Evidence of Validity and Normative Values of a New Auditory Backward Masking Test
Abstract
:1. Introduction
2. Materials & Methods
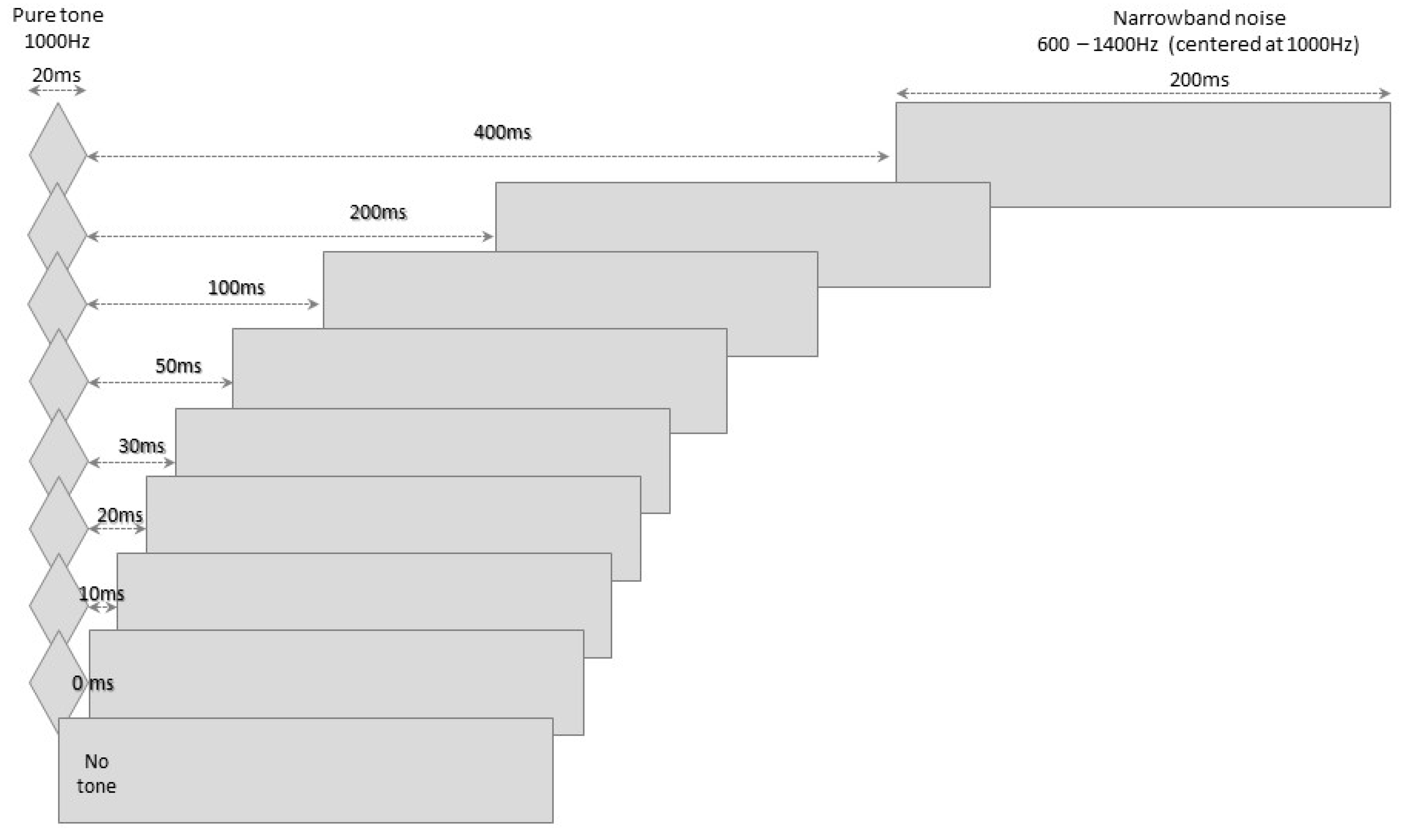
2.1. The BMT
2.2. Procedures
- (a)
- (b)
- basic hearing assessment (pure-tone audiometry, tympanometry and acoustic reflexes measurement), with a type A tympanogram, the presence of ipsi- and contralateral acoustic reflexes and hearing thresholds between 250 and 8000 Hz below 20 dBHL as inclusion criteria;
- (c)
- behavioral assessment of ATP, using tests well established in clinical practice: the pitch pattern (PP; [43]), duration pattern (DP; [43]) and Gaps-in-Noise (GIN; [3]) tests, which assess temporal ordering and resolution. The tests were applied at an intensity of 50 dBSL, that is, 50 dB above the speech recognition threshold (SRT) of each individual;
- (d)
- the backward masking test (BMT) developed in the present study, as specified in the previous section.
2.3. Sample Characterization
2.4. Results Analysis
3. Results
Correlation with Other ATP Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eggermont, J.J. Auditory Temporal Processing and Its Disorders, 1st ed.; Oxford University: Oxford, UK, 2015. [Google Scholar]
- Shinn, J.B. Temporal Processing: The Basics. Hear. J. 2003, 56, 52. [Google Scholar] [CrossRef]
- Musiek, F.E.; Shinn, J.B.; Jirsa, R.; Bamiou, D.-E.; Baran, J.A.; Zaida, E. GIN (Gaps-In-Noise) Test Performance in Subjects with Confirmed Central Auditory Nervous System Involvement. Ear Hear. 2005, 26, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.B. Temporal Processing Tests. In Handbook of Central Auditory Processing Disorder: Auditory Neuroscience and Diagnosis; Musiek, F.E., Chermak, G.D., Eds.; Plural Publishing: San Diego, CA, USA, 2014; pp. 405–434. [Google Scholar]
- Musiek, F.E.; Chermak, G.D. Psychophysical and Behavioral Peripheral and Central Auditory Tests. Handb Clin. Neurol. 2015, 129, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Tallal, P. Improving Language and Literacy Is a Matter of Time. Nat. Rev. Neurosci. 2004, 5, 721–728. [Google Scholar] [CrossRef]
- Hämäläinen, J.A.; Salminen, H.K.; Leppänen, P.H.T. Basic Auditory Processing Deficits in Dyslexia. J. Learn. Disabil. 2013, 46, 413–427. [Google Scholar] [CrossRef]
- Tierney, A.; Kraus, N. Auditory-Motor Entrainment and Phonological Skills: Precise Auditory Timing Hypothesis (PATH). Front. Hum. Neurosci. 2014, 8, 949. [Google Scholar] [CrossRef] [Green Version]
- Cancer, A.; Antonietti, A. Music-Based and Auditory-Based Interventions for Reading Difficulties: A Literature Review. Heliyon 2022, 8, e09293. [Google Scholar] [CrossRef]
- McArthur, G.M.; Bishop, D.V.M. Speech and Non-Speech Processing in People with Specific Language Impairment: A Behavioural and Electrophysiological Study. Brain Lang. 2005, 94, 260–273. [Google Scholar] [CrossRef]
- Fortunato-Tavares, T.; Rocha, C.N.; de Andrade, C.R.F.; Befi-Lopes, D.M.; Schochat, E.; Hestvik, A.; Schwartz, R.G. Linguistic and Auditory Temporal Processing in Children with Specific Language Impairment. Pró-Fono Rev. Atualização Científica 2009, 21, 279–284. [Google Scholar] [CrossRef]
- Batista, P.B.; Lemos, S.M.A.; Rodrigues, L.O.C.; de Rezende, N.A. Auditory Temporal Processing Deficits and Language Disorders in Patients with Neurofibromatosis Type 1. J. Commun. Disord. 2014, 48, 18–26. [Google Scholar] [CrossRef]
- Wright, B.A.; Lombardino, L.J.; King, W.M.; Puranik, C.S.; Leonard, C.M.; Merzenich, M.M. Deficits in Auditory Temporal and Spectral Resolution in Language-Impaired Children. Nature 1997, 387, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.T.; Kraus, N. The Ability to Tap to a Beat Relates to Cognitive, Linguistic, and Perceptual Skills. Brain Lang. 2013, 124, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Schlauch, R.S.; Sasisekaran, J. Backward Masking of Tones and Speech in People Who Do and Do Not Stutter. J. Fluen. Disord. 2018, 57, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, Y.; Dastgerdi, Z.H.; Farazi, M.; Moossavi, A.; Bakhshi, E. Auditory Temporal Processing Assessment in Children with Developmental Stuttering. Int. J. Pediatr. Otorhinolaryngol. 2020, 132, 109935. [Google Scholar] [CrossRef]
- Nittrouer, S.; Shune, S.; Lowenstein, J.H. What Is the Deficit in Phonological Processing Deficits: Auditory Sensitivity, Masking, or Category Formation? J. Exp. Child Psychol. 2011, 108, 762–785. [Google Scholar] [CrossRef] [Green Version]
- Vandewalle, E.; Boets, B.; Ghesquière, P.; Zink, I. Auditory Processing and Speech Perception in Children with Specific Language Impairment: Relations with Oral Language and Literacy Skills. Res. Dev. Disabil. 2012, 33, 635–644. [Google Scholar] [CrossRef]
- Boets, B.; Vandermosten, M.; Poelmans, H.; Luts, H.; Wouters, J.; Ghesquière, P. Preschool Impairments in Auditory Processing and Speech Perception Uniquely Predict Future Reading Problems. Res. Dev. Disabil. 2011, 32, 560–570. [Google Scholar] [CrossRef]
- Tomlin, D.; Dillon, H.; Sharma, M.; Rance, G. The Impact of Auditory Processing and Cognitive Abilities in Children. Ear Hear. 2015, 36, 527–542. [Google Scholar] [CrossRef]
- Neijenhuis, K.; Campbell, N.G.; Cromb, M.; Luinge, M.R.; Moore, D.R.; Rosen, S.; de Wit, E. An Evidence-Based Perspective on “Misconceptions” Regarding Pediatric Auditory Processing Disorder. Front. Neurol. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Back, N.C.F.; Crippa, A.C.D.S.; Riechi, T.I.J.D.S.; Pereira, L.D. Central Auditory Processing and Cognitive Functions in Children. Int. Arch. Otorhinolaryngol. 2022, 26, e020–e031. [Google Scholar] [CrossRef]
- Samelli, A.G.; Schochat, E. Processamento Auditivo, Resolução Temporal e Teste de Detecção de Gap: Revisão da Literatura. Rev. CEFAC 2008, 10, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Marler, J.A.; Champlin, C.A.; Gillam, R.B. Auditory Memory for Backward Masking Signals in Children with Language Impairment. Psychophysiology 2002, 39, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, C.R.; Morris, R.D.; Sevcik, R.A.; Clarkson, M.G. Auditory Backward Masking Deficits in Children with Reading Disabilities. Brain Lang. 2005, 95, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Howell, P.; Rosen, S.; Hannigan, G.; Rustin, L. Auditory Backward-Masking Performance by Children Who Stutter and Its Relation to Dysfluency Rate. Percept. Mot. Skills 2000, 90, 355–363. [Google Scholar] [CrossRef]
- MacDonald, J.A. Using the Ideal Observer to Predict Performance in Perceptual Tasks: An Example from the Auditory Temporal Masking Domain. Atten. Percept. Psychophys. 2011, 73, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, M.M.; Donell, B.M.; Rosen, M.J. Late Maturation of Backward Masking in Auditory Cortex. J. Neurophysiol. 2018, 120, 1558–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, H.L.; Spitzer, E.R.; Buss, E.; Leibold, L.J.; Grose, J.H. Forward and Backward Masking of Consonants in School-Age Children and Adults. J. Speech Lang. Hear. Res. 2018, 61, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Grose, J.H.; Menezes, D.C.; Porter, H.L.; Griz, S. Masking Period Patterns and Forward Masking for Speech-Shaped Noise. Ear Hear. 2016, 37, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Filippini, R.; Schochat, E. A New Paradigm for Temporal Masking Assessment: Pilot Study. CoDAS 2014, 26, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Rishiq, D.A.; Harkrider, A.W.; Hedrick, M.S. Acceptable Noise Level and Psychophysical Masking. Am. J. Audiol. 2012, 21, 199–205. [Google Scholar] [CrossRef]
- Carcagno, S.; Plack, C.J. Relations between Speech-reception, Psychophysical Temporal Processing, and Subcortical Electrophysiological Measures of Auditory Function in Humans. Hear. Res. 2022, 417, 108456. [Google Scholar] [CrossRef] [PubMed]
- Strait, D.L.; Kraus, N. Biological Impact of Auditory Expertise across the Life Span: Musicians as a Model of Auditory Learning. Hear. Res. 2014, 308, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.L. Development of Auditory Narrow-Band Frequency Contours. J. Acoust. Soc. Am. 1967, 42, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.L. Backward and Forward Masking of Probe Tones of Different Frequencies. J. Acoust. Soc. Am. 1962, 34, 1116–1117. [Google Scholar] [CrossRef]
- Elliott, L.L. Backward Masking: Monotic and Dichotic Conditions. J. Acoust. Soc. Am. 1962, 34, 1108–1115. [Google Scholar] [CrossRef]
- Soderquist, D.R.; Carstens, A.A.; Frank, G.J. Backward, Simultaneous, and Forward Masking as a Function of Signal Delay and Frequency. J. Audit. Res. 1981, 21, 227–245. [Google Scholar]
- Gelfand, S.A. Hearing: An Introduction to Psychological and Physiological Acoustics, 6th ed.; CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Memória, C.M.; Yassuda, M.S.; Nakano, E.Y.; Forlenza, O.V. Brief Screening for Mild Cognitive Impairment: Validation of the Brazilian Version of the Montreal Cognitive Assessment. Int. J. Geriatr. Psychiatry 2013, 28, 34–40. [Google Scholar] [CrossRef]
- Musiek, F.E. Frequency (Pitch) and Duration Pattern Tests. J. Am. Acad. Audiol. 1994, 5, 265–268. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A 1972, 135, 370. [Google Scholar] [CrossRef]
- Ng, V.K.Y.; Cribbie, R.A. Using the Gamma Generalized Linear Model for Modeling Continuous, Skewed and Heteroscedastic Outcomes in Psychology. Curr. Psychol. 2017, 36, 225–235. [Google Scholar] [CrossRef] [Green Version]
- AAA (American Academy of Audiology). Clinical Practice Guidelines: Diagnosis, Treatment and Management of Children and Adults with Central Auditory Processing Disorder. Available online: https://audiology-web.s3.amazonaws.com/migrated/CAPD Guidelines 8-2010.pdf_539952af956c79.73897613.pdf (accessed on 26 March 2020).
- ASHA (American Speech-Language-Hearing Association). Central Auditory Processing Disorder (Practice Portal). Available online: www.asha.org/Practice-Portal/Clinical-Topics/Central-Auditory-Processing-Disorder/ (accessed on 12 March 2021).
- BSA (British Society of Audiology). Practice Guidance—An Overview of Current Management of Auditory Processing Disorder (APD). Available online: http://www.thebsa.org.uk/wp-content/uploads/2014/04/BSA_APD_Management_1Aug11_FINAL_amended17Oct11.pdf (accessed on 12 March 2021).
- BSA (British Society of Audiology). Position Statement and Practice Guidance—Auditory Processing Disorder (APD). Available online: www.thebsa.org (accessed on 12 March 2021).
- Nickisch, A.; Kiese-Himmel, C.; Wiesner, T.; Schönweiler, R. Guideline: Auditory Processing and Perception Disorders: Differential Diagnosis: S1 Guideline of the German Society of Phoniatrics and Pediatric Audiology. HNO 2019, 67, 576–583. [Google Scholar] [CrossRef]
- Iliadou, V. (Vivian); Ptok, M.; Grech, H.; Pedersen, E.R.; Brechmann, A.; Deggouj, N.; Kiese-Himmel, C.; Śliwińska-Kowalska, M.; Nickisch, A.; Demanez, L.; et al. A European Perspective on Auditory Processing Disorder-Current Knowledge and Future Research Focus. Front. Neurol. 2017, 8, 622. [Google Scholar] [CrossRef] [Green Version]
- Keith, W.J.; Purdy, S.C.; Baily, M.R.; Kay, F.M. New Zealand Guidelines on Auditory Processing Disorder. Available online: https://www.audiology.org.nz/ (accessed on 12 March 2021).
- Neijenhuis, K.A.; Stollman, M.H.; Snik, A.F.; Van der Broek, P. Development of a Central Auditory Test Battery for Adults. Audiology 2001, 40, 69–77. [Google Scholar] [CrossRef]
- Demanez, L.; Demanez, J.P. Central Auditory Processing Assessment. Acta Otorhinolaryngol. Belg. 2003, 57, 243–252. [Google Scholar]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Hartley, D.E.H.; Moore, D.R. Auditory Processing Efficiency Deficits in Children with Developmental Language Impairments. J. Acoust. Soc. Am. 2002, 112, 2962–2966. [Google Scholar] [CrossRef]
- Moore, D.R. Listening Difficulties in Children: Bottom-up and Top-down Contributions. J. Commun. Disord. 2012, 45, 411–418. [Google Scholar] [CrossRef]
- Ahmmed, A.U.; Ahmmed, A.A.; Bath, J.R.; Ferguson, M.A.; Plack, C.J.; Moore, D.R. Assessment of Children with Suspected Auditory Processing Disorder. Ear Hear. 2014, 35, 295–305. [Google Scholar] [CrossRef]
- Uttl, B. Measurement of Individual Differences: Lessons from Memory Assessment in Research and Clinical Practice. Psychol. Sci. 2005, 16, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Leite Filho, C.A.; da Silva, F.F.; Pradella-Hallinan, M.; Xavier, S.D.; Miranda, M.C.; Pereira, L.D. Auditory Behavior and Auditory Temporal Resolution in Children with Sleep-Disordered Breathing. Sleep Med. 2017, 34, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.M.; Weihing, J.A.; Schochat, E. Temporal Resolution in Individuals with Neurological Disorders. Clinics 2015, 70, 606–611. [Google Scholar] [CrossRef]
- Matas, C.G.; Padilha, F.Y.O.; Angrisani, R.M.; Samelli, A.G. Oxidative Stress as a Risk Factor for Hearing Changes in HIV-Positive Normal Listeners. Clinics 2020, 75, e1845. [Google Scholar] [CrossRef]
- Said, E.A. Electrophysiological Differences in Sensorineural Hearing Loss Patients with and without Problem-Tinnitus. Egypt. J. Otolaryngol. 2012, 28, 22–34. [Google Scholar] [CrossRef]
- De Carvalho, N.G.; do Amaral, M.I.R.; Colella-Santos, M.F. AudBility: Effectiveness of an Online Central Auditory Processing Screening Program. PLoS ONE 2021, 16, e0256593. [Google Scholar] [CrossRef]
- Power, M.; Fell, G.; Wright, M. Principles for High-Quality, High-Value Testing. Evid. Based. Med. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Musiek, F.E.; Chermak, G.D.; Weihing, J.; Zappulla, M.; Nagle, S. Diagnostic Accuracy of Established Central Auditory Processing Test Batteries in Patients with Documented Brain Lesions. J. Am. Acad. Audiol. 2011, 22, 342–358. [Google Scholar] [CrossRef]

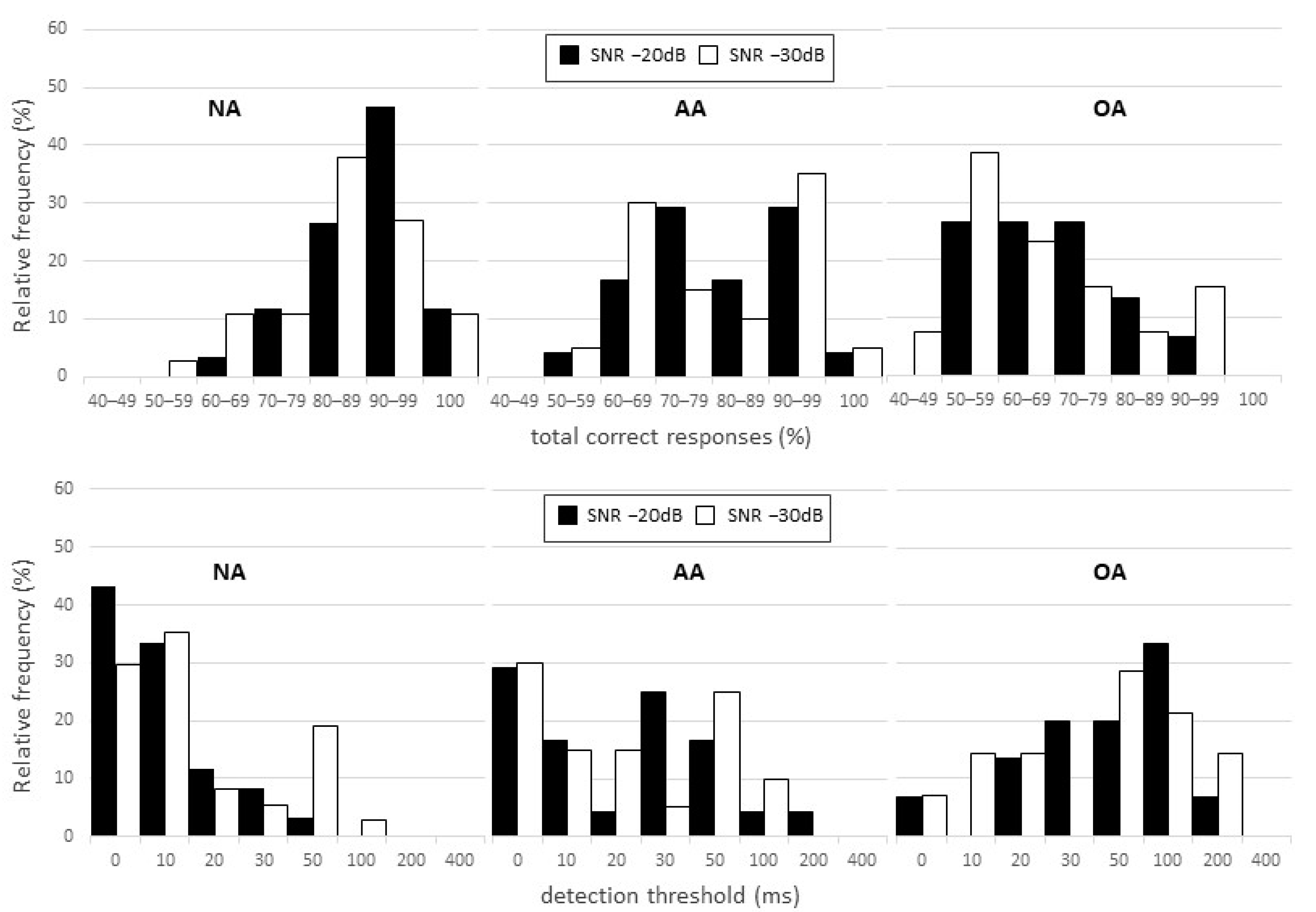
| SNR (dB) | Ear | Mean | SD | Minimum | Median | Maximum | |
|---|---|---|---|---|---|---|---|
| BMTP | −20 | Right | 88.70 | 9.40 | 63.00 | 90.00 | 100 |
| Left | 89.66 | 9.04 | 65.00 | 93.33 | 100 | ||
| −30 | Right | 87.85 | 10.34 | 55.00 | 88.33 | 100 | |
| Left | 85.86 | 11.39 | 61.67 | 87.50 | 100 | ||
| BMTT | −20 | Right | 10.65 | 13.89 | 0 | 10 | 50 |
| Left | 8.97 | 9.76 | 0 | 10 | 30 | ||
| −30 | Right | 13.55 | 20.58 | 0 | 10 | 100 | |
| Left | 18.33 | 20.07 | 0 | 10 | 50 |
| SNR (dB) | Mean | SD | Minimum | Median | Maximum | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| AA/OA vs. NA | OA vs. AA | ||||||||
| BMTP | −20 | NA | 89.16 | 9.16 | 63.00 | 90.80 | 100 | -- | -- |
| AA | 80.61 | 13.33 | 55.00 | 80.82 | 100 | 0.003 * | 0.006 * | ||
| OA | 70.98 | 14.22 | 51.66 | 70.00 | 96.66 | <0.001 * | |||
| −30 | NA | 87.12 | 10.66 | 55.00 | 88.33 | 100 | -- | -- | |
| AA | 80.23 | 14.81 | 56.66 | 81.66 | 100 | 0.040 * | 0.002 * | ||
| OA | 66.47 | 16.01 | 45.00 | 61.66 | 93.33 | <0.001 * | |||
| BMTT | −20 | NA | 9.83 | 12.00 | 0 | 10 | 50 | -- | -- |
| AA | 30.83 | 43.43 | 0 | 25 | 200 | 0.014 * | 0.138 | ||
| OA | 58.24 | 52.11 | 0 | 50 | 200 | 0.013 * | |||
| −30 | NA | 15.31 | 20.32 | 0 | 10 | 100 | -- | -- | |
| AA | 28.50 | 31.33 | 0 | 20 | 100 | 0.058 | 0.001 * | ||
| OA | 65.88 | 61.14 | 0 | 50 | 200 | <0.001 * | |||
| SNR (dB) | Method | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) | Efficiency (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | OA | AA | OA | AA | OA | AA | OA | ||||
| BMTP | −20 | M − 2SD | 95 | 29 | 59 | 85 | 92 | 57 | 70 | 62 | 77 |
| P | 93 | 38 | 59 | 84 | 89 | 60 | 69 | 66 | 76 | ||
| −30 | M − 2SD | 94 | 30 | 59 | 83 | 91 | 57 | 70 | 62 | 76 | |
| P | 92 | 30 | 59 | 79 | 88 | 57 | 69 | 61 | 75 | ||
| BMTT | −20 | M + 2SD | 97 | 25 | 53 | 88 | 94 | 56 | 67 | 61 | 75 |
| P | 97 | 25 | 53 | 88 | 94 | 56 | 67 | 61 | 75 | ||
| −30 | M + 2SD | 98 | 10 | 35 | 83 | 95 | 52 | 60 | 54 | 67 | |
| P | 98 | 10 | 35 | 83 | 95 | 52 | 60 | 54 | 67 | ||
| GP | GT | PP | DP | ||
|---|---|---|---|---|---|
| BMTP (SNR −20 dB) | Coeff. | 0.572 | −0.545 | 0.289 | 0.355 |
| p | <0.001 * | <0.001 * | 0.008 * | <0.001 * | |
| BMTT (SNR −20 dB) | Coeff. | −0.549 | 0.486 | −0.366 | −0.211 |
| p | <0.001 * | <0.001 * | <0.001 * | 0.039 * | |
| BMTP (SNR −30 dB) | Coeff. | 0.549 | −0.510 | 0.421 | 0.419 |
| p | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| BMTT (SNR −30 dB) | Coeff. | −0.508 | 0.500 | −0.403 | −0.286 |
| p | <0.001 * | <0.001 * | <0.001 * | 0.009 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, R.; Leite Filho, C.A.; Barros, G.M.S.B.; Musiek, F.E.; Schochat, E. Evidence of Validity and Normative Values of a New Auditory Backward Masking Test. J. Clin. Med. 2022, 11, 4933. https://doi.org/10.3390/jcm11174933
Filippini R, Leite Filho CA, Barros GMSB, Musiek FE, Schochat E. Evidence of Validity and Normative Values of a New Auditory Backward Masking Test. Journal of Clinical Medicine. 2022; 11(17):4933. https://doi.org/10.3390/jcm11174933
Chicago/Turabian StyleFilippini, Renata, Carlos Alberto Leite Filho, Gabriela Melo Santos Bonassa Barros, Frank E. Musiek, and Eliane Schochat. 2022. "Evidence of Validity and Normative Values of a New Auditory Backward Masking Test" Journal of Clinical Medicine 11, no. 17: 4933. https://doi.org/10.3390/jcm11174933
APA StyleFilippini, R., Leite Filho, C. A., Barros, G. M. S. B., Musiek, F. E., & Schochat, E. (2022). Evidence of Validity and Normative Values of a New Auditory Backward Masking Test. Journal of Clinical Medicine, 11(17), 4933. https://doi.org/10.3390/jcm11174933






