Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases
Abstract
1. Introduction
1.1. The Gut Microbiota
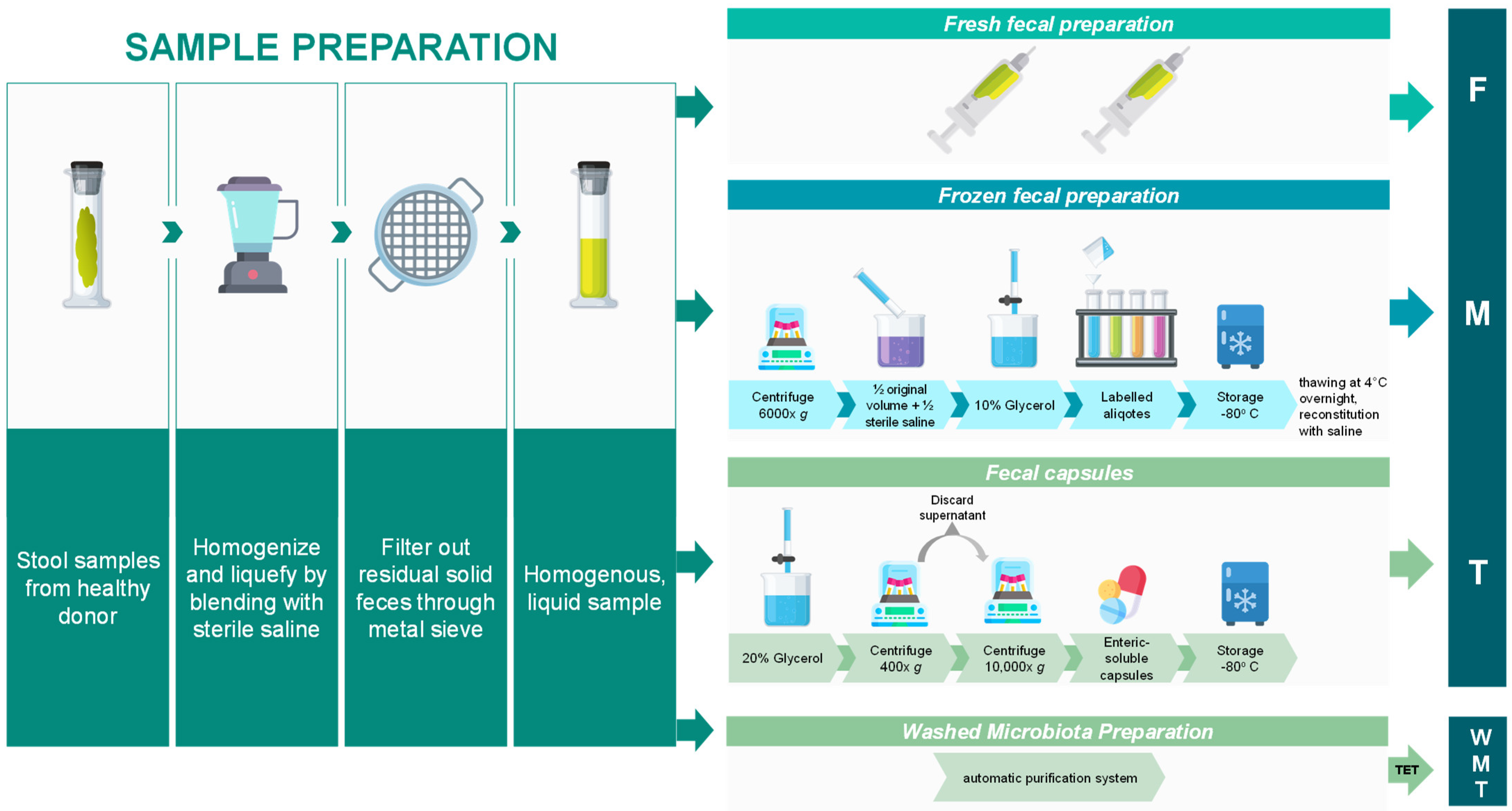
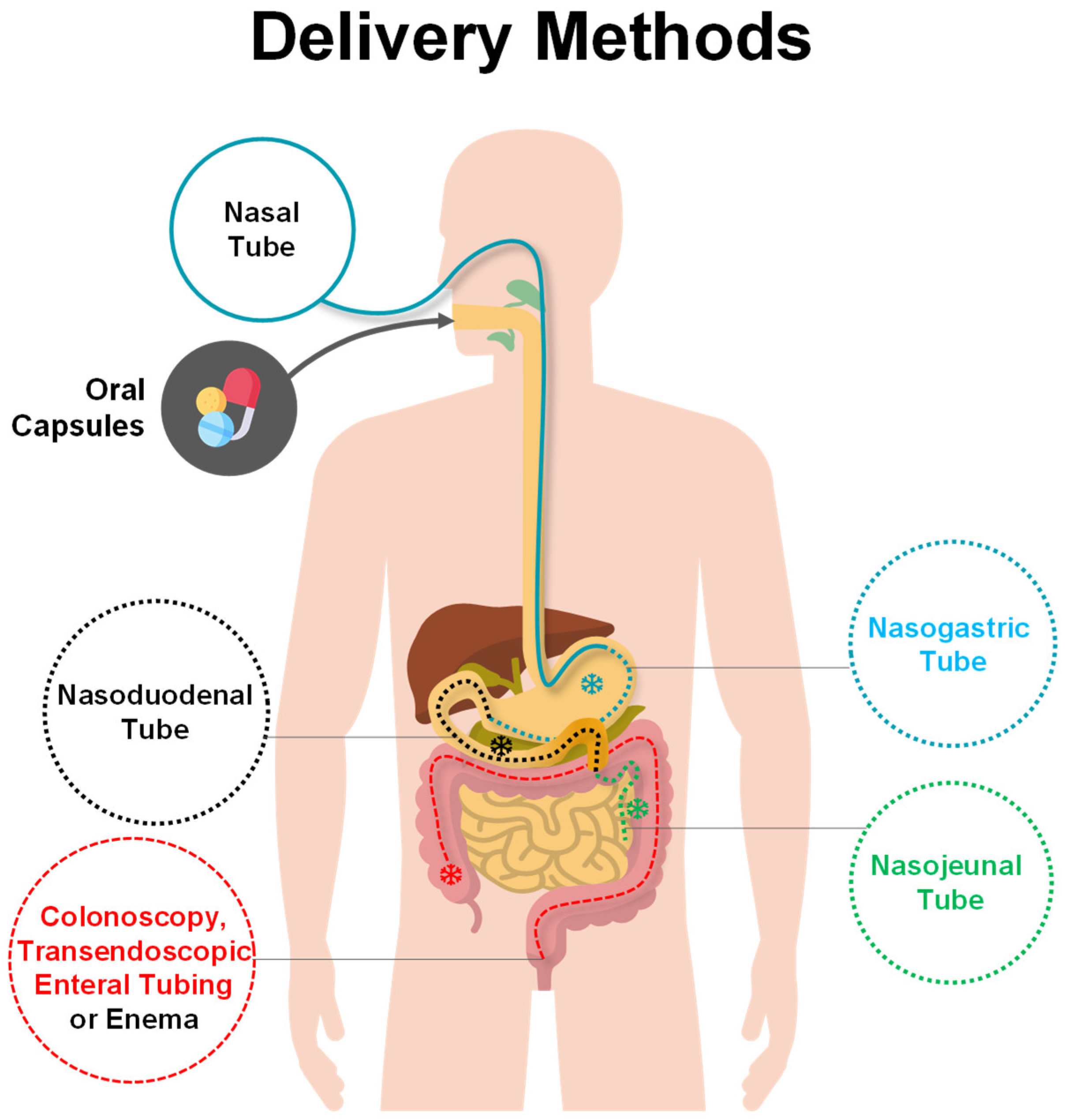
1.2. Fecal Microbiota Transplantation: Advantages, Preparation and Delivery Methods
2. Fecal Microbiota Transplantation in Human Diseases
2.1. Fecal Microbiota Transplantation: First Use for C. difficile Infections
2.2. Fecal Microbiota Transplantation in Gastrointestinal Diseases
2.2.1. Inflammatory Bowel Disease
2.2.2. Irritable Bowel Syndrome
2.2.3. Other Gastrointestinal Diseases
2.3. Fecal Microbiota Transplantation in Liver Diseases
2.4. Fecal Microbiota Transplantation in Obesity and Metabolic Disorders
2.5. Fecal Microbiota Transplantation in Cancer Diseases
2.6. Fecal Microbiota Transplantation in Auto-Immune, Inflammatory and Infectious Diseases
2.7. Fecal Microbiota Transplantation in Cardio-Vascular Diseases
2.8. Fecal Microbiota Transplantation in Brain Diseases
3. Divergent Clinical Response to Fecal Microbiota Transplantation and the Super-Donor Phenomenon
4. Limitations of Current Microbiota Profiling Based on 16S rRNA Gene Sequencing
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Mao, Y.K.; Kasper, D.L.; Wang, B.; Forsythe, P.; Bienenstock, J.; Kunze, W.A. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 2013, 4, 1465. [Google Scholar] [CrossRef]
- Knights, D.; Silverberg, M.S.; Weersma, R.K.; Gevers, D.; Dijkstra, G.; Huang, H.; Tyler, A.D.; van Sommeren, S.; Imhann, F.; Stempak, J.M.; et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Leung, R.K.; Guan, W.; Au, W.W. Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018, 10, 3. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Gulden, E.; Wong, F.S.; Wen, L. The gut microbiota and Type 1 Diabetes. Clin. Immunol. 2015, 159, 143–153. [Google Scholar] [CrossRef]
- Pop, M.; Walker, A.W.; Paulson, J.; Lindsay, B.; Antonio, M.; Hossain, M.A.; Oundo, J.; Tamboura, B.; Mai, V.; Astrovskaya, I.; et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014, 15, R76. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salojarvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; de Vos, W.M. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef]
- Manrique, P.; Dills, M.; Young, M.J. The Human Gut Phage Community and Its Implications for Health and Disease. Viruses 2017, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Deidda, G.; Biazzo, M. Gut and Brain: Investigating Physiological and Pathological Interactions Between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases. Front. Neurosci. 2021, 15, 753915. [Google Scholar] [CrossRef]
- Gorski, A.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Rogoz, P.; Jonczyk-Matysiak, E.; Dabrowska, K.; Majewska, J.; Borysowski, J. Phage Therapy: Combating Infections with Potential for Evolving from Merely a Treatment for Complications to Targeting Diseases. Front. Microbiol. 2016, 7, 1515. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Gupta, R.; Prasad, Y. Efficacy of polyvalent bacteriophage P-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr. Microbiol. 2011, 62, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.W. Dead bugs don’t mutate: Susceptibility issues in the emergence of bacterial resistance. Emerg. Infect. Dis. 2003, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Thomas-Abedon, C. Phage therapy pharmacology. Curr. Pharm. Biotechnol. 2010, 11, 28–47. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Bakker, G.J.; Nieuwdorp, M. Fecal Microbiota Transplantation: Therapeutic Potential for a Multitude of Diseases beyond Clostridium difficile. Microbiol. Spectr. 2017, 5, 1–14. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. Fecal Microbiota Transplantation and Its Usage in Neuropsychiatric Disorders. Clin. Psychopharmacol. Neurosci. Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2016, 14, 231–237. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Ofosu, A.; Ofori, E.; Reddy, M. Fecal microbiota transplantation: Donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann. Gastroenterol. 2019, 32, 30–38. [Google Scholar] [CrossRef]
- Rossen, N.G.; MacDonald, J.K.; de Vries, E.M.; D’Haens, G.R.; de Vos, W.M.; Zoetendal, E.G.; Ponsioen, C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 2015, 21, 5359–5371. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Zhou, Y.L.; Zhao, H.L.; Xu, J.; Shou, D.W.; Liu, Y.D.; Zhou, Y.J.; Nie, Y.Q. Fecal Microbiota Transplantation: A New Therapeutic Attempt from the Gut to the Brain. Gastroenterol. Res. Pract. 2021, 2021, 6699268. [Google Scholar] [CrossRef] [PubMed]
- Marotz, C.A.; Zarrinpar, A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J. Biol. Med. 2016, 89, 383–388. [Google Scholar] [PubMed]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Korzenik, J.R.; Hamilton, M.J. Fecal microbiota transplantation via colonoscopy for recurrent C. difficile Infection. J. Vis. Exp. 2014, 94, 52154. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Salzman, N.H.; Acharya, C.; Sterling, R.K.; White, M.B.; Gavis, E.A.; Fagan, A.; Hayward, M.; Holtz, M.L.; Matherly, S.; et al. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology 2019, 70, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, G.; Zhao, Z.; Liu, Y.; Shen, Q.; Li, P.; Chen, Y.; Yin, H.; Wang, H.; Marcella, C.; et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell. 2020, 11, 251–266. [Google Scholar] [CrossRef]
- Nanjing consensus on methodology of washed microbiota transplantation Chin. Med. J. 2020, 133, 2330–2332. [CrossRef]
- Jimoh, O.; Gachette, D.; Bakhit, M.; Kelly, C.R. Fecal Microbiota Transplant via Retention Enema in Severe Clostridium difficile Infection: 203. Off. J. Am. Coll. Gastroenterol. 2018, 113, S119–S120. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Buch, H.; Wen, Q.; Long, C.; Cui, B.; Zhang, F. Colonic Transendoscopic Enteral Tubing: Route for a Novel, Safe, and Convenient Delivery of Washed Microbiota Transplantation in Children. Gastroenterol. Res. Pract. 2021, 2021, 6676962. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xiang, J.; He, Z.; Zhang, T.; Xu, L.; Cui, B.; Li, P.; Huang, G.; Ji, G.; Nie, Y.; et al. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc. Int. Open 2016, 4, E610–E613. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Vazquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome with Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef] [PubMed]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: A randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755. [Google Scholar] [CrossRef]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Oines, M.N.; Wiig, H.; Rose, O.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef]
- Friedman-Korn, T.; Livovsky, D.M.; Maharshak, N.; Aviv Cohen, N.; Paz, K.; Bar-Gil Shitrit, A.; Goldin, E.; Koslowsky, B. Fecal Transplantation for Treatment of Clostridium Difficile Infection in Elderly and Debilitated Patients. Dig. Dis. Sci. 2018, 63, 198–203. [Google Scholar] [CrossRef]
- Fischer, M.; Kao, D.; Kassam, Z.; Smith, J.; Louie, T.; Sipe, B.; Torbeck, M.; Xu, H.; Ouyang, F.; Mozaffarian, D.; et al. Stool Donor Body Mass Index Does Not Affect Recipient Weight After a Single Fecal Microbiota Transplantation for Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Kelly, C.R.; Brandt, L.J.; Khoruts, A.; Sadowsky, M.J. Complete Microbiota Engraftment Is Not Essential for Recovery from Recurrent Clostridium difficile Infection following Fecal Microbiota Transplantation. mBio 2016, 7, e01965-16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Steiner, T.; Petrof, E.O.; Smieja, M.; Roscoe, D.; Nematallah, A.; Weese, J.S.; Collins, S.; Moayyedi, P.; Crowther, M.; et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients with Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2016, 315, 142–149. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Weingarden, A.R.; Sadowsky, M.J.; Khoruts, A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 761–767. [Google Scholar] [CrossRef]
- Satokari, R.; Mattila, E.; Kainulainen, V.; Arkkila, P.E. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection—An observational cohort study. Aliment. Pharmacol. Ther. 2015, 41, 46–53. [Google Scholar] [CrossRef]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbo, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Ajami, N.J.; Petrosino, J.F.; Jun, G.; Hanis, C.L.; Shah, M.; Hochman, L.; Ankoma-Sey, V.; DuPont, A.W.; Wong, M.C.; et al. Randomised clinical trial: Faecal microbiota transplantation for recurrent Clostridum difficile infection—Fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment. Pharmacol. Ther. 2017, 45, 899–908. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Jalanka, J.; Hillamaa, A.; Satokari, R.; Mattila, E.; Anttila, V.J.; Arkkila, P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2018, 47, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dahl Jorgensen, S.M.; Jorgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Shaukat, A.; Zhang, J.H.; Reinink, A.R.; Nugent, S.; Dominitz, J.A.; Davis-Karim, A.; Gerding, D.N.; Kyriakides, T.C. Microbiota or placebo after antimicrobial therapy for recurrent Clostridioides difficile at home: A clinical trial with novel home-based enrollment. Clin. Trials 2021, 18, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Maynard, C.L.; Eipers, P.; Goldsmith, K.T.; Ptacek, T.; Grubbs, J.A.; Dixon, P.; Howard, D.; Crossman, D.K.; Crowley, M.R.; et al. Colonization potential to reconstitute a microbe community in patients detected early after fecal microbe transplant for recurrent C. difficile. BMC Microbiol. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Yu, H.; MacIsaac, D.; Wong, J.J.; Sellers, Z.M.; Wren, A.A.; Bensen, R.; Kin, C.; Park, K.T. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment. Pharmacol. Ther. 2018, 47, 364–370. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346 e310. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Cianci, R.; Bibbo, S.; Gasbarrini, A.; Curro, D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol. Ther. 2015, 149, 191–212. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Damman, C.J.; Miller, S.I.; Surawicz, C.M.; Zisman, T.L. The microbiome and inflammatory bowel disease: Is there a therapeutic role for fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Joossens, M.; Verbeke, K.; Wang, J.; Machiels, K.; Sabino, J.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Raes, J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 387–394. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Imaeda, H.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J. Gastroenterol. 2017, 52, 476–482. [Google Scholar] [CrossRef]
- Jacob, V.; Crawford, C.; Cohen-Mekelburg, S.; Viladomiu, M.; Putzel, G.G.; Schneider, Y.; Chabouni, F.; O’Neil, S.; Bosworth, B.; Woo, V.; et al. Single Delivery of High-Diversity Fecal Microbiota Preparation by Colonoscopy Is Safe and Effective in Increasing Microbial Diversity in Active Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 903–911. [Google Scholar] [CrossRef]
- Damman, C.J.; Brittnacher, M.J.; Westerhoff, M.; Hayden, H.S.; Radey, M.; Hager, K.R.; Marquis, S.R.; Miller, S.I.; Zisman, T.L. Low Level Engraftment and Improvement following a Single Colonoscopic Administration of Fecal Microbiota to Patients with Ulcerative Colitis. PLoS ONE 2015, 10, e0133925. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.; Huuskonen, L.; Korpela, K.; Salojarvi, J.; Aalvink, S.; de Vos, W.M.; D’Haens, G.R.; et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.; Pham, A.; Bonczyk, S.; Crumb, T.; Duba, M.; Conrad, H., Jr.; Cloney, D.; Kugathasan, S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J. Pediatric Gastroenterol. Nutr. 2013, 56, 597–601. [Google Scholar] [CrossRef]
- Kellermayer, R.; Nagy-Szakal, D.; Harris, R.A.; Luna, R.A.; Pitashny, M.; Schady, D.; Mir, S.A.; Lopez, M.E.; Gilger, M.A.; Belmont, J.; et al. Serial fecal microbiota transplantation alters mucosal gene expression in pediatric ulcerative colitis. Am. J. Gastroenterol. 2015, 110, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Veereman, G.; van der Werff Ten Bosch, J.; Goossens, A.; Pierard, D.; Samsom, J.N.; Escher, J.C. Fecal Microbial Transplantation in Early-Onset Colitis: Caution Advised. J. Pediatric Gastroenterol. Nutr. 2015, 61, e12–e14. [Google Scholar] [CrossRef]
- Shimizu, H.; Arai, K.; Abe, J.; Nakabayashi, K.; Yoshioka, T.; Hosoi, K.; Kuroda, M. Repeated fecal microbiota transplantation in a child with ulcerative colitis. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2016, 58, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Yokoyama, K.; Imagawa, T.; Inoue, S.; Tulyeu, J.; Tanaka, M.; Yamagata, T. Failure of Fecal Microbiota Transplantation in a Three-Year-Old Child with Severe Refractory Ulcerative Colitis. Pediatric Gastroenterol. Hepatol. Nutr. 2016, 19, 214–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pai, N.; Popov, J.; Hill, L.; Hartung, E.; Grzywacz, K.; Moayyedi, P.; McMaster Pediatric Fecal Microbiota Transplant Research Collaboration. Results of the First Pilot Randomized Controlled Trial of Fecal Microbiota Transplant In Pediatric Ulcerative Colitis: Lessons, Limitations, and Future Prospects. Gastroenterology 2021, 161, 388–393 e383. [Google Scholar] [CrossRef]
- Sager, K.; Alam, S.; Bond, A.; Chinnappan, L.; Probert, C.S. Review article: Cytomegalovirus and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 725–733. [Google Scholar] [CrossRef]
- Bonta, J.; Zeitz, J.; Frei, P.; Biedermann, L.; Sulz, M.C.; Vavricka, S.R.; Scharl, S.; Fried, M.; Rogler, G.; Scharl, M. Cytomegalovirus disease in inflammatory bowel disease: Epidemiology and disease characteristics in a large single-centre experience. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1329–1334. [Google Scholar] [CrossRef]
- Zagorowicz, E.; Bugajski, M.; Wieszczy, P.; Pietrzak, A.; Magdziak, A.; Mroz, A. Cytomegalovirus Infection in Ulcerative Colitis is Related to Severe Inflammation and a High Count of Cytomegalovirus-positive Cells in Biopsy Is a Risk Factor for Colectomy. J. Crohn’s Colitis 2016, 10, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Gwee, A.; Curtis, N.; Connell, T.G.; Garland, S.; Daley, A.J. Ganciclovir for the treatment of congenital cytomegalovirus: What are the side effects? Pediatric Infect. Dis. J. 2014, 33, 115. [Google Scholar] [CrossRef] [PubMed]
- Karolewska-Bochenek, K.; Lazowska-Przeorek, I.; Grzesiowski, P.; Dziekiewicz, M.; Dembinski, L.; Albrecht, P.; Radzikowski, A.; Banaszkiewicz, A. Faecal Microbiota Transfer—A new concept for treating cytomegalovirus colitis in children with ulcerative colitis. Ann. Agric. Environ. Med. 2021, 28, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Yang, Z.; Bu, C.; Yuan, W.; Shen, Z.; Quan, Y.; Wu, S.; Zhu, C.; Wang, X. Fecal Microbiota Transplant via Endoscopic Delivering Through Small Intestine and Colon: No Difference for Crohn’s Disease. Dig. Dis. Sci. 2020, 65, 150–157. [Google Scholar] [CrossRef]
- He, Z.; Li, P.; Zhu, J.; Cui, B.; Xu, L.; Xiang, J.; Zhang, T.; Long, C.; Huang, G.; Ji, G.; et al. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn’s disease complicated with inflammatory mass. Sci. Rep. 2017, 7, 4753. [Google Scholar] [CrossRef]
- Vaughn, B.P.; Vatanen, T.; Allegretti, J.R.; Bai, A.; Xavier, R.J.; Korzenik, J.; Gevers, D.; Ting, A.; Robson, S.C.; Moss, A.C. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2182–2190. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Xiao, Y.; Tian, L.; Cui, B.; Ji, G.; Liu, Y.Y.; Zhang, F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl. Microbiol. Biotechnol. 2019, 103, 349–360. [Google Scholar] [CrossRef]
- Wang, H.; Cui, B.; Li, Q.; Ding, X.; Li, P.; Zhang, T.; Yang, X.; Ji, G.; Zhang, F. The Safety of Fecal Microbiota Transplantation for Crohn’s Disease: Findings from A Long-Term Study. Adv. Ther. 2018, 35, 1935–1944. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Kaplan, G.G.; Harris, M.L.; Brant, S.R. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 2008, 103, 1443–1450. [Google Scholar] [CrossRef]
- Razik, R.; Rumman, A.; Bahreini, Z.; McGeer, A.; Nguyen, G.C. Recurrence of Clostridium difficile Infection in Patients with Inflammatory Bowel Disease: The RECIDIVISM Study. Am. J. Gastroenterol. 2016, 111, 1141–1146. [Google Scholar] [CrossRef]
- Kelsen, J.R.; Kim, J.; Latta, D.; Smathers, S.; McGowan, K.L.; Zaoutis, T.; Mamula, P.; Baldassano, R.N. Recurrence rate of clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 50–55. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Guzman-Perez, R.; Gainer, V.; Cai, T.; Churchill, S.; Kohane, I.; Plenge, R.M.; Murphy, S. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 35, 789–795. [Google Scholar] [CrossRef]
- Gravito-Soares, M.; Gravito-Soares, E.; Portela, F.; Ferreira, M.; Sofia, C. Fecal microbiota transplantation in recurrent Clostridium difficile infection in a patient with concomitant inflammatory bowel disease. Rev. Esp. Enferm. Dig. Organo Of. Soc. Esp. Patol. Dig. 2017, 109, 473–476. [Google Scholar] [CrossRef]
- Ianiro, G.; Bibbo, S.; Porcari, S.; Settanni, C.R.; Giambo, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: Experience of a large-volume European FMT center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef]
- Pai, N.; Popov, J.; Hill, L.; Hartung, E. Protocol for a double-blind, randomised, placebo-controlled pilot study for assessing the feasibility and efficacy of faecal microbiota transplant in a paediatric Crohn’s disease population: PediCRaFT Trial. BMJ Open 2019, 9, e030120. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Simren, M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr. Gastroenterol. Rep. 2013, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpusch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Goll, R.; Johnsen, P.H.; Hjerde, E.; Diab, J.; Valle, P.C.; Hilpusch, F.; Cavanagh, J.P. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes 2020, 12, 1794263. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; Hov, J.R.; Valeur, J.; Sangnes, D.A.; El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Lied, G.A. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand. J. Gastroenterol. 2019, 54, 690–699. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillila, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Brathen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2021, 33, e13983. [Google Scholar] [CrossRef]
- El-Salhy, M.; Casen, C.; Valeur, J.; Hausken, T.; Hatlebakk, J.G. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J. Gastroenterol. 2021, 27, 2219–2237. [Google Scholar] [CrossRef]
- Halkjaer, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Gunther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Oneto, C.; Feuerstadt, P.; Sherman, A.; Wolkoff, A.W.; Kassam, Z.; Sadovsky, R.G.; Elliott, R.J.; Budree, S.; et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: A double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 675–685. [Google Scholar] [CrossRef]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; Konig, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef] [PubMed]
- Holster, S.; Repsilber, D.; Geng, D.; Hyotylainen, T.; Salonen, A.; Lindqvist, C.M.; Rajan, S.K.; de Vos, W.M.; Brummer, R.J.; Konig, J. Correlations between microbiota and metabolites after faecal microbiota transfer in irritable bowel syndrome. Benef. Microbes 2021, 12, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Holster, S.; Hooiveld, G.J.; Repsilber, D.; Vos, W.M.; Brummer, R.J.; Konig, J. Allogenic Faecal Microbiota Transfer Induces Immune-Related Gene Sets in the Colon Mucosa of Patients with Irritable Bowel Syndrome. Biomolecules 2019, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hausken, T.; Hatlebakk, J.G. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019, 11, 1415. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef]
- Ding, X.W.; Liu, Y.X.; Fang, X.C.; Liu, K.; Wei, Y.Y.; Shan, M.H. The relationship between small intestinal bacterial overgrowth and irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5191–5196. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Srivastava, D.; Misra, A.; Ghoshal, U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: A randomized, double-blind, placebo-controlled trial. Eur. J. Gastroenterol. Hepatol. 2016, 28, 281–289. [Google Scholar] [CrossRef]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Carrellas, M.; Mullish, B.H.; Marchesi, J.R.; Pechlivanis, A.; Smith, M.; Gerardin, Y.; Timberlake, S.; Pratt, D.S.; et al. Fecal Microbiota Transplantation in Patients with Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am. J. Gastroenterol. 2019, 114, 1071–1079. [Google Scholar] [CrossRef]
- Wijdicks, E.F. Hepatic Encephalopathy. N. Engl. J. Med. 2016, 375, 1660–1670. [Google Scholar] [CrossRef]
- Bass, N.M.; Mullen, K.D.; Sanyal, A.; Poordad, F.; Neff, G.; Leevy, C.B.; Sigal, S.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Fagan, A.; Gavis, E.A.; Kassam, Z.; Sikaroodi, M.; Gillevet, P.M. Long-term Outcomes of Fecal Microbiota Transplantation in Patients with Cirrhosis. Gastroenterology 2019, 156, 1921–1923 e1923. [Google Scholar] [CrossRef]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology from the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Gavis, E.A.; Fagan, A.; Wade, J.B.; Thacker, L.R.; Fuchs, M.; Patel, S.; Davis, B.; Meador, J.; Puri, P.; et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology 2021, 73, 1688–1700. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I. Natural history of chronic hepatitis B REVEALed. J. Gastroenterol. Hepatol. 2011, 26, 628–638. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Zhao, Y.; Peng, J.; Feng, Q.; Dai, J.; Sun, S.; et al. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front. Microbiol. 2017, 8, 2222. [Google Scholar] [CrossRef]
- Chou, H.H.; Chien, W.H.; Wu, L.L.; Cheng, C.H.; Chung, C.H.; Horng, J.H.; Ni, Y.H.; Tseng, H.T.; Wu, D.; Lu, X.; et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, 2175–2180. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumar, R.; Sharma, S.; Mahanta, M.; Vayuuru, S.K.; Nayak, B.; Kumar, S.; Shalimar. Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig. Dis. Sci. 2021, 66, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.V.; Wilding, J.P.H.; Skjoth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409. [Google Scholar] [CrossRef]
- Wolf, A.M.; Colditz, G.A. Current estimates of the economic cost of obesity in the United States. Obes. Res. 1998, 6, 97–106. [Google Scholar] [CrossRef]
- Panca, M.; Viner, R.M.; White, B.; Pandya, T.; Melo, H.; Adamo, M.; Batterham, R.; Christie, D.; Kinra, S.; Morris, S. Cost-effectiveness of bariatric surgery in adolescents with severe obesity in the UK. Clin. Obes. 2018, 8, 105–113. [Google Scholar] [CrossRef]
- Klebanoff, M.J.; Chhatwal, J.; Nudel, J.D.; Corey, K.E.; Kaplan, L.M.; Hur, C. Cost-effectiveness of Bariatric Surgery in Adolescents with Obesity. JAMA Surg. 2017, 152, 136–141. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Bray, G.A.; Home, P.D.; Garvey, W.T.; Klein, S.; Pi-Sunyer, F.X.; Hu, F.B.; Raz, I.; Van Gaal, L.; Wolfe, B.M.; et al. Advances in the Science, Treatment, and Prevention of the Disease of Obesity: Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care 2015, 38, 1567–1582. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr. Rev. 2018, 39, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, T.N.; Chiavaroli, V.; Holland, D.J.; Cutfield, W.S.; O’Sullivan, J.M. The New Era of Treatment for Obesity and Metabolic Disorders: Evidence and Expectations for Gut Microbiome Transplantation. Front. Cell. Infect. Microbiol. 2016, 6, 15. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojarvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- De Groot, P.; Scheithauer, T.; Bakker, G.J.; Prodan, A.; Levin, E.; Khan, M.T.; Herrema, H.; Ackermans, M.; Serlie, M.J.M.; de Brauw, M.; et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut 2020, 69, 502–512. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Jayasinghe, T.N.; Leong, K.S.W.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; et al. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome 2021, 9, 107. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents with Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173 e110. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Kaplan, A.; Zelicha, H.; Rubin, E.; Koren, O.; Shai, I. Autologous fecal microbiota transplantation can retain the metabolic achievements of dietary interventions. Eur. J. Intern. Med. 2021, 92, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; Goldszmid, R.; Honda, K.; Trinchieri, G.; Wargo, J.; Zitvogel, L. Can we harness the microbiota to enhance the efficacy of cancer immunotherapy? Nat. Rev. Immunol. 2020, 20, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Vekhoff, A.; Lapusan, S.; Isnard, F.; D’Incan-Corda, E.; Rey, J.; Saillard, C.; Thomas, X.; Ducastelle-Lepretre, S.; Paubelle, E.; et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat. Commun. 2021, 12, 3084. [Google Scholar] [CrossRef] [PubMed]
- Mjaess, G.; Karam, A.; Aoun, F.; Albisinni, S.; Roumeguere, T. Fecal microbiota transplantation for immunotherapy-resistant urological tumors: Is it time? An update of the recent literature. Cancer 2022, 128, 14–19. [Google Scholar] [CrossRef]
- Ianiro, G.; Rossi, E.; Thomas, A.M.; Schinzari, G.; Masucci, L.; Quaranta, G.; Settanni, C.R.; Lopetuso, L.R.; Armanini, F.; Blanco-Miguez, A.; et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 2020, 11, 4333. [Google Scholar] [CrossRef]
- De Castro, C.G., Jr.; Ganc, A.J.; Ganc, R.L.; Petrolli, M.S.; Hamerschlack, N. Fecal microbiota transplant after hematopoietic SCT: Report of a successful case. Bone Marrow Transplant. 2015, 50, 145. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef]
- Coates, L.C.; Gossec, L.; Ramiro, S.; Mease, P.; van der Heijde, D.; Smolen, J.S.; Ritchlin, C.; Kavanaugh, A. New GRAPPA and EULAR recommendations for the management of psoriatic arthritis. Rheumatology 2017, 56, 1251–1253. [Google Scholar] [CrossRef]
- Scher, J.U.; Littman, D.R.; Abramson, S.B. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 35–45. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Moller, S.; et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: An exploratory randomised placebo-controlled trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef]
- Kragsnaes, M.S.; Sodergren, S.T.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Klinkby, C.S.; de Wit, M.; Ahlmark, N.G.; Tjornhoj-Thomsen, T.; et al. Experiences and perceptions of patients with psoriatic arthritis participating in a trial of faecal microbiota transplantation: A nested qualitative study. BMJ Open 2021, 11, e039471. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.G.; Bridgewood, C.; Marzo-Ortega, H. Correspondence on ‘Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: An exploratory randomised placebo-controlled trial’. Ann. Rheum. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [PubMed]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Davidsen, J.R.; Nilsson, A.C.; Rottger, R.; et al. Response to: ‘Correspondence on ‘Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: An exploratory randomised placebo-controlled trial’’ by McGonagle et al. Ann. Rheum. Dis. 2021. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Thoua, N.M.; Bunce, C.; Brough, G.; Forbes, A.; Emmanuel, A.V.; Denton, C.P. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology 2010, 49, 1770–1775. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Hoffmann-Vold, A.M.; Chang, Y.L.; Jacobs, J.P.; Tillisch, K.; Mayer, E.A.; Clements, P.J.; Hov, J.R.; Kummen, M.; Midtvedt, O.; et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017, 4, e000134. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Baekkevold, E.S.; Midtvedt, O.; Brunborg, C.; Holm, K.; Valeur, J.; Tennoe, A.H.; Garen, T.; et al. Fecal microbiota transplantation in systemic sclerosis: A double-blind, placebo-controlled randomized pilot trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Roep, B.O.; Wheeler, D.C.S.; Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019, 7, 65–74. [Google Scholar] [CrossRef]
- De Groot, P.F.; Belzer, C.; Aydin, O.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef] [PubMed]
- De Goffau, M.C.; Luopajarvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Harkonen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91 e75. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Oledzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaci, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Hong, H.C.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S28–S36. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Huang, J.T.; Abrams, M.; Tlougan, B.; Rademaker, A.; Paller, A.S. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009, 123, e808-814. [Google Scholar] [CrossRef]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Pan, H.H.; Lue, K.H.; Sun, H.L.; Ku, M.S. Gastroenteritis during infancy is a novel risk factor for allergic disease. Medicine 2019, 98, e16540. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays News Rev. Mol. Cell. Dev. Biol. 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2021, 10, e570. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Hase, K. Commensal microbiota regulates T cell fate decision in the gut. Seminars in immunopathology 2015, 37, 17–25. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4615–4622. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Meydani, S.N.; Das, S.K.; Pieper, C.F.; Lewis, M.R.; Klein, S.; Dixit, V.D.; Gupta, A.K.; Villareal, D.T.; Bhapkar, M.; Huang, M.; et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: A randomized controlled trial in non-obese humans. Aging 2016, 8, 1416–1431. [Google Scholar] [CrossRef]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235 e1226. [Google Scholar] [CrossRef]
- Sandler, N.G.; Douek, D.C. Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 2012, 10, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013, 5, 193ra191. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Dong, Z.; Hecht, D.K.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014, 7, 983–994. [Google Scholar] [CrossRef]
- Gori, A.; Rizzardini, G.; Van’t Land, B.; Amor, K.B.; van Schaik, J.; Torti, C.; Quirino, T.; Tincati, C.; Bandera, A.; Knol, J.; et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: Results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011, 4, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; de Lagarde, M.; Vazquez-Castellanos, J.; Vallejo, A.; Bernadino, J.I.; Madrid, N.; Matarranz, M.; Diaz-Santiago, A.; Gutierrez, C.; Cabello, A.; et al. Effects of Immunonutrition in Advanced Human Immunodeficiency Virus Disease: A Randomized Placebo-controlled Clinical Trial (Promaltia Study). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 120–130. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Talavera-Rodriguez, A.; Gosalbes, M.J.; Madrid, N.; Perez-Molina, J.A.; Elliott, R.J.; Navia, B.; Lanza, V.F.; Vallejo, A.; Osman, M.; et al. Fecal microbiota transplantation in HIV: A pilot placebo-controlled study. Nat. Commun. 2021, 12, 1139. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Somsouk, M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Curr. HIV/AIDS Rep. 2019, 16, 204–213. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Ye, Z.N.; Peng, P.; Xie, W.R.; Xu, J.T.; Zhang, X.Y.; Xia, H.H.; He, X.X. Efficacy and Safety of Washed Microbiota Transplantation to Treat Patients with Mild-to-Severe COVID-19 and Suspected of Having Gut Microbiota Dysbiosis: Study Protocol for a Randomized Controlled Trial. Curr. Med. Sci. 2021, 41, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Hazen, S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation 2017, 135, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients with Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc. Res. 2021, 118, 785–797. [Google Scholar] [CrossRef]
- Hu, X.F.; Zhang, W.Y.; Wen, Q.; Chen, W.J.; Wang, Z.M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.X.; Yang, J.; et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol. Res. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic transmission in development and neurodevelopmental disorders: Investigating physiology and pathology to gain therapeutic perspectives. Front. Cell. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Goo, N.; Bae, H.J.; Park, K.; Kim, J.; Jeong, Y.; Cai, M.; Cho, K.; Jung, S.Y.; Kim, D.H.; Ryu, J.H. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020, 262, 118497. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, M.; Chen, H.; Tang, W.; Nie, Y.; Zhou, Y. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Medicine 2019, 98, e16163. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.; Zlotnik, Y.; Moyal-Atias, K.; Abuhasira, R.; Ifergane, G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease—A case series. Clin. Neurol. Neurosurg. 2021, 207, 106791. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cui, B.T.; Zhang, T.; Li, P.; Long, C.Y.; Ji, G.Z.; Zhang, F.M. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef]
- Molloy, C.A.; Manning-Courtney, P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism Int. J. Res. Pract. 2003, 7, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.W.; Adams, J.B. Gut bacteria in children with autism spectrum disorders: Challenges and promise of studying how a complex community influences a complex disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lin, P.; Jiang, P.; Li, C. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: A systematic review. Shanghai Arch. Psychiatry 2013, 25, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, P.; Laczmanska, I.; Laczmanski, L. Future Directions in Reducing Gastrointestinal Disorders in Children with ASD Using Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2021, 11, 630052. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, 1–17. [Google Scholar] [CrossRef]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Xi, L.; Shi, Y.; Peng, L.; Wang, C.; Zou, L.; Yang, Y. Fecal microbiota transplantation for children with autism spectrum disorder. Gastrointest. Endosc. 2019, 6, AB512–AB513. [Google Scholar] [CrossRef]
- Carlucci, C.; Petrof, E.O.; Allen-Vercoe, E. Fecal Microbiota-based Therapeutics for Recurrent Clostridium difficile Infection, Ulcerative Colitis and Obesity. EBioMedicine 2016, 13, 37–45. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; FMT-standardization Study Group. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, J.H.; Shin, J.; Kim, J.S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Hazan, S. Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: A case report. J. Int. Med. Res. 2020, 48, 300060520925930. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.L.S.; Campbell, J.; Torres, M.; Nowak, A. Fecal Microbiota Transplantation (FMT) in Multiple Sclerosis (MS). Am. J. Gastroenterol. 2011, 106, S352. [Google Scholar] [CrossRef]
- Makkawi, S.; Camara-Lemarroy, C.; Metz, L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e459. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.D.; Cold, F.; Petersen, A.M.; Halkjaer, S.I.; Christensen, A.H.; Gunther, S.; Hestbjerg Hansen, L. Engraftment of strictly anaerobic oxygen-sensitive bacteria in irritable bowel syndrome patients following fecal microbiota transplantation does not improve symptoms. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Ohara, T. Identification of the microbial diversity after fecal microbiota transplantation therapy for chronic intractable constipation using 16s rRNA amplicon sequencing. PLoS ONE 2019, 14, e0214085. [Google Scholar] [CrossRef]
- Chu, N.D.; Crothers, J.W.; Nguyen, L.T.T.; Kearney, S.M.; Smith, M.B.; Kassam, Z.; Collins, C.; Xavier, R.; Moses, P.L.; Alm, E.J. Dynamic Colonization of Microbes and Their Functions after Fecal Microbiota Transplantation for Inflammatory Bowel Disease. mBio 2021, 12, e0097521. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Shapiro, B.; Hofreiter, M. A paleogenomic perspective on evolution and gene function: New insights from ancient DNA. Science 2014, 343, 1236573. [Google Scholar] [CrossRef] [PubMed]
- Der Sarkissian, C.; Allentoft, M.E.; Avila-Arcos, M.C.; Barnett, R.; Campos, P.F.; Cappellini, E.; Ermini, L.; Fernandez, R.; da Fonseca, R.; Ginolhac, A.; et al. Ancient genomics. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2015, 370, 20130387. [Google Scholar] [CrossRef] [PubMed]
- Patin, N.V.; Kunin, V.; Lidstrom, U.; Ashby, M.N. Effects of OTU clustering and PCR artifacts on microbial diversity estimates. Microb. Ecol. 2013, 65, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef]
- Sims, D.; Sudbery, I.; Ilott, N.E.; Heger, A.; Ponting, C.P. Sequencing depth and coverage: Key considerations in genomic analyses. Nat. Rev. Genet. 2014, 15, 121–132. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Paramsothy, S.; Borody, T.J.; Lin, E.; Finlayson, S.; Walsh, A.J.; Samuel, D.; van den Bogaerde, J.; Leong, R.W.; Connor, S.; Ng, W.; et al. Donor Recruitment for Fecal Microbiota Transplantation. Inflamm. Bowel Dis. 2015, 21, 1600–1606. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. https://doi.org/10.3390/jcm11144119
Biazzo M, Deidda G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. Journal of Clinical Medicine. 2022; 11(14):4119. https://doi.org/10.3390/jcm11144119
Chicago/Turabian StyleBiazzo, Manuele, and Gabriele Deidda. 2022. "Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases" Journal of Clinical Medicine 11, no. 14: 4119. https://doi.org/10.3390/jcm11144119
APA StyleBiazzo, M., & Deidda, G. (2022). Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. Journal of Clinical Medicine, 11(14), 4119. https://doi.org/10.3390/jcm11144119





