.670 | [0.298–1.504] | 0.332 |
| Malignancies or ID | 0.979 | [0.322–2.977] | 0.970 |
| Respiratory diseases | 0.955 | [0.353–2.585] | 0.928 |
| ED management | |||
| Respiratory rate (/min) | 1.102 | [1.043–1.166] | 0.001 * |
| Glasgow score scale | 1.531 | [0.918–2.555] | 0.103 |
| First O2 saturation (%) | 0.963 | [0.913–1.017] | 0.175 |
| O2 requirement (L/min) | 0.979 | [0.900–1.066] | 0.632 |
| Heart rate (/min) | 1.007 | [0.981–1.035] | 0.591 |
| Systolic BP (mmHg) | 1.006 | [0.987–1.024] | 0.549 |
| Temperature (°C) | 1.521 | [1.011–2.288] | 0.044 * |
| Shock | 4.030 | [0.750–21.650] | 0.104 |
| Confusion | 0.128 | [0.008–2.018] | 0.144 |
| Duration since onset symptom (days) | 0.929 | [0.830–1.040] | 0.199 |
| Intubation in the ED | 3.844 | [1.199–12.319] | 0.023 * |
| Laboratory findings | |||
| Creatinine (>100 μmol/L) | 0.325 | [0.105–1.008] | 0.052 |
| CRP (>100 mg/L) | 0.792 | [0.323–1.939] | 0.609 |
| Lymphocytes (1000/μL) | 1.845 | [0.799–4.261] | 0.151 |
| Lactate (mmol/L) | 0.663 | [0.384–1.146] | 0.141 |
| pH | 0.171 | [0.000–86.023] | 0.578 |
| pO2 (mmHg) | 1.016 | [1.002–1.031] | 0.031 * |
| pCO2 (mmHg) | 1.027 | [0.950–1.110] | 0.501 |
| HCO3- (mmol/L) | 0.857 | [0.738–0.995] | 0.043 * |
| Radiological findings | |||
| Typical CT-scan | 0.331 | [0.128–0.857] | 0.023 * |
| Extension > 50% | 3.017 | [1.270–7.166] | 0.012 * |
| ICU management | |||
| SAPS II | 0.990 | [0.961–1.019] | 0.492 |
| ARDS | |||
| 200 < PaO2/FiO2 ≤ 300 | 1.748 | [0.354–8.624] | 0.493 |
| 100 < PaO2/FiO2 ≤ 200 | 1.556 | [0.457–5.303] | 0.479 |
| PaO2/FiO2 ≤ 100 | 0.518 | [0.123–2.172] | 0.368 |
| Mechanical ventilation (days) | 1.027 | [0.976–1.081] | 0.306 |
| Prone position | 0.527 | [0.211–1.312] | 0.169 |
| Renal replacement therapy | 1.835 | [0.462–7.289] | 0.388 |
| ECMO | 3.852 | [0.516–28.747] | 0.188 |
| Thromboembolic events | 0.940 | [0.321–2.756] | 0.911 |
| Outcome | |||
| ICU LOS (days) | 0.978 | [0.928–1.031] | 0.405 |
| In-hospital LOS (days) | 1.019 | [0.981–1.057] | 0.330 |
| ICU mortality (days) | 2.385 | [0.715–7.950] | 0.157 |
| In-hospital mortality (days) | 3.079 | [0.932–10.171] | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamé, K.; Lemaitre, E.L.; Abensur Vuillaume, L.; Noizet, M.; Gottwalles, Y.; Chouihed, T.; Lavoignet, C.-E.; Bérard, L.; Molter, L.; Gennai, S.; et al. Silent Hypoxemia in the Emergency Department: A Retrospective Cohort of Two Clinical Phenotypes in Critical COVID-19. J. Clin. Med. 2022, 11, 5034. https://doi.org/10.3390/jcm11175034
Alamé K, Lemaitre EL, Abensur Vuillaume L, Noizet M, Gottwalles Y, Chouihed T, Lavoignet C-E, Bérard L, Molter L, Gennai S, et al. Silent Hypoxemia in the Emergency Department: A Retrospective Cohort of Two Clinical Phenotypes in Critical COVID-19. Journal of Clinical Medicine. 2022; 11(17):5034. https://doi.org/10.3390/jcm11175034
Chicago/Turabian StyleAlamé, Karine, Elena Laura Lemaitre, Laure Abensur Vuillaume, Marc Noizet, Yannick Gottwalles, Tahar Chouihed, Charles-Eric Lavoignet, Lise Bérard, Lise Molter, Stéphane Gennai, and et al. 2022. "Silent Hypoxemia in the Emergency Department: A Retrospective Cohort of Two Clinical Phenotypes in Critical COVID-19" Journal of Clinical Medicine 11, no. 17: 5034. https://doi.org/10.3390/jcm11175034
APA StyleAlamé, K., Lemaitre, E. L., Abensur Vuillaume, L., Noizet, M., Gottwalles, Y., Chouihed, T., Lavoignet, C.-E., Bérard, L., Molter, L., Gennai, S., Ugé, S., Lefebvre, F., Bilbault, P., & Le Borgne, P., on behalf of the CREMS Network (Clinical Research in Emergency Medicine and Sepsis) . (2022). Silent Hypoxemia in the Emergency Department: A Retrospective Cohort of Two Clinical Phenotypes in Critical COVID-19. Journal of Clinical Medicine, 11(17), 5034. https://doi.org/10.3390/jcm11175034





