Patients with Cardiac Implantable Electronic Device Undergoing Radiation Therapy: Insights from a Ten-Year Tertiary Center Experience
Abstract
:1. Introduction
2. Methods
2.1. Patient Sample
2.2. CIED Characteristics
2.3. Oncological Characteristics, Radiotherapy and Radiological Exposure
2.4. Device Programming
2.5. Post-Radiotherapy Outcomes
2.6. Statistical Analysis
3. Results
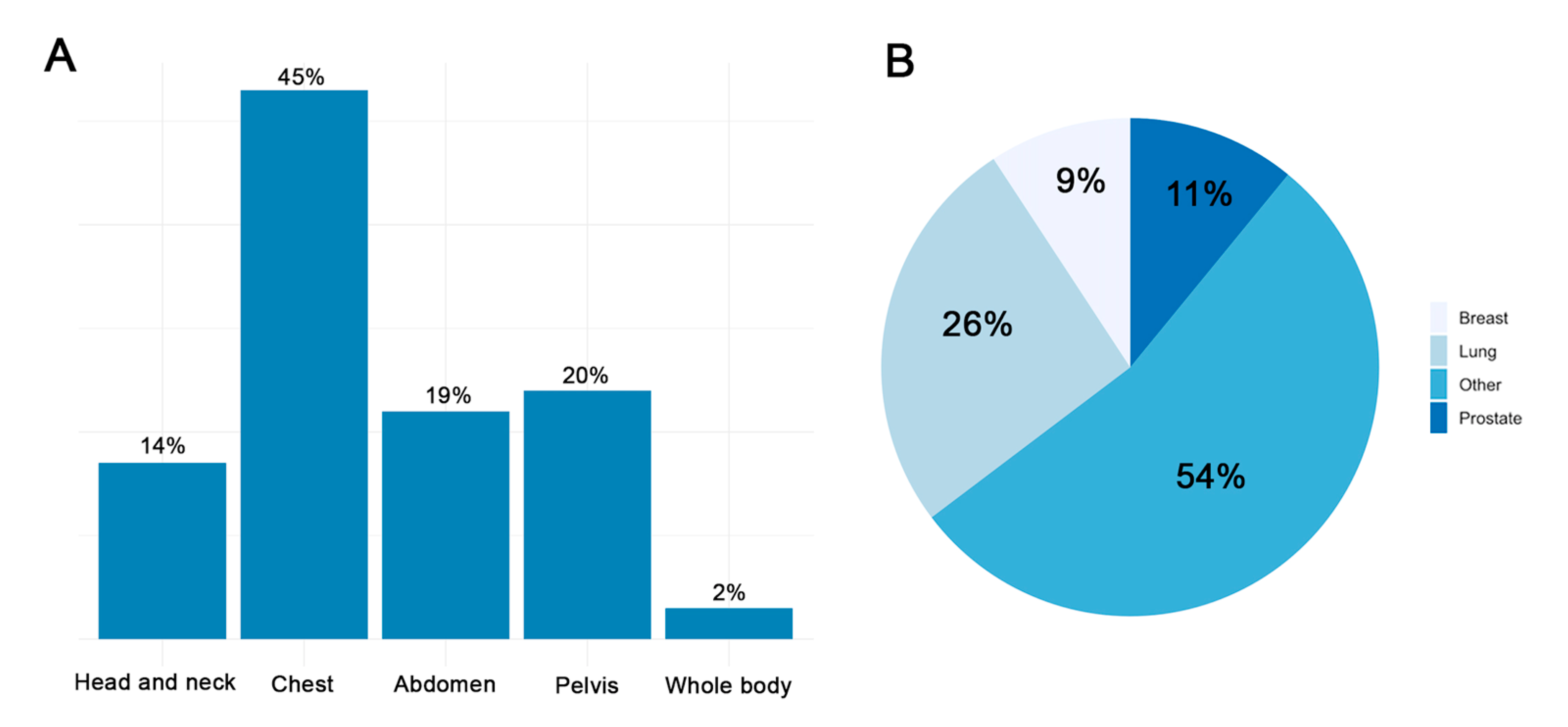
3.1. Baseline Characteristics
3.2. Changes in Device Parameters
3.3. Post-Radiotherapy Outcomes
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval
Abbreviations
| CIED | Cardiac Implantable Electronic Device |
| ICD | Implantable Cardioverter Defibrillator |
| PM | Pacemaker |
| RT | Radiotherapy |
References
- Last, A. Radiotherapy in patients with cardiac pacemakers. Br. J. Radiol. 1998, 71, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gossman, M.S.; Wilkinson, J.D.; Mallick, A. Treatment approach, delivery, and follow-up evaluation for cardiac rhythm disease management patients receiving radiation therapy: Retrospective physician surveys including chart reviews at numerous centers. Med. Dosim. 2014, 39, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Lefebvre, B.; Carver, J.; Cheung, J.W.; Feigenberg, S.J.; Lampert, R.; Liu, J.; Rajagopalan, B.; Lenihan, D.J. How to Manage Patients with Cardiac Implantable Electronic Devices Undergoing Radiation Therapy. JACC CardioOncol. 2021, 3, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, M.; Severgnini, M.; Fiorentino, A.; Malavasi, V.L.; Menegotti, L.; Alongi, F.; Catanzariti, D.; Jereczek-Fossa, B.A.; Stasi, M.; Russi, E.; et al. Management of patients with cardiac implantable electronic devices (CIED) undergoing radiotherapy: A consensus document from Associazione Italiana Aritmologia e Cardiostimolazione (AIAC), Associazione Italiana Radioterapia Oncologica (AIRO), Associazione Italiana Fisica Medica (AIFM). Int. J. Cardiol. 2018, 255, 175–183. [Google Scholar] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Chacko, S.; Glover, B.; Campbell, D.; Crystal, E.; Ben-Dov, N.; Baranchuk, A. Radiotherapy for Patients with Cardiovascular Implantable Electronic Devices: A Review. Can. J. Cardiol. 2018, 34, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, J.T.; Blamek, S.; Gadula-Gacek, E.; Gorol, J.; Kurek, A.; Witek, M.; Wojtaszczyk, A.; Plaza, P.; Miszczyk, L.; Gąsior, M.; et al. Radiation therapy in patients with cardiac implantable electronic devices. Kardiol. Pol. 2021, 79, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Soejima, T.; Yoden, E.; Nishimura, Y.; Ono, S.; Yoshida, A.; Fukuda, H.; Fukuhara, N.; Sasaki, R.; Tsujino, K.; Norihisa, Y. Radiation therapy in patients with implanted cardiac pacemakers and implantable cardioverter defibrillators: A prospective survey in Japan. J. Radiat. Res. 2011, 52, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadehgan, A.; Laurans, M.; Thuillot, M.; Huertas, A.; Baudinaud, P.; Narayanan, K.; Mirabel, M.; Bibault, J.-E.; Frey, P.; Waldmann, V.; et al. Radiotherapy in Patients with a Cardiac Implantable Electronic Device. Am. J. Cardiol. 2020, 128, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rav Acha, M.; Soifer, E.; Hasin, T. Cardiac Implantable Electronic Miniaturized and Micro Devices. Micromachines 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, A.; Vitali-Serdoz, L.; Fischlein, T.; Kirste, W.; Buia, V.; Walaschek, J.; Rittger, H.; Bastian, D. Perioperative Sensor and Algorithm Programming in Patients with Implanted ICDs and Pacemakers for Cardiac Resynchronization Therapy. Sensors 2021, 21, 8346. [Google Scholar] [CrossRef]

| Total Patients (n = 107) | Pacemaker Carriers (n = 63) | Implantable Cardioverter Defibrillator Carriers (n = 44) | p Value | |
|---|---|---|---|---|
| Age (years) | 75.8 ± 7.0 | 77.4 ± 7.5 | 74.2 ± 6.3 | 0.07 |
Manufacturer
| ||||
| 45 (42.1) | 31 (49.2) | 14 (31.8) | ||
| 16 (15.0) | 7 (11.1) | 9 (20.5) | ||
| 26 (24.3) | 10 (15.9) | 16 (36.4) | ||
| 15 (14.0) | 11 (17.5) | 4 (9.1) | ||
| 5 (4.7) | 4 (6.3) | 1 (2.3) | ||
| RT sessions | 16.4 ± 10.7 | 17.2 ± 10.6 | 15.1 ± 11.0 | 0.38 |
| RT total dose (Gy) | 46.4 ± 15.5 | 46.9 ± 15.4 | 45.7 ± 15.7 | 0.79 |
| RT fractions | 16.0 ± 10.3 | 16.3 ± 10.7 | 15.7 ± 9.9 | 0.87 |
| Device Maximum Dose (Gy) | 2.8 ± 3.8 | 3.0 ± 4.2 | 2.6 ± 3.1 | 0.83 |
| Device Mean Dose (Gy) | 1.0 ± 1.3 | 0.9 ± 1.1 | 1.0 ± 1.6 | 0.69 |
| Lead Maximum Dose (Gy) | 22.5 ± 18.8 | 22.5 ± 18.8 | 22.5 ± 19.2 | 0.89 |
| Lead Mean Dose (Gy) | 5.4 ± 6.5 | 5.7 ± 6.5 | 4.9 ± 6.5 | 0.30 |
| Before Radiotherapy (n = 107) | After Radiotherapy (n = 107) | p Value | |
|---|---|---|---|
| Atrial capture threshold * | 1.2 ± 0.6 | 1.2 ± 0.5 | 0.92 |
| P-wave amplitude | 4.3 ± 2.2 | 4.3 ± 2.1 | 0.97 |
| Atrial lead impedance | 611.5 ± 155.2 | 614.6 ± 152.5 | 0.83 |
| RV capture threshold * | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.26 |
| RV R-wave amplitude | 11.0 ± 4.6 | 11.2 ± 4.5 | 0.93 |
| RV lead impedance | 623.3 ± 158.1 | 622.8 ± 158.1 | 0.97 |
| LV capture threshold * | 1.3 ± 0.9 | 1.3 ± 1.0 | 0.93 |
| LV R-wave amplitude | 12.8 ± 4.7 | 12.8 ± 4.7 | 0.89 |
| LV lead impedance | 779.9 ± 138.0 | 824.4 ± 155.5 | 0.60 |
| Pacemakers (n = 63) | |||
|---|---|---|---|
| Before Radiotherapy (n = 63) | After Radiotherapy (n = 63) | p Value | |
| Atrial capture threshold * | 1.2 ± 0.4 | 1.2 ± 0.6 | 0.94 |
| P-wave amplitude | 4.5 ± 2.0 | 4.6 ± 2.0 | 0.80 |
| Atrial lead impedance | 622.6 ± 146.3 | 619.5 ± 147.6 | 0.87 |
| RV capture threshold * | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.90 |
| RV R-wave amplitude | 10.7 ± 3.6 | 10.7 ± 4.3 | 0.92 |
| RV lead impedance | 644.1 ± 174.6 | 646.0 ± 179.4 | 0.94 |
| LV capture threshold * | 4.5 ± 0.9 | 3.8 ± 1 | 0.32 |
| LV R-wave amplitude | 12.8 ± 4.7 | 12.8 ± 4.7 | 0.89 |
| LV lead impedance | 570.0 ± 138.5 | 608.0 ± 155.5 | 0.32 |
| Implantable Cardioverter Defibrillator (n = 44) | |||
| Before Radiotherapy (n = 44) | After Radiotherapy (n = 44) | p Value | |
| Atrial capture threshold * | 1.2 ± 0.5 | 1.2 ± 0.5 | 0.92 |
| P-wave amplitude | 4.0 ± 2.2 | 4.0 ± 2.3 | 0.75 |
| Atrial lead impedance | 603.6 ± 161.9 | 600.8 ± 166.1 | 0.88 |
| RV capture threshold * | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.09 |
| RV R-wave amplitude | 11.8 ± 5.3 | 11.4 ± 5.0 | 0.88 |
| RV lead impedance | 593.3 ± 128.1 | 591.8 ± 117.5 | 0.97 |
| LV capture threshold * | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.91 |
| LV R-wave amplitude | 12.8 ± 4.7 | 12.8 ± 4.7 | 0.89 |
| LV lead impedance | 847.5 ± 139.8 | 797.1 ± 132.5 | 0.60 |
| Total Patients (n = 107) | Pacemaker Carriers (n = 63) | Implantable Cardioverter Defibrillator Carriers (n = 44) | p Value | |
|---|---|---|---|---|
| Generator failures | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Power-on resets | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Changes in pacing threshold requiring system revision or programming changes | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Changes in sensing threshold requiring system revision or programming changes | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Battery depletions | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Pacing inhibitions | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Inappropriate therapies | 0 (0) | 0 (0) | 0 (0) | 0.99 |
| Atrial Arrhythmias during RT session period | 14 (13.1) | 10 (15.9) | 4 (9.1) | 0.39 |
| Ventricular Arrhythmias during RT session period | 10 (9.9) | 5 (8.5) | 5 (11.9) | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulletta, S.; Falasconi, G.; Cianfanelli, L.; Centola, A.; Paglino, G.; Cireddu, M.; Radinovic, A.; D’Angelo, G.; Marzi, A.; Sala, S.; et al. Patients with Cardiac Implantable Electronic Device Undergoing Radiation Therapy: Insights from a Ten-Year Tertiary Center Experience. J. Clin. Med. 2022, 11, 4990. https://doi.org/10.3390/jcm11174990
Gulletta S, Falasconi G, Cianfanelli L, Centola A, Paglino G, Cireddu M, Radinovic A, D’Angelo G, Marzi A, Sala S, et al. Patients with Cardiac Implantable Electronic Device Undergoing Radiation Therapy: Insights from a Ten-Year Tertiary Center Experience. Journal of Clinical Medicine. 2022; 11(17):4990. https://doi.org/10.3390/jcm11174990
Chicago/Turabian StyleGulletta, Simone, Giulio Falasconi, Lorenzo Cianfanelli, Alice Centola, Gabriele Paglino, Manuela Cireddu, Andrea Radinovic, Giuseppe D’Angelo, Alessandra Marzi, Simone Sala, and et al. 2022. "Patients with Cardiac Implantable Electronic Device Undergoing Radiation Therapy: Insights from a Ten-Year Tertiary Center Experience" Journal of Clinical Medicine 11, no. 17: 4990. https://doi.org/10.3390/jcm11174990
APA StyleGulletta, S., Falasconi, G., Cianfanelli, L., Centola, A., Paglino, G., Cireddu, M., Radinovic, A., D’Angelo, G., Marzi, A., Sala, S., Fierro, N., Bisceglia, C., Peretto, G., Di Muzio, N., Della Bella, P., Vergara, P., & Dell’Oca, I. (2022). Patients with Cardiac Implantable Electronic Device Undergoing Radiation Therapy: Insights from a Ten-Year Tertiary Center Experience. Journal of Clinical Medicine, 11(17), 4990. https://doi.org/10.3390/jcm11174990







