Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
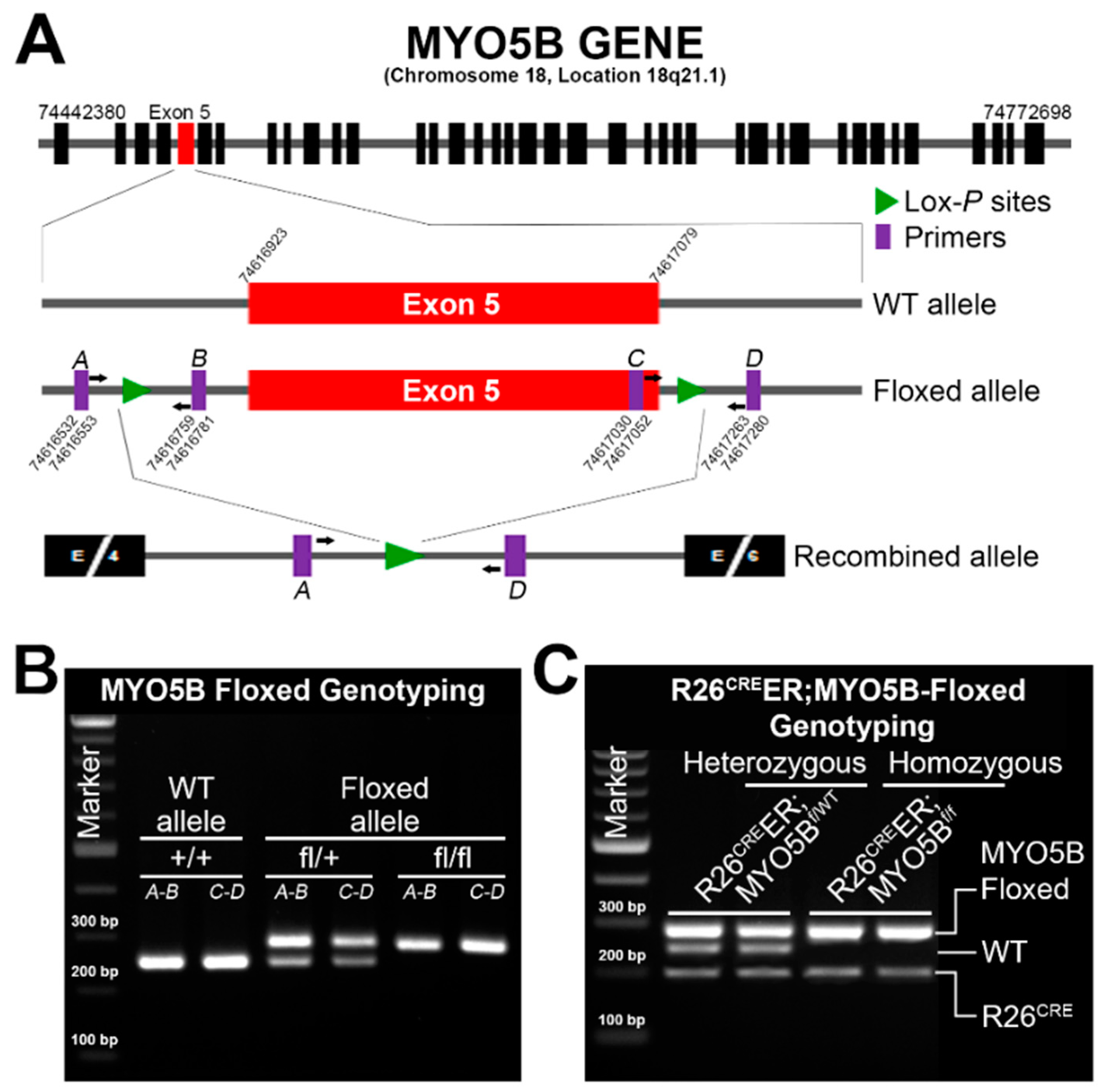
2.2. Generation of Tamoxifen-Inducible R26CreER;MYO5Bf/f Conditional Single Knockout (cMYO5BKO) and R26CreER;MYO5Bf/f;SGK1f/f Double Knockout (cSGK1/MYO5B-DKO)
2.3. Semiquantitative RT-PCR Analysis
2.4. Immunoblot Analysis
2.5. Hematoxylin and Eosin (H&E) Staining
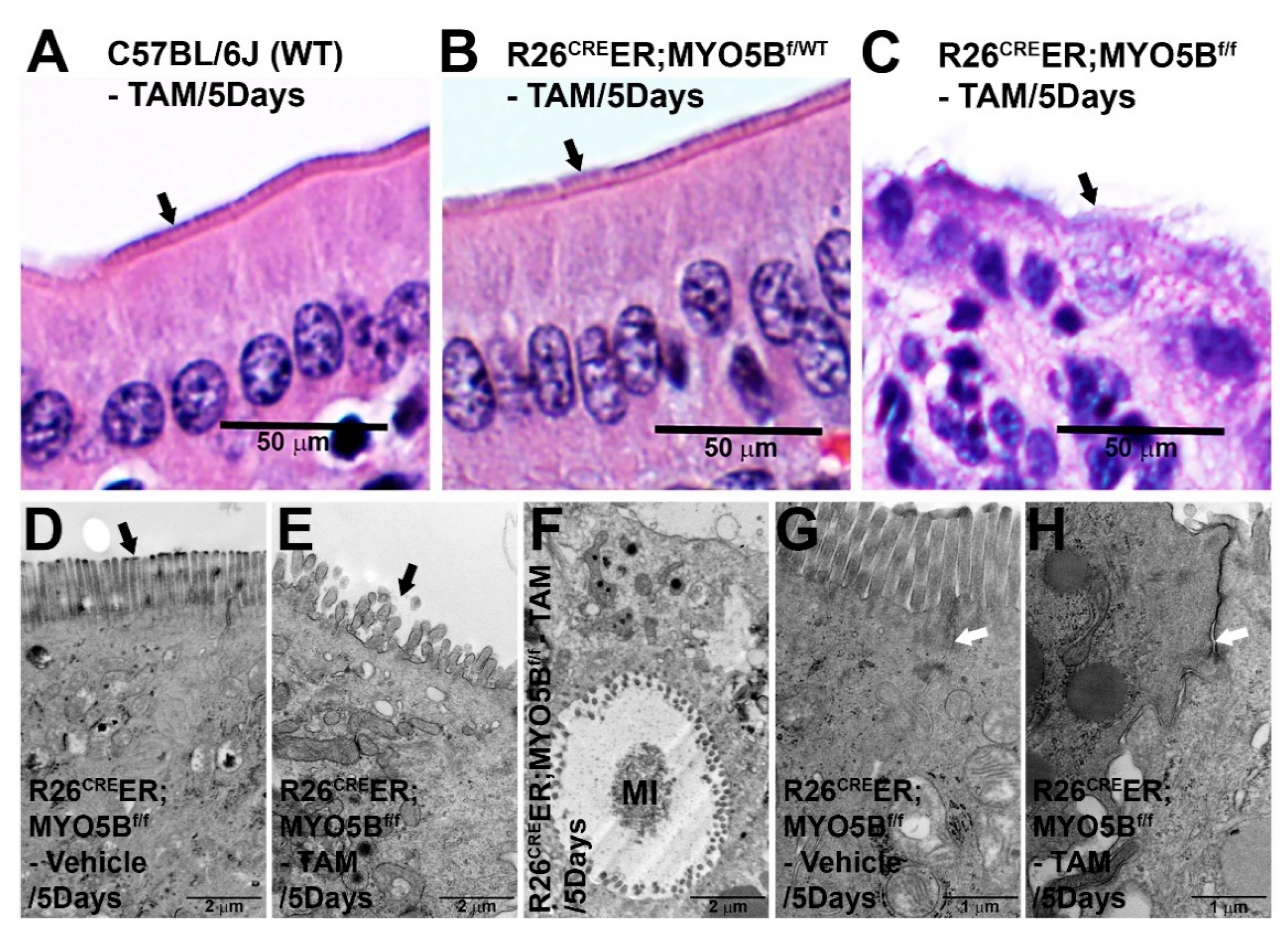
2.6. Transmission Electron Microscopy
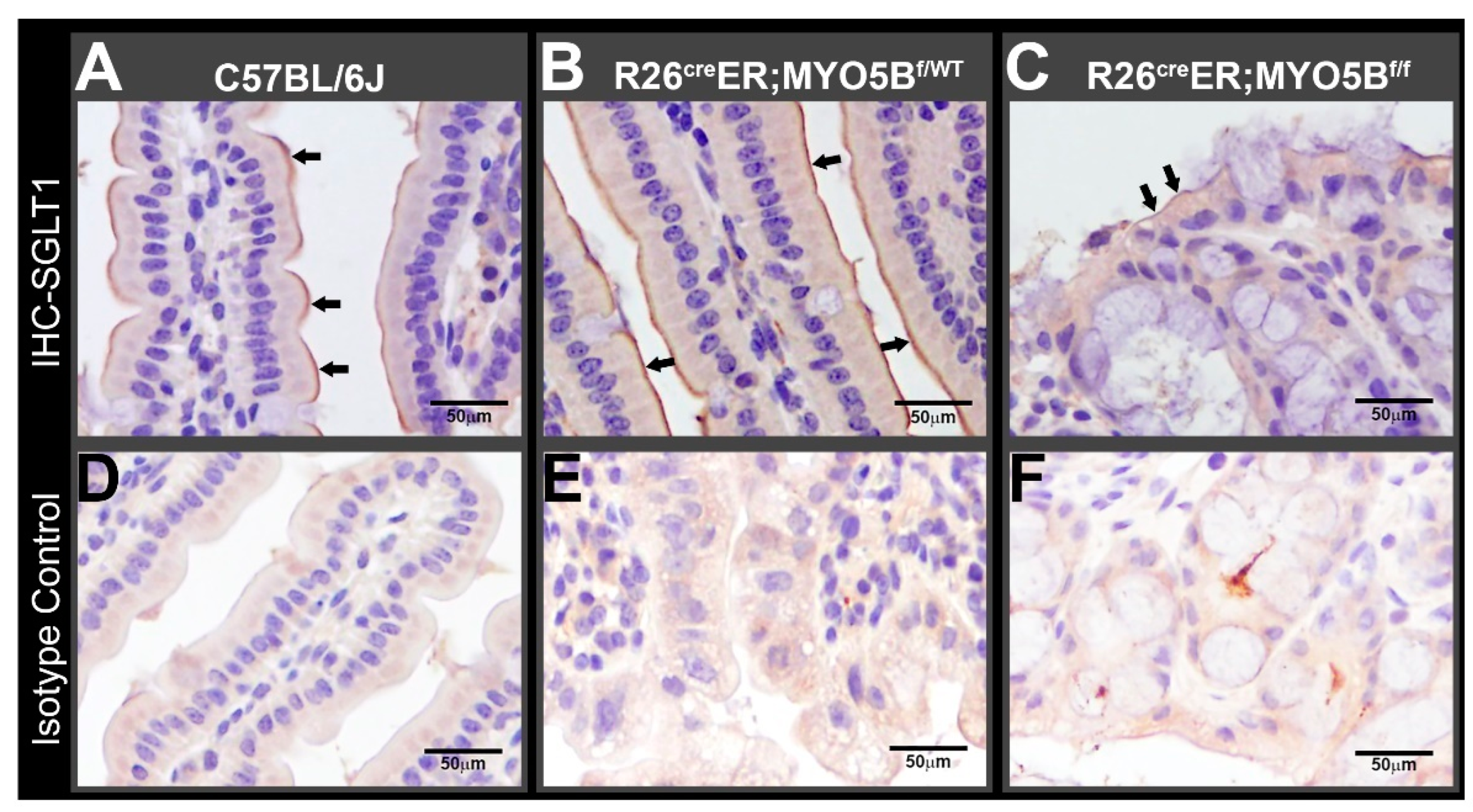
2.7. Immunohistochemistry
2.8. Tissue Preparation and Immunofluorescence Labeling
2.9. Fluorescence Microscopy
2.10. Glucose Measurement
2.11. Statistical Analysis
3. Results
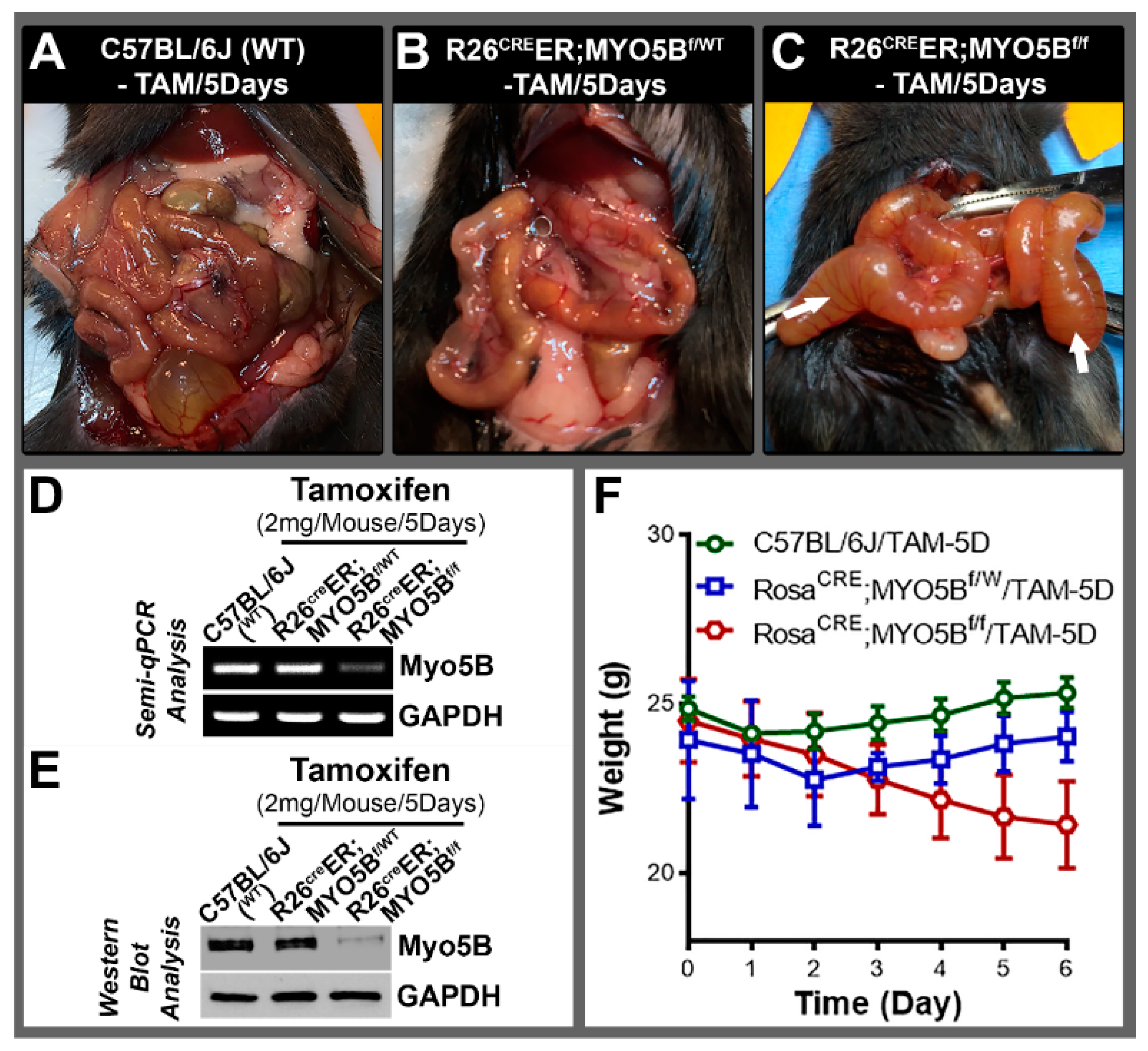
3.1. Generation and Characterization of a Conditional Tamoxifen-Inducible MYO5B Knock out Mouse Model of MVID
3.2. Tamoxifen Induction in R26CreER;MYO5Bf/f (cMYO5BKO) Mice Downregulates SGLT1 Expression and Supports Carbohydrate Malabsorption
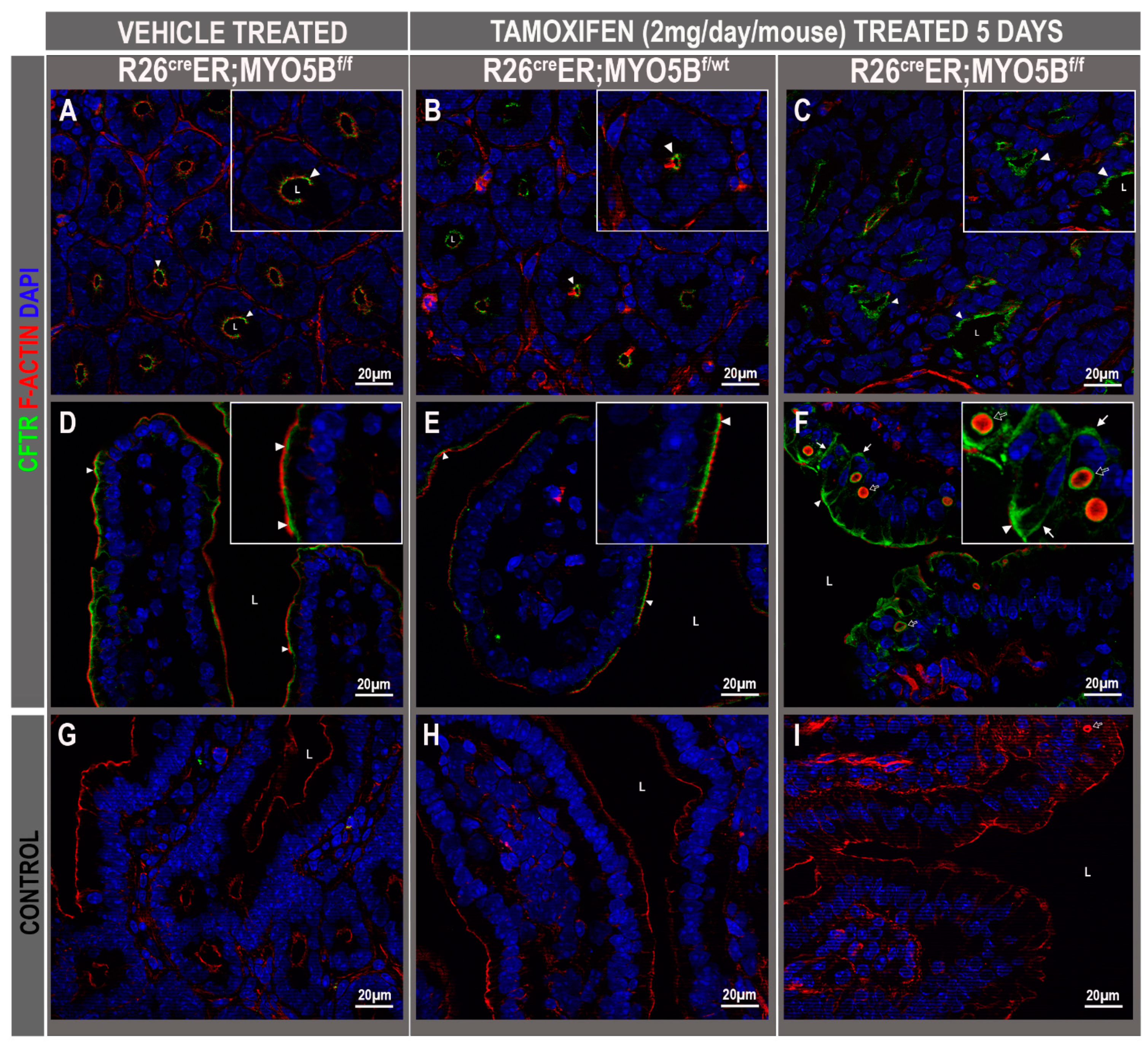
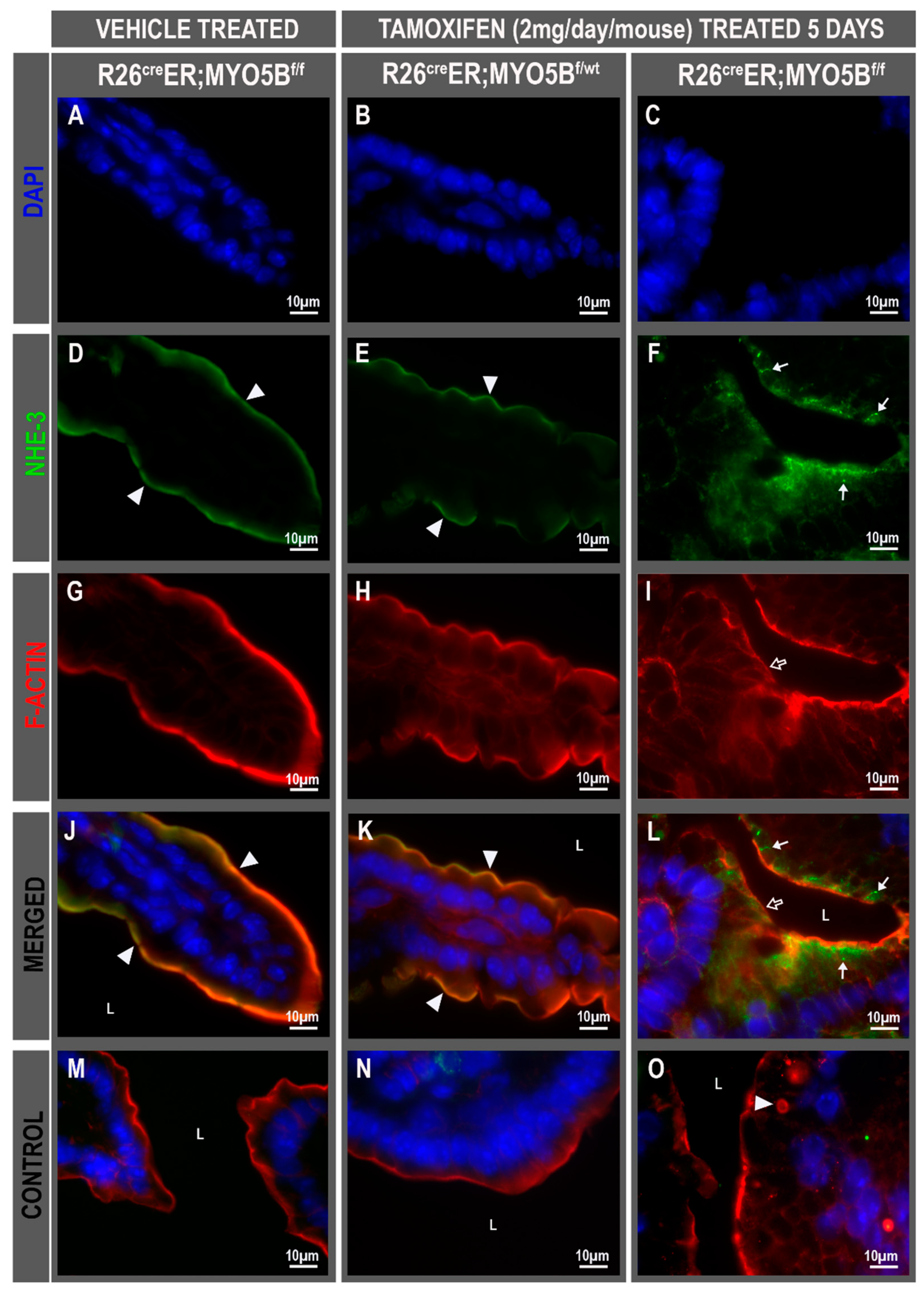
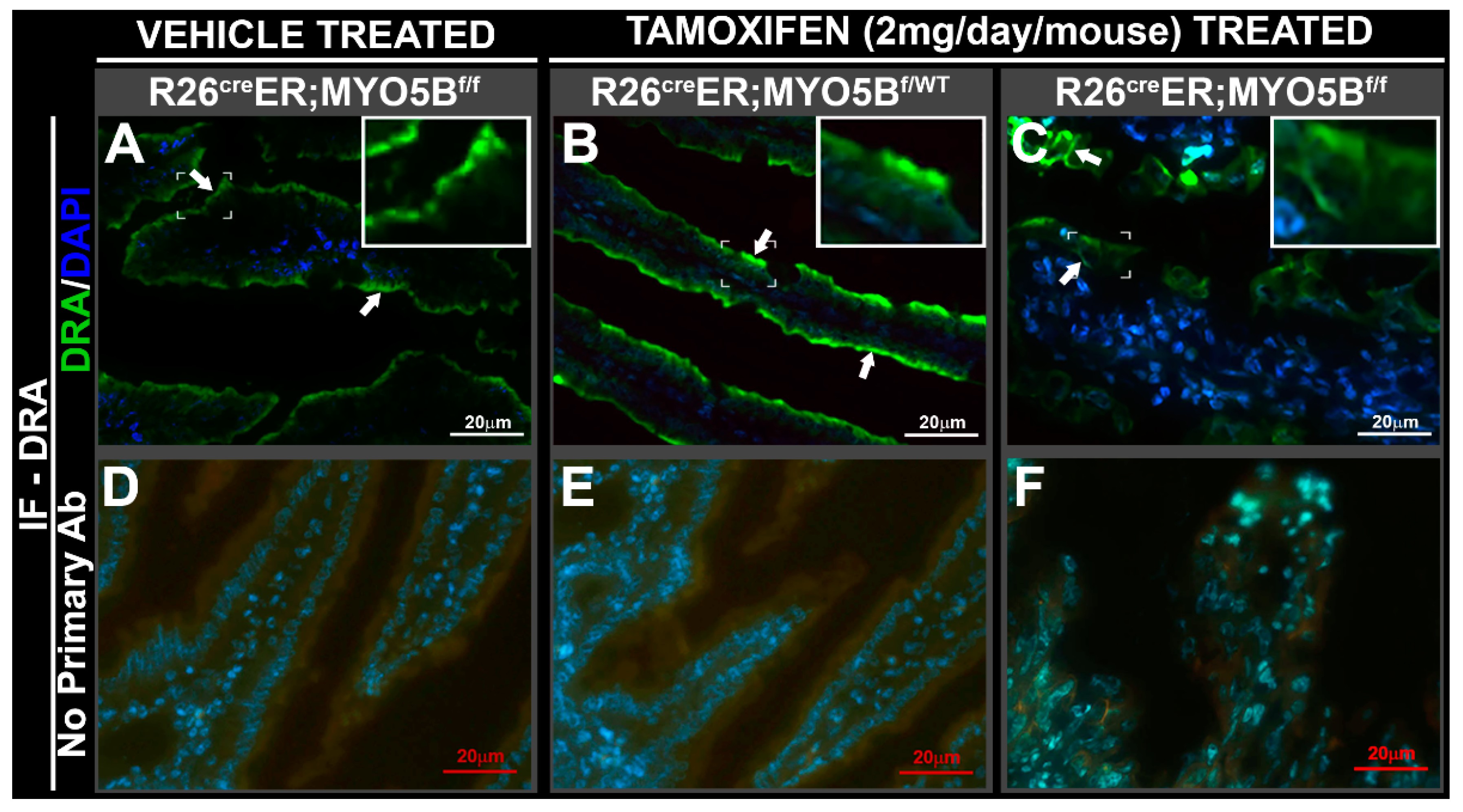
3.3. Tamoxifen Induction in R26CreER;MYO5Bf/f (cMYO5BKO) Mice Promotes Basolateral Redistribution of CFTR, Reduces Na+/H+ Exchanger 3 (NHE3) and Down-Regulated in Adenoma (DRA) Expression on the Villus Enterocyte Brush Border
3.4. Tamoxifen Induction in R26CreER;MYO5Bf/f (cMYO5BKO) Mice Increases SGK1 Phosphorylation in the Intestine
3.5. Immunoblot Changes in Protein Expression of Ion Transporters and Signaling Kinases in the Small Intestine of R26CreER;MYO5Bf/f (cMYO5B KO) Mice
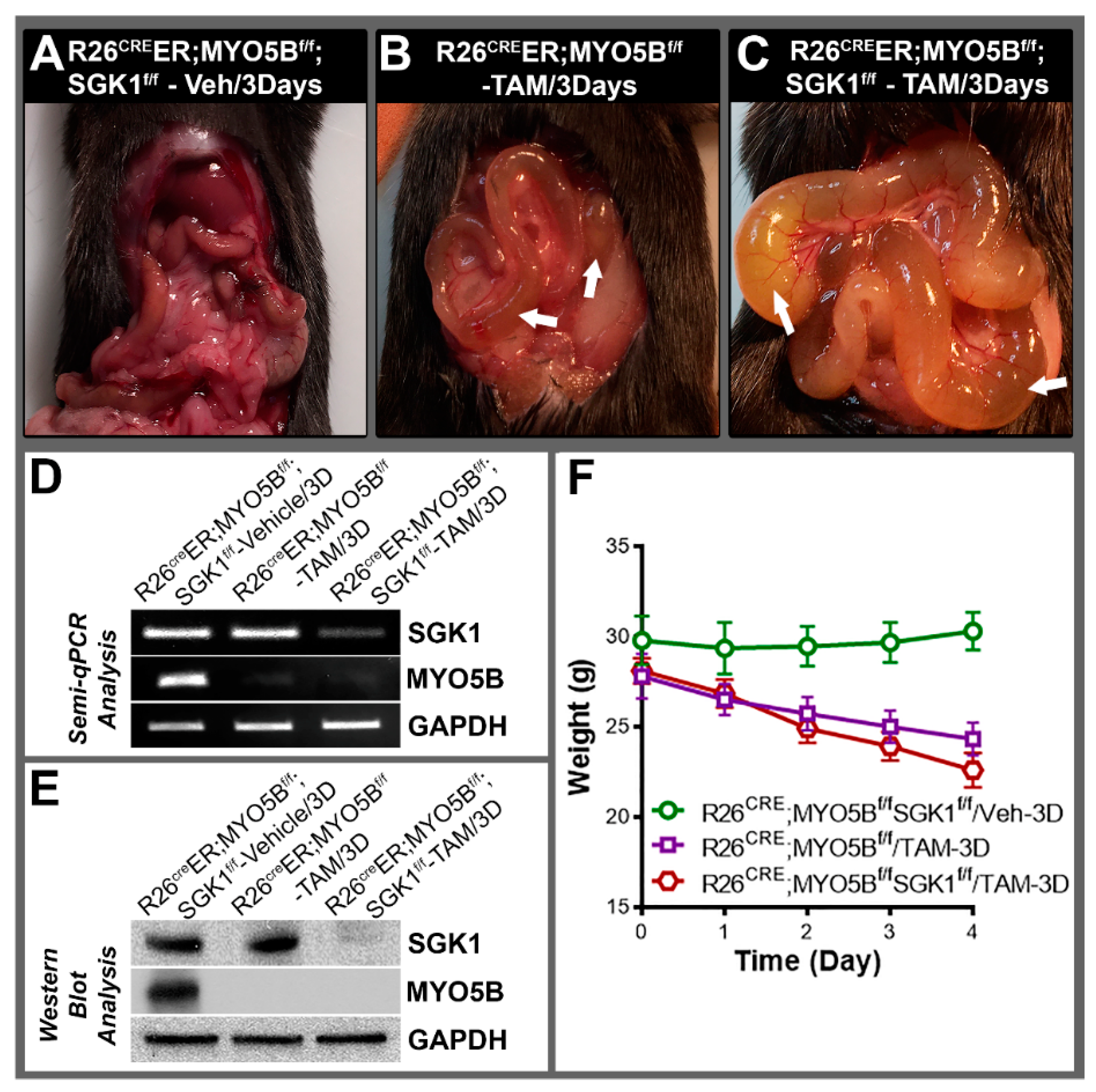
3.6. Conditional MYO5B-SGK1-DKO (cSGK1/MYO5B-DKO) Mice Display More Severe Diarrhea Compared with cMYO5BKO Mice
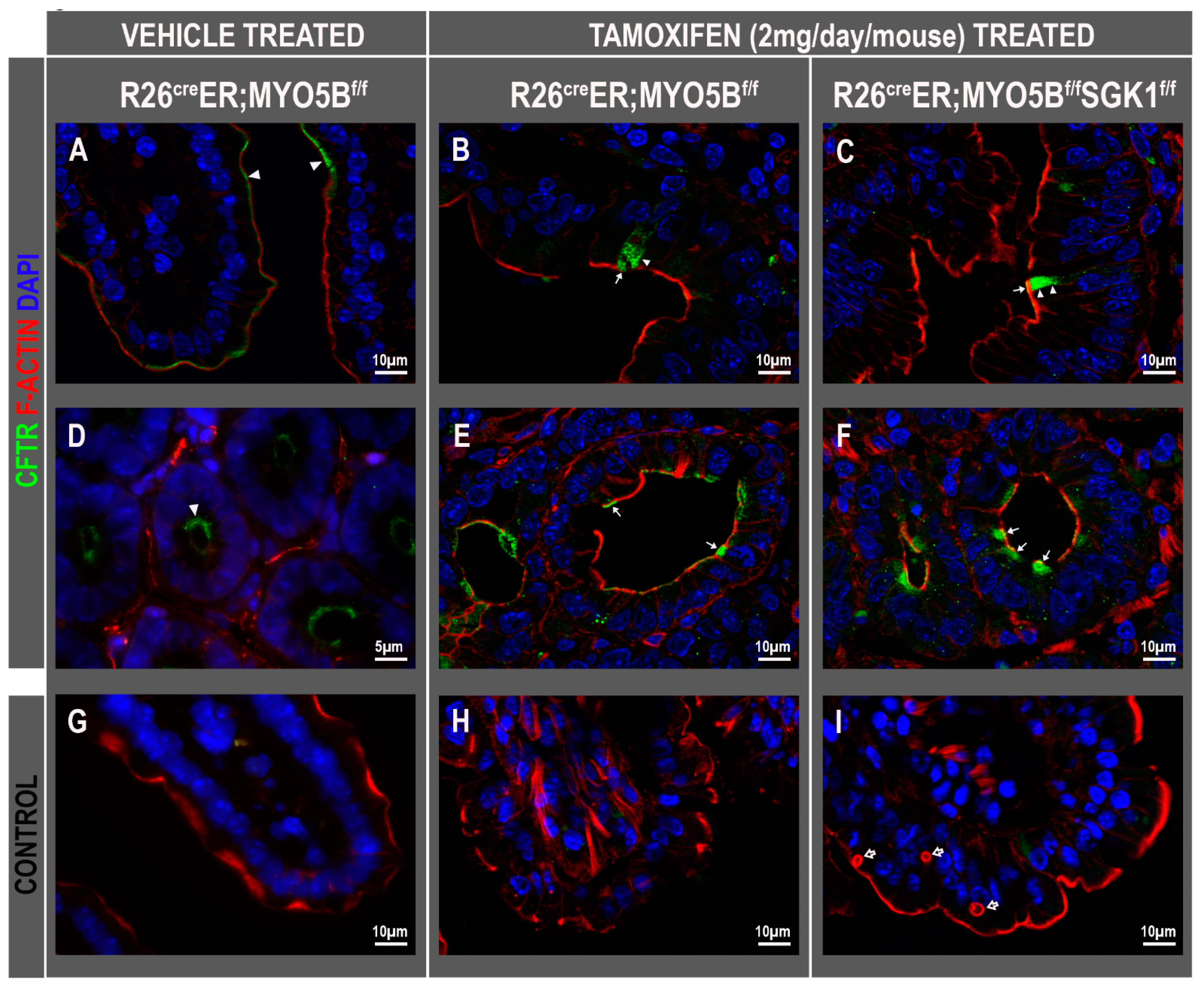
3.7. CFTR High Expresser (CHE) Cells Are Present in cMYO5BKO and cMYO5B-SGK1-DKO Mice
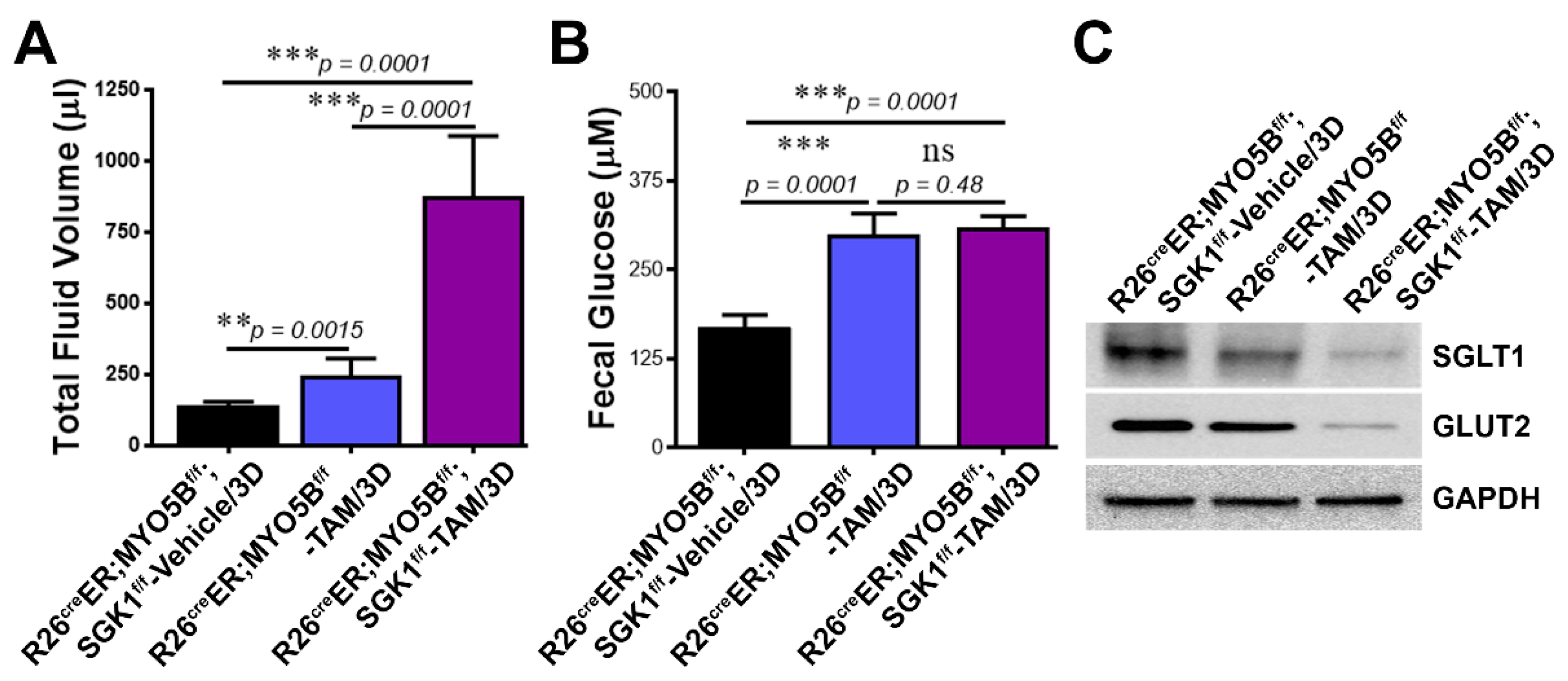
3.8. Loss of SGK1 Leads to Further Reduction of Glucose Transporter Expression and Increased Intestinal Fluid Loss in MVID
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruemmele, F.M.; Muller, T.; Schiefermeier, N.; Ebner, H.L.; Lechner, S.; Pfaller, K.; Thoni, C.E.; Goulet, O.; Lacaille, F.; Schmitz, J.; et al. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum. Mutat. 2010, 31, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Hess, M.W.; Schiefermeier, N.; Pfaller, K.; Ebner, H.L.; Heinz-Erian, P.; Ponstingl, H.; Partsch, J.; Rollinghoff, B.; Kohler, H.; et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 2008, 40, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Cutz, E.; Rhoads, J.M.; Drumm, B.; Sherman, P.M.; Durie, P.R.; Forstner, G.G. Microvillus inclusion disease: An inherited defect of brush-border assembly and differentiation. N. Engl. J. Med. 1989, 320, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Vogler, R.C.; Lacey, S.R.; Reddick, R.L.; Keku, E.O.; Azizkhan, R.G.; Berschneider, H.M. Microvillus inclusion disease. In vitro jejunal electrolyte transport. Gastroenterology 1991, 100, 811–817. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Engevik, M.A.; Meyer, A.R.; Weis, V.G.; Goldstein, A.; Hess, M.W.; Muller, T.; Koepsell, H.; Dudeja, P.K.; et al. Loss of MYO5B Leads to Reductions in Na(+) Absorption With Maintenance of CFTR-Dependent Cl(−) Secretion in Enterocytes. Gastroenterology 2018, 155, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.C.; Roland, J.T.; Krishnan, M.; Tyska, M.J.; Lapierre, L.A.; Dickman, P.S.; Goldenring, J.R.; Shub, M.D. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J. Clin. Inves. 2014, 124, 2947–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiatecka-Urban, A.; Talebian, L.; Kanno, E.; Moreau-Marquis, S.; Coutermarsh, B.; Hansen, K.; Karlson, K.H.; Barnaby, R.; Cheney, R.E.; Langford, G.M.; et al. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J. Biol. Chem. 2007, 282, 23725–23736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen, N.; Salas, P. Microvillus Inclusion Disease: A Genetic Defect Affecting Apical Membrane Protein Traffic in Intestinal Epithelium. Traffic 2000, 1, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, D.; Mashukova, A.; Forteza, R.; Rodriguez, M.M.; Ameen, N.A.; Salas, P.J. Myosin 5b loss of function leads to defects in polarized signaling: Implication for microvillus inclusion disease pathogenesis and treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G992–G1001. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.; Shumilina, E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J. 2013, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Daidie, D.; Li, H.; Pao, A.C.; LaGrange, L.P.; Wang, J.; Vandewalle, A.; Stockand, J.D.; Staub, O.; Pearce, D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol. Endocrinol. 2005, 19, 3073–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahammer, F.; Henke, G.; Sandu, C.; Rexhepaj, R.; Hussain, A.; Friedrich, B.; Risler, T.; Metzger, M.; Just, L.; Skutella, T.; et al. Intestinal function of gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1114–G1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rexhepaj, R.; Rotte, A.; Pasham, V.; Gu, S.; Kempe, D.S.; Lang, F. PI3 kinase and PDK1 in the regulation of the electrogenic intestinal dipeptide transport. Cell. Physiol. Biochem. 2010, 25, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.F.; Klee, K.M.C.; Janecke, A.; Muller, T.; Hess, M.W.; Huber, L.A. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7 and Syntaxin 3. J. Cell Biol. 2015, 211, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, D.V.; Ahsan, M.K.; Kumari, V.; van Ijzendoorn, S.C.; Reyes-Mugica, M.; Kumar, A.; Gujral, T.; Dudeja, P.K.; Ameen, N.A. Identification of intestinal ion transport defects in microvillus inclusion disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G142–G155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engevik, A.C.; Coutts, A.W.; Kaji, I.; Rodriguez, P.; Ongaratto, F.; Saqui-Salces, M.; Medida, R.L.; Meyer, A.R.; Kolobova, E.; Engevik, M.A.; et al. Editing Myosin VB Gene to Create Porcine Model of Microvillus Inclusion Disease, with Microvillus-Lined Inclusions and Alterations in Sodium Transporters. Gastroenterology 2020, 158, 2236–2249. [Google Scholar] [CrossRef]
- Schneeberger, K.; Vogel, G.F.; Teunissen, H.; van Ommen, D.D.; Begthel, H.; El Bouazzaoui, L.; van Vugt, A.H.; Beekman, J.M.; Klumperman, J.; Muller, T.; et al. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc. Natl. Acad. Sci. USA 2015, 112, 12408–12413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiagarajah, J.R.; Ko, E.A.; Tradtrantip, L.; Donowitz, M.; Verkman, A.S. Discovery and development of antisecretory drugs for treating diarrheal diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Muller, T.; Wijmenga, C.; Phillips, A.D.; Janecke, A.; Houwen, R.H.; Fischer, H.; Ellemunter, H.; Fruhwirth, M.; Offner, F.; Hofer, S.; et al. Congenital sodium diarrhea is an autosomal recessive disorder of sodium/proton exchange but unrelated to known candidate genes. Gastroenterology 2000, 119, 1506–1513. [Google Scholar] [CrossRef]
- Janecke, A.R.; Heinz-Erian, P.; Yin, J.; Petersen, B.S.; Franke, A.; Lechner, S.; Fuchs, I.; Melancon, S.; Uhlig, H.H.; Travis, S.; et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 2015, 24, 6614–6623. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.K.; Figueroa-Hall, L.; Baratta, V.; Garcia-Milian, R.; Lam, T.T.; Hoque, K.; Salas, P.J.; Ameen, N.A. Glucocorticoids and serum- and glucocorticoid-inducible kinase 1 are potent regulators of CFTR in the native intestine: Implications for stress-induced diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G121–G132. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Jaenisch, R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 2014, 9, 1956–1968. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press Cold Spring: Harbor, NY, USA, 2003. [Google Scholar]
- Collaco, A.; Jakab, R.; Hegan, P.; Mooseker, M.; Ameen, N. Alpha-AP-2 directs myosin VI-dependent endocytosis of cystic fibrosis transmembrane conductance regulator chloride channels in the intestine. J. Biol. Chem. 2010, 285, 17177–17187. [Google Scholar] [CrossRef] [Green Version]
- Carton-Garcia, F.; Overeem, A.W.; Nieto, R.; Bazzocco, S.; Dopeso, H.; Macaya, I.; Bilic, J.; Landolfi, S.; Hernandez-Losa, J.; Schwartz, S.; et al. Myo5b knockout mice as a model of microvillus inclusion disease. Sci. Rep. 2015, 5, 12312. [Google Scholar] [CrossRef] [PubMed]
- Weis, G.V.; Knowles, B.C.; Choi, E.; Goldstein, A.E.; Williams, J.A.; Manning, E.H.; Roland, J.T.; Lapierre, L.A.; Goldenring, J.R. Loss of MYO5B in mice recapitulates Microvillus Inclusion Disease and reveals an apical trafficking pathway distinct to neonatal duodenum. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 131–157. [Google Scholar] [CrossRef] [Green Version]
- Forteza, R.; Ahsan, M.K.; Carton-Garcia, F.; Arango, D.; Ameen, N.A.; Salas, P.J. Glucocorticoids and myosin5b loss of function induce heightened PKA signaling in addition to membrane traffic defects. Mol. Biol. Cell. 2019, 30, 3076–3089. [Google Scholar] [CrossRef]
- Dieter, M.; Palmada, M.; Rajamanickam, J.; Aydin, A.; Busjahn, A.; Boehmer, C.; Luft, F.C.; Lang, F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes. Res. 2004, 12, 862–870. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Webster, M.K.; Goya, L.; Ge, Y.; Maiyar, A.C.; Firestone, G.L. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993, 13, 2031–2040. [Google Scholar]
- Webster, M.K.; Goya, L.; Firestone, G.L. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J. Biol. Chem. 1993, 268, 11482–11485. [Google Scholar] [CrossRef]
- Lang, F.; Bohmer, C.; Palmada, M.; Seebohm, G.; Strutz-Seebohm, N.; Vallon, V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 2006, 86, 1151–1178. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Stournaras, C.; Alesutan, I. Regulation of transport across cell membranes by the serum- and glucocorticoid-inducible kinase SGK1. Mol. Membr. Biol. 2014, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Baines, D.L.; Wilson, S.M. The phosphorylation of endogenous Nedd4-2 In Na(+)-absorbing human airway epithelial cells. Eur. J. Pharmacol. 2014, 732, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Kawabe, H.; Rotin, D. The Ubiquitin Ligase Nedd4L Regulates the Na/K/2Cl Co-transporter NKCC1/SLC12A2 in the Colon. J. Biol. Chem. 2017, 292, 3137–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Sun, H.; Lang, F.; Yun, C.C. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am. J. Physiol. Cell. Physiol. 2005, 289, C802–C810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhang, H.; Lang, F.; Yun, C.C. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am. J. Physiol. Cell. Physiol. 2007, 292, C396–C404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomberger, J.M.; Coutermarsh, B.A.; Barnaby, R.L.; Sato, J.D.; Chapline, M.C.; Stanton, B.A. Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval. PLoS ONE 2014, 9, e89599. [Google Scholar] [CrossRef] [Green Version]
- Ameen, N.A.; Ardito, T.; Kashgarian, M.; Marino, C.R. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 1995, 108, 1016–1023. [Google Scholar] [CrossRef]
- Ameen, N.A.; Alexis, J.; Salas, P.J. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem. Cell Biol. 2000, 114, 69–75. [Google Scholar] [CrossRef]
- Jakab, R.L.; Collaco, A.M.; Ameen, N.A. Characterization of CFTR High Expresser cells in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G453–G465. [Google Scholar] [CrossRef] [Green Version]
- Ameen, N.A.; van Donselaar, E.; Posthuma, G.; de Jonge, H.; McLaughlin, G.; Geuze, H.J.; Marino, C.; Peters, P.J. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem. Cell. Biol. 2000, 114, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ameen, N.A.; Martensson, B.; Bourguinon, L.; Marino, C.; Isenberg, J.; McLaughlin, G.E. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J. Cell. Sci. 1999, 112, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Collaco, A.M.; Ameen, N.A. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G82–G98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiagarajah, J.R.; Kamin, D.S.; Acra, S.; Goldsmith, J.D.; Roland, J.T.; Lencer, W.I.; Muise, A.M.; Goldenring, J.R.; Avitzur, Y.; Martin, M.G.; et al. Advances in Evaluation of Chronic Diarrhea in Infants. Gastroenterology 2018, 154, 2045–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Mosa, M.H.; Nicolle, O.; Maschalidi, S.; Sepulveda, F.E.; Bidaud-Meynard, A.; Menche, C.; Michels, B.E.; Michaux, G.; de Saint Basile, G.; Farin, H.F. Dynamic Formation of Microvillus Inclusions During Enterocyte Differentiation in Munc18-2-Deficient Intestinal Organoids. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 477–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busslinger, G.A.; Weusten, B.L.A.; Bogte, A.; Begthel, H.; Brosens, L.A.A.; Clevers, H. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell. Rep. 2021, 34, 108819. [Google Scholar] [CrossRef]
- Tuo, B.; Wen, G.; Zhang, Y.; Liu, X.; Wang, X.; Dong, H. Involvement of phosphatidylinositol 3-kinase in cAMP- and cGMP-induced duodenal epithelial CFTR activation in mice. Am. J. Physiol. Cell. Physiol. 2009, 297, C503–C515. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, D.; Alrefai, W.A.; Dudeja, P.K.; Gill, R.K. Recent advances in understanding and managing malabsorption: Focus on microvillus inclusion disease. F1000 Res. 2019, 8, 20762.1. [Google Scholar] [CrossRef] [Green Version]


| Recombination Template Sequences |
|---|
| MYO5B 5′loxP template: (forward strand) 5′GAAGACCCTTGTTCTTATCAGAAAACTTATAGGGAATTCAGTGGCACATAGTAGGCCTGTATAACTTCGTATAATGTATGCTATACGAAGTTATTGTAGGAACGAAGACAAAAGGATAGATGTTCTTAGGACAATGGGCATCATGTCCCAGTTT 3′ |
| MYO5B 3′loxP template: (reverse strand) 5′CCATCACTACAGCACGCACAGGGTCCAAGGACACAGAGAGCATGAAGACGGTCGACCTAAATAACTTCGTATAGCATACATTATACGAAGTTATCACAGGGCCCTGCAGGACGATTATCTTTAACCCTGGGTTTCAGAGGTACCTCCACCCTCT 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.K.; dos Reis, D.C.; Barbieri, A.; Sumigray, K.D.; Nottoli, T.; Salas, P.J.; Ameen, N.A. Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID). J. Clin. Med. 2022, 11, 4179. https://doi.org/10.3390/jcm11144179
Ahsan MK, dos Reis DC, Barbieri A, Sumigray KD, Nottoli T, Salas PJ, Ameen NA. Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID). Journal of Clinical Medicine. 2022; 11(14):4179. https://doi.org/10.3390/jcm11144179
Chicago/Turabian StyleAhsan, Md Kaimul, Diego Carlos dos Reis, Andrea Barbieri, Kaelyn D. Sumigray, Timothy Nottoli, Pedro J. Salas, and Nadia A. Ameen. 2022. "Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID)" Journal of Clinical Medicine 11, no. 14: 4179. https://doi.org/10.3390/jcm11144179
APA StyleAhsan, M. K., dos Reis, D. C., Barbieri, A., Sumigray, K. D., Nottoli, T., Salas, P. J., & Ameen, N. A. (2022). Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID). Journal of Clinical Medicine, 11(14), 4179. https://doi.org/10.3390/jcm11144179






