Pancreatic Adeno-MiNEN, a Rare Newly Defined Entity with Challenging Diagnosis and Treatment: A Case Report with Systematic Literature Review and Pooled Analysis
Abstract
1. Introduction
2. Case Report
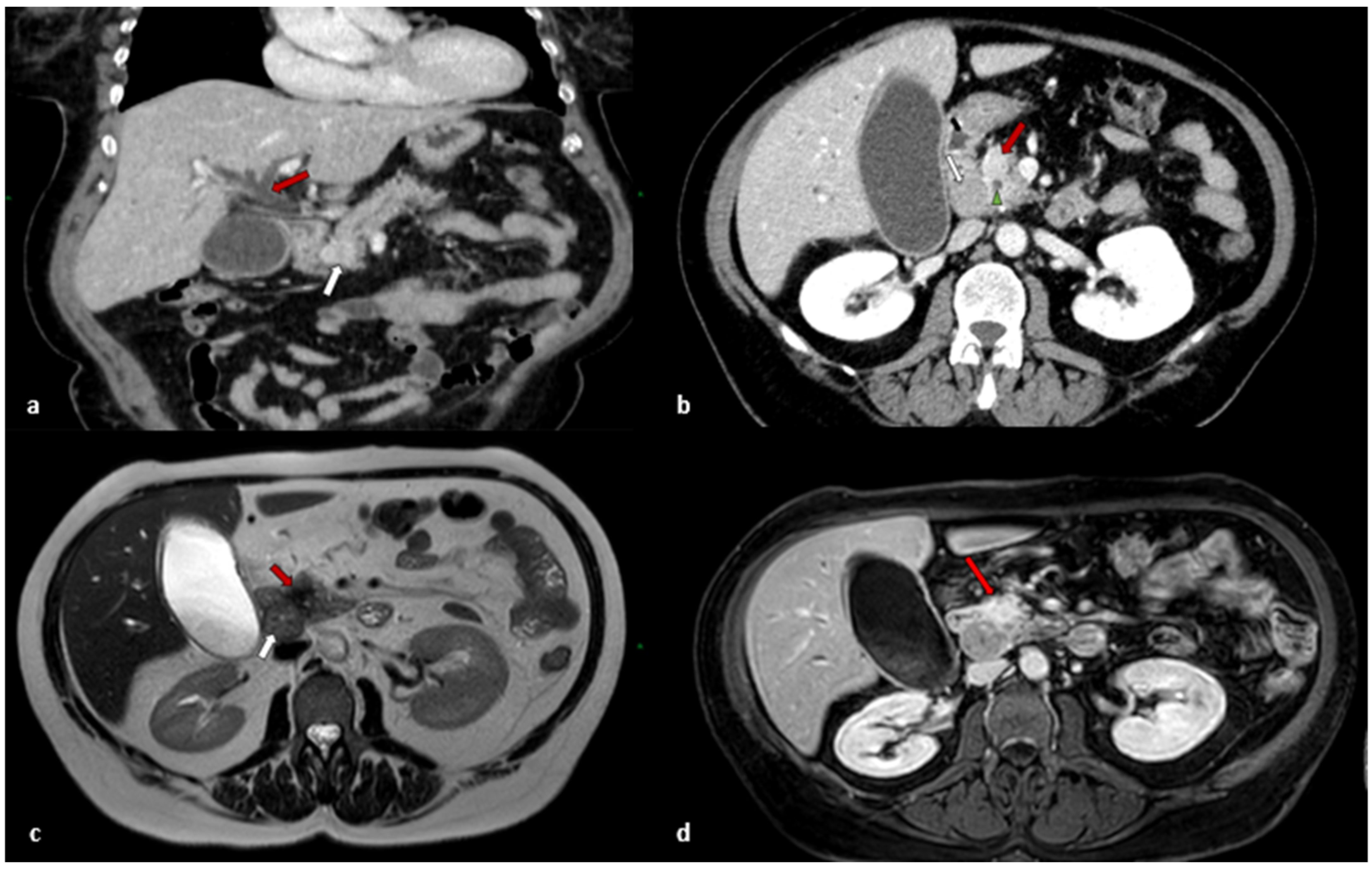
2.1. Clinical Presentation
2.2. Treatment
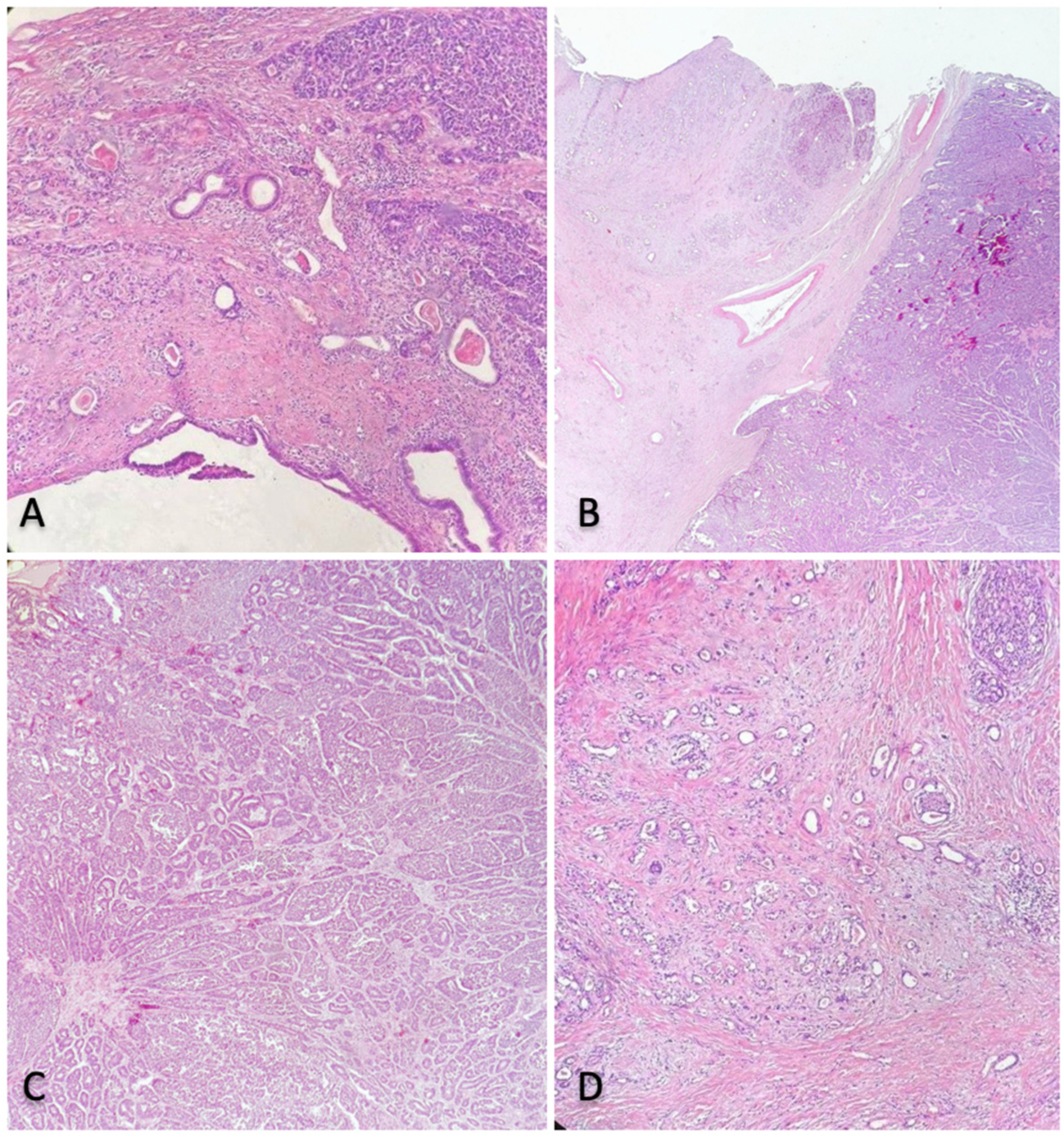
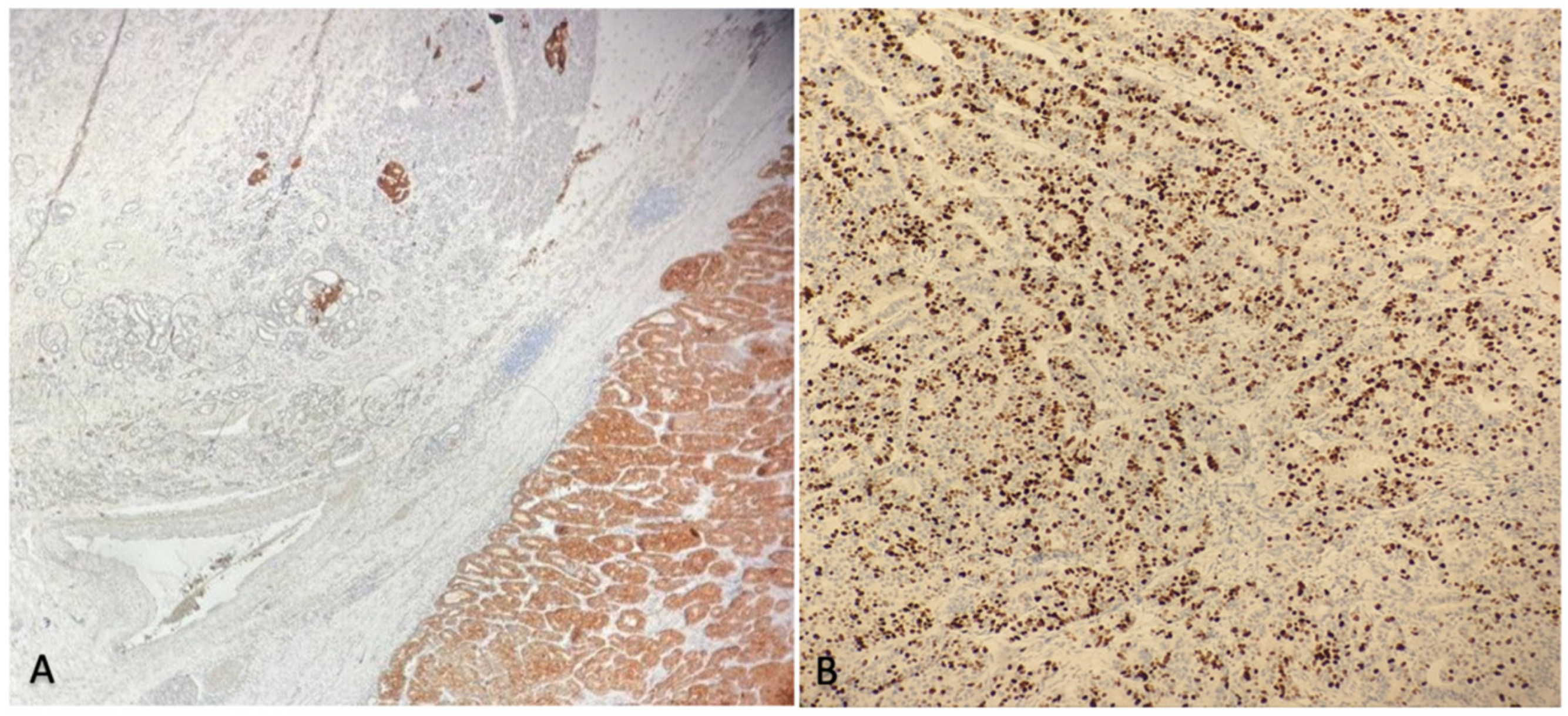
2.3. Histological Findings
2.4. Outcomes and Follow-Up
3. Materials and Methods
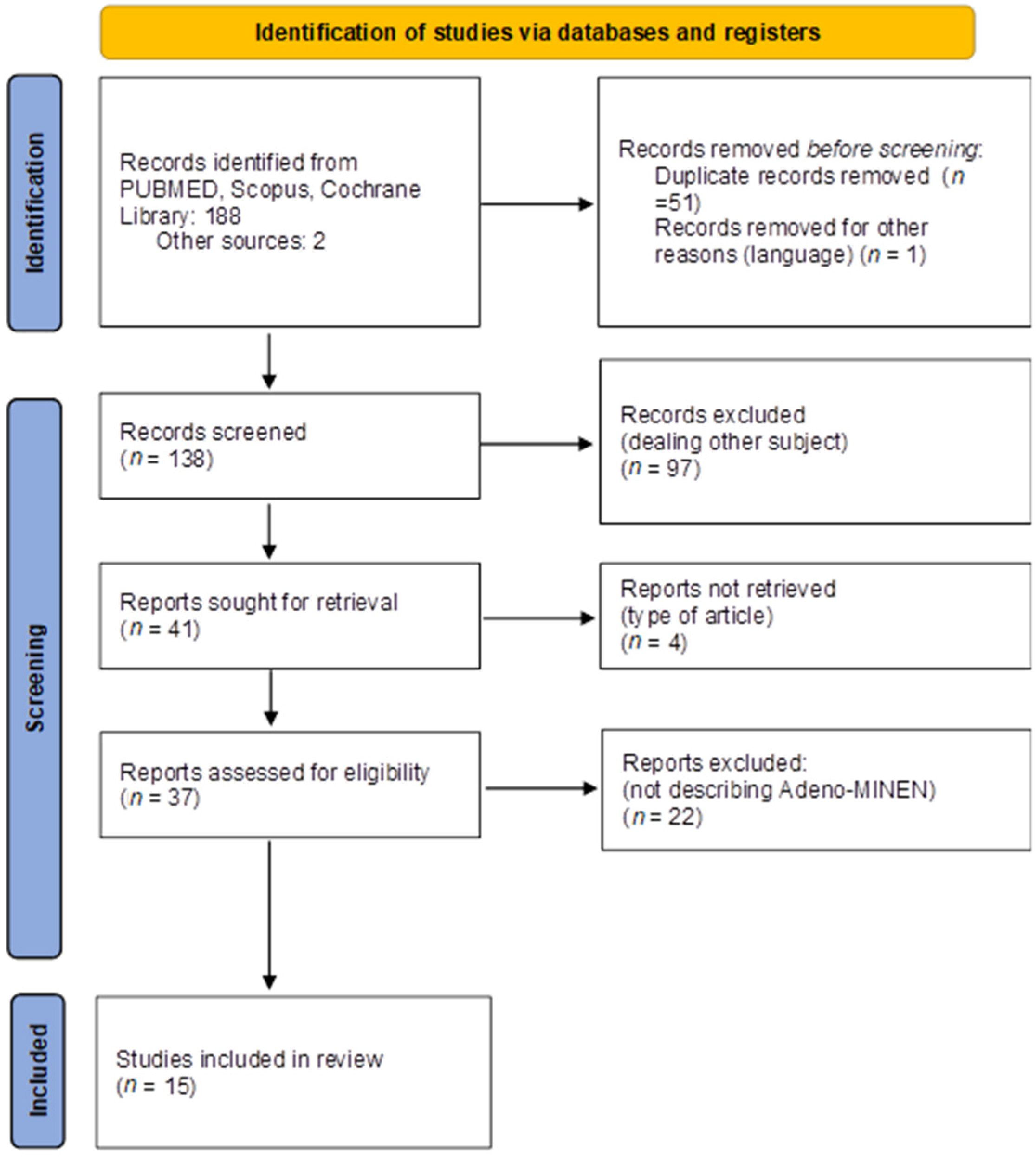
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Data Extraction and Synthesis
3.4. Endpoint
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 209–240. [Google Scholar]
- de Mestier, L.; Cros, J.; Neuzillet, C.; Hentic, O.; Egal, A.; Muller, N.; Bouché, O.; Cadiot, G.; Ruszniewski, P.; Couvelard, A.; et al. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology 2017, 105, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Matsunaga, Y.; Maeta, H.; Endo, K.; Horie, S.; Ohta, T. Mixed ductal-endocrine carcinoma of the pancreas presenting as gastrinoma with Zollinger-Ellison syndrome: An autopsy case with a 24-year survival period. Virchows Arch. 1999, 435, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, D.; Parc, Y.; Christin-Maitre, S.; Parc, R.; Flejou, J.F. Mixed ductal-pancreatic polypeptide-cell carcinoma of the pancreas. Histopathology 2002, 41, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Kawaguchi, M.; Furukawa, K.; Sekido, Y.; Osamura, Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol. Int. 2002, 52, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Ballas, K.D.; Rafailidis, S.F.; Demertzidis, C.; Alatsakis, M.B.; Pantzaki, A.; Sakadamis, A.K. Mixed exocrine-endocrine tumor of the pancreas. JOP 2005, 6, 449–454. [Google Scholar] [PubMed]
- Hashimoto, Y.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Ohge, H.; Sueda, T.; Shimamoto, F.; Hiyama, E. Mixed ductal-endocrine carcinoma derived from intraductal papillary mucinous neoplasm (IPMN) of the pancreas identified by human telomerase reverse transcriptase (hTERT) expression. J. Surg. Oncol. 2008, 97, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Mumtaz, S.; Fatima, S.; Qureshi, A. Mixed ductal-endocrine carcinoma of pancreas. BMJ Case Rep. 2011, 2011, bcr0220113861. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Shimura, T.; Kobayashi, T.; Saito, K.; Wada, W.; Sasaki, S.; Suzuki, H.; Kashiwabara, K.; Nakajima, T.; Kuwano, H. Mixed ductal-endocrine carcinoma of the pancreas occurring as a double cancer: Report of a case. Int. Surg. 2011, 96, 153–158. [Google Scholar] [CrossRef]
- Hirano, H.; Terada, N.; Yamada, N.; Yamanegi, Y.; Oyama, H.; Nishigami, T.; Nakasho, K. A case of mixed ductal-endocrine carcinoma of the pancreas. Med. Mol. Morphol. 2011, 44, 58–62. [Google Scholar] [CrossRef]
- Yang, M.; Ke, N.-W.; Zhang, Y.; Zeng, L.; Tan, C.-L.; Zhang, H.; Mai, G.; Tian, B.-L.; Liu, X.-B. Survival analyses for patients with surgically resected pancreatic neuroendocrine tumors by world health organization 2010 grading classifications and american joint committee on cancer 2010 staging systems. Medicine 2015, 94, e2156. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, K.; Fukuda, S.; Tazawa, H.; Kuga, Y.; Mochizuki, T.; Hirata, Y.; Fujisaki, S.; Takahashi, M.; Nishida, T.; Sakimoto, H. A mixed adenoneuroendocrine carcinoma of the pancreas: A case report. Surg. Case Rep. 2016, 2, 133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murata, M.; Takahashi, H.; Yamada, M.; Song, M.; Hiratsuka, M. A case of mixed adenoneuroendocrine carcinoma of the pancreas: Immunohistochemical analysis for histogenesis. Medicine 2017, 96, e6225. [Google Scholar] [CrossRef]
- Düzköylü, Y.; Aras, O.; Bostancı, E.B.; Keklik Temuçin, T.; Ulaş, M. Mixed Adeno-Neuroendocrine Carcinoma; Case Series of Ten Patients with Review of the Literature. Balk. Med. J. 2018, 35, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Nießen, A.; Schimmack, S.; Weber, T.F.; Mayer, P.; Bergmann, F.; Hinz, U.; Büchler, M.W.; Strobel, O. Presentation and outcome of mixed neuroendocrine non-neuroendocrine neoplasms of the pancreas. Pancreatology 2021, 21, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Schiavo Lena, M.; Cangi, M.G.; Pecciarini, L.; Francaviglia, I.; Grassini, G.; Maire, R.; Partelli, S.; Falconi, M.; Perren, A.; Doglioni, C. Evidence of a common cell origin in a case of pancreatic mixed intraductal papillary mucinous neoplasm-neuroendocrine tumor. Virchows Arch. 2021, 478, 1215–1219. [Google Scholar] [CrossRef]
- Varshney, B.; Bharti, J.N.; Varshney, V.K.; Yadav, T. Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN) of pancreas: A rare entity-worth to note. BMJ Case Rep. 2020, 13, e234855. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Sessa, F.; Uccella, S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr. Pathol. 2016, 27, 284–311. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Tumours Editorial Board, Digestive System Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- La Rosa, S.; Marando, A.; Sessa, F.; Capella, C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers 2012, 4, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Couvelard, A.; Scoazec, J.Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Cros, J. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Ann. Endocrinol. 2019, 80, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ito, T.; Jensen, R.T. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: Recent advances and controversies. Expert Rev. Anticancer Ther. 2019, 19, 1029–1050. [Google Scholar] [CrossRef]
- Yu, R.; Jih, L.; Zhai, J.; Nissen, N.N.; Colquhoun, S.; Wolin, E.; Dhall, D. Mixed acinar-endocrine carcinoma of the pancreas: New clinical and pathological features in a contemporary series. Pancreas 2013, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
| Authors and Year | Type of Study | N of Pts | Sex | Age (Years) | Presentation | Preoperative Diagnosis | TNM-Staging | G | Pancreatic Localization | Surgical Treatment | Adjuvant Treatment | Tumour Recurrence | Follow-Up | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terada et al., 1999 [3] | Case report | 1 | M | 24 | Anaemia | No | nr | nr | Head | PD | nr | Yes, local, lung, liver | DFS 216 months OS 288 months | Yes |
| Chatelain et al., 2002 [4] | Case report | 1 | F | 76 | Incidental finding | No | T3N0M0 Stage II | nr | Tail | DPS | No | nr | nr | nr |
| Terada et al., 2002 [5] | Case report | 1 | M | 34 | Abdominal pain | Yes | T1N0M0 Stage I | G2 | Body | DPS | No | nr | nr | nr |
| Ballas et al., 2005 [6] | Case report | 1 | F | 65 | Abdominal pain, anaemia, nausea | No | T4N0M0 Stage III | nr | Tail | DPS | nr | No | DFS 18 months OS 18 months | No |
| Hashimoto et al., 2008 [7] | Case report | 1 | M | 75 | Obstructive jaundice | No | T3N1M0 Stage III | G2 | Head | PD | No | Yes, liver | DFS nr OS 6 months | Yes |
| Ahmad et al., 2011 [8] | Case report | 1 | M | 73 | Epigastric pain, weight loss | No | T4N0M0 Stage III | nr | Body | Unspecified resection | No | No | DFS 6 months OS 6 months | No |
| Araki et al., 2011 [9] | Case report | 1 | M | 68 | Incidental finding | No | T2N0M0 Stage II | G3 | Head | PD | No | No | DFS 52 months OS 52 months | No |
| Hirano et al., 2011 [10] | Case report | 1 | M | 66 | Jaundice, weight loss | No | T2N0M0 Stage II | G3 | Head | PD | No, patient refused | Yes, unspecified | DFS 1 year OS 1 year | Yes |
| Yang et al., 2015 [11] | Retrospective study | 6 | nr | 47.7 Mean | nr | Nr | Stage III 2 Stage IV 4 | G3 6 100% | nr | 2 PD 2 DPS 2 debulking biopsy | nr | nr | Mean DFS nr Mean OS 15.3 | 6 death |
| Imaoka et al., 2017 [12] | Case report | 1 | M | 63 | Incidental finding | No | T4N2M0 Stage III | G3 | Head | PD | 1 course of cisplatin and irinotecan, afterwards patient refusal | Yes, local, peritoneal carcinosis | DFS nr OS 6 months | Yes |
| Murata et al., 2017 [13] | Case report | 1 | M | 66 | Obstructive jaundice | No | T3N1M0 Stage III | G3 | Head | PD | (2 courses of Tegafur gimercil oteracil (S-1) monotherapy | Yes, liver | DFS nr OS 12 months | Yes |
| Düzkoylü et al., 2018 [14] | Case report | 1 | M | 72 | nr | No | T3N0M0 Stage II | G1 | Tail | DPS | Yes, unspecified | No | DFS 31 months OS 31 months | No |
| Niessen et al., 2020 [15] | Case series | 8 | 7 M 1 F | Mean 70.5 (range 30–82) | nr | nr | Stage I 1 Stage III 6 Stage IV 1 | G3 8100% | 5 head 2 body 1 tail | 5 PD 2 TP 1 DPS | 7/8 4 gemcitabine 1 capecitabine 1 carboplatin + etoposide 1 cisplatin + etoposide | 1 liver | DFS 6 pt 0 month 1 pt 88 months 1 pt 11 months Median OS 40 month | 5 dead |
| Schiavo Lena et al., 2020 [16] | Case report | 1 | M | 56 | nr | Yes | T2N0M0 Stage II | G2 | Head | PD | No | No | DFS 27 months OS 27 months | No |
| Varshney et al., 2020 [17] | Case report | 1 | M | 81 | Abdominal pain | No | nr | G1 | Head | PD | 4 cycles of with gemcitabine and cisplatin | No | DFS 12 months OS 12 months | No |
| Current Case | Case Report | 1 | F | 59 | Obstructive jaundice | No | T2N1M0 Stage III | G3 | Head | PD | No, patient refused | No | DFS 12 months OS 12 months | No |
| Total 28 pt | -M 18/22 (82.8%) -F 4/22 (18.2%) | Mean 61.7 (range 24–82) | -Abdominal pain 4/12 (33.3%) -Jaundice 4/12 (33.3%) -Incidental 3/12 (25%) -Weight loss 2/12 (16.6%) -Anaemia 2/12 (16.6%) -Nausea and vomiting 1/12 (8.3%) | Preoperative diagnosis 2/14 (14.2%) | -Stage I 2/26 (7.7%) -Stage II 5/26 (19.2%) -Stage III 14/26 (53.8) -Stage IV 5/26 (19.3%) | -G1 2/24 (8.3%) -G2 3/24–(12.5%) -G3 19/24 (79.2%) | -Head 14/22 (63.6%) -Body 4/22 (18.2%) -Tail 4/22 (18.2%) | -PD 16/28 (57.1%) -DPS 7/28 (25%) -TP 2/28 (7.1%) -Debulking procedure 2/28 (7.1%) -Unspecified resection 1/28 (3.7%) | -Neoadjuvant therapy 0% -Adjuvant therapy 11/20 (55%) | Recurrence 6/20 (30%) | Mean DFS 2.5 months median DFS 12 months (17 pt) range 0–216 months 1-year DFS 62.5% (10/16 pt) 2-year DFS 41.6% (5/12 pt) 5-year DFS 16.6% (2/12 pt) Mean OS 40.2 months median OS 12 months (26 pt) range 0–288 months 1-year OS 84% (21/25 pt) 3-year OS 27.7% (5/18 pt) 5-year OS 23.5% (4/17) | Dead 16/26 (61.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelico, R.; Siragusa, L.; Pathirannehalage Don, C.B.; Sensi, B.; Billeci, F.; Vattermoli, L.; Padial, B.; Palmieri, G.; Anselmo, A.; Coppola, A.; et al. Pancreatic Adeno-MiNEN, a Rare Newly Defined Entity with Challenging Diagnosis and Treatment: A Case Report with Systematic Literature Review and Pooled Analysis. J. Clin. Med. 2022, 11, 5021. https://doi.org/10.3390/jcm11175021
Angelico R, Siragusa L, Pathirannehalage Don CB, Sensi B, Billeci F, Vattermoli L, Padial B, Palmieri G, Anselmo A, Coppola A, et al. Pancreatic Adeno-MiNEN, a Rare Newly Defined Entity with Challenging Diagnosis and Treatment: A Case Report with Systematic Literature Review and Pooled Analysis. Journal of Clinical Medicine. 2022; 11(17):5021. https://doi.org/10.3390/jcm11175021
Chicago/Turabian StyleAngelico, Roberta, Leandro Siragusa, Cristine Brooke Pathirannehalage Don, Bruno Sensi, Federica Billeci, Leonardo Vattermoli, Belen Padial, Giampiero Palmieri, Alessandro Anselmo, Alessandro Coppola, and et al. 2022. "Pancreatic Adeno-MiNEN, a Rare Newly Defined Entity with Challenging Diagnosis and Treatment: A Case Report with Systematic Literature Review and Pooled Analysis" Journal of Clinical Medicine 11, no. 17: 5021. https://doi.org/10.3390/jcm11175021
APA StyleAngelico, R., Siragusa, L., Pathirannehalage Don, C. B., Sensi, B., Billeci, F., Vattermoli, L., Padial, B., Palmieri, G., Anselmo, A., Coppola, A., Tisone, G., & Manzia, T. M. (2022). Pancreatic Adeno-MiNEN, a Rare Newly Defined Entity with Challenging Diagnosis and Treatment: A Case Report with Systematic Literature Review and Pooled Analysis. Journal of Clinical Medicine, 11(17), 5021. https://doi.org/10.3390/jcm11175021









