Preoperative Inflammatory Markers and the Risk of Postoperative Delirium in Patients Undergoing Lumbar Spinal Fusion Surgery
Abstract
:1. Introduction
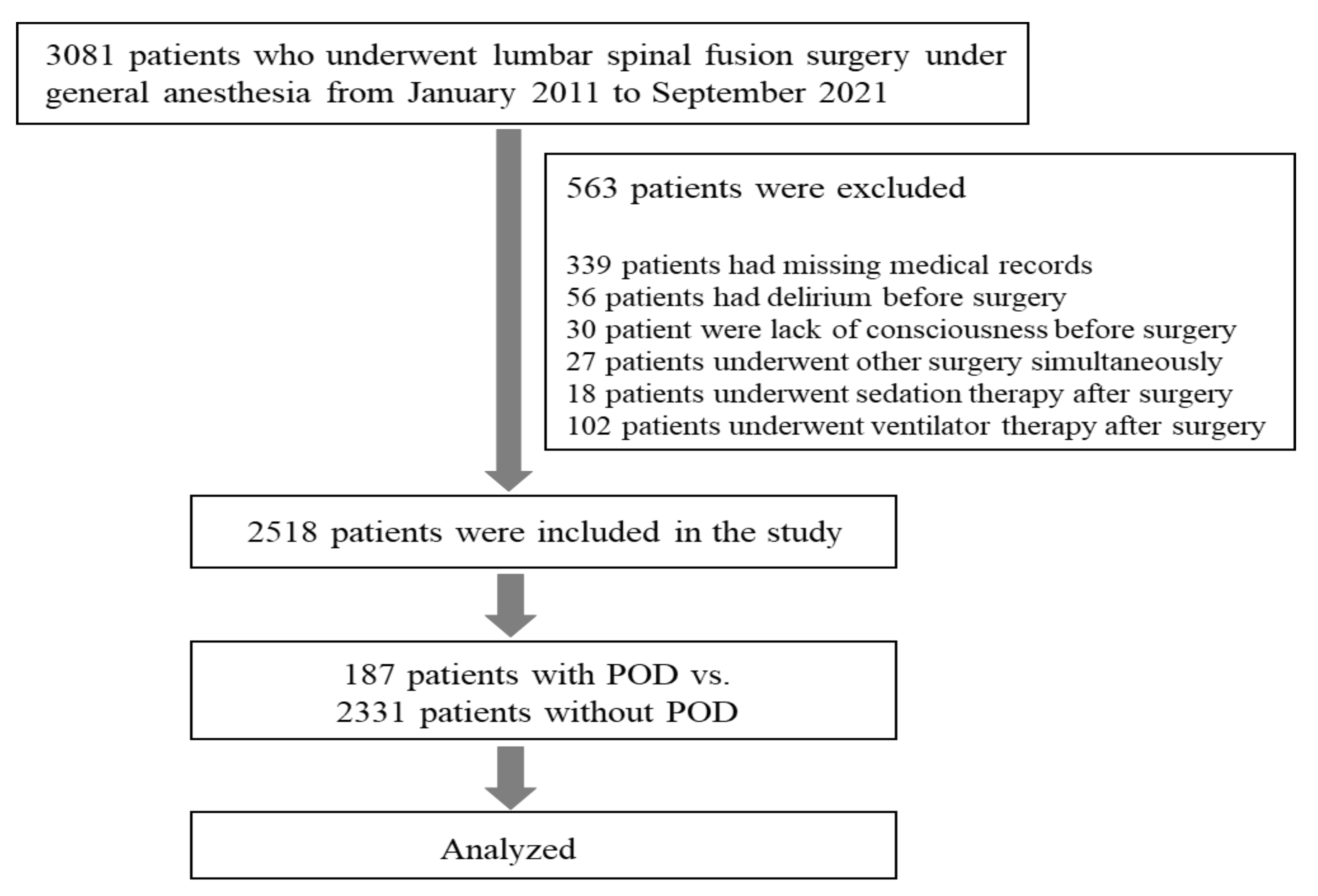
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Parameters
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ushida, T.; Yokoyama, T.; Kishida, Y.; Hosokawa, M.; Taniguchi, S.; Inoue, S.; Takemasa, R.; Suetomi, K.; Arai, Y.-C.P.; McLaughlin, M.; et al. Incidence and Risk Factors of Postoperative Delirium in Cervical Spine Surgery. Spine 2009, 34, 2500–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, M.; Takeuchi, H.; Fujisawa, D.; Miyajima, K.; Yoshimura, K.; Hashiguchi, S.; Ozawa, S.; Ando, N.; Shirahase, J.; Kitagawa, Y.; et al. Incidence and Risk Factors of Postoperative Delirium in Patients with Esophageal Cancer. Ann. Surg. Oncol. 2012, 19, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Brauer, C.; Morrison, R.S.; Silberzweig, S.B.; Siu, A.L. The Cause of Delirium in Patients with Hip Fracture. Arch. Intern. Med. 2000, 160, 1856–1860. [Google Scholar] [CrossRef] [Green Version]
- Saczynski, J.S.; Marcantonio, E.R.; Quach, L.; Fong, T.G.; Gross, A.; Inouye, S.K.; Jones, R.N. Cognitive Trajectories after Postoperative Delirium. N. Engl. J. Med. 2012, 367, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.H.; LaFlam, A.; Max, L.; Wyrobek, J.; Neufeld, K.J.; Kebaish, K.M.; Cohen, D.B.; Walston, J.D.; Hogue, C.W.; Riley, L.H. Delirium After Spine Surgery in Older Adults: Incidence, Risk Factors, and Outcomes. J. Am. Geriatr. Soc. 2016, 64, 2101–2108. [Google Scholar] [CrossRef]
- Maldonado, J.R. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am. J. Geriatr. Psychiatry 2013, 21, 1190–1222. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Ngo, L.; Inouye, S.K.; Libermann, T.A.; Jones, R.N.; Alsop, D.C.; Guess, J.; Jastrzebski, S.; McElhaney, J.E.; Kuchel, G.; et al. Cytokines and Postoperative Delirium in Older Patients Undergoing Major Elective Surgery. J. Gerontol. Ser. A 2015, 70, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Ritter, C.; Tomasi, C.D.; Dal-Pizzol, F.; Pinto, B.B.; Dyson, A.; de Miranda, A.S.; Comim, C.M.; Soares, M.; Teixeira, A.L.; Quevedo, J.; et al. Inflammation biomarkers and delirium in critically ill patients. Crit. Care 2014, 18, R106. [Google Scholar] [CrossRef] [Green Version]
- Noah, A.M.; Almghairbi, D.; Evley, R.; Moppett, I.K. Preoperative inflammatory mediators and postoperative delirium: Systematic review and meta-analysis. Br. J. Anaesth. 2021, 127, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Y.; Zhu, S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS ONE 2018, 13, e0195659. [Google Scholar] [CrossRef] [PubMed]
- Kulaksizoglu, B.; Kulaksizoglu, S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2016, 12, 1999–2005. [Google Scholar] [CrossRef] [Green Version]
- Egberts, A.; Mattace-Raso, F.U. Increased neutrophil–lymphocyte ratio in delirium: A pilot study. Clin. Interv. Aging 2017, 12, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- De Jager, C.P.C.; Wever, P.C.; Gemen, E.F.A.; Kusters, R.; Van Gageldonk-Lafeber, A.B.; Van Der Poll, T.; Laheij, R.J.F. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef]
- Núñez, J.; Nunez, E.; Bodí, V.; Sanchis, J.; Minana, G.; Mainar, L.; Santas, E.; Merlos, P.; Rumiz, E.; Darmofal, H.; et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am. J. Cardiol. 2008, 101, 747–752. [Google Scholar] [CrossRef]
- Oyama, T.; Kinoshita, H.; Takekawa, D.; Saito, J.; Kushikata, T.; Hirota, K. Higher neutrophil-to-lymphocyte ratio, mean platelet volume, and platelet distribution width are associated with postoperative delirium in patients undergoing esophagectomy: A retrospective observational study. J. Anesth. 2022, 36, 58–67. [Google Scholar] [CrossRef]
- Kinoshita, H.; Saito, J.; Takekawa, D.; Ohyama, T.; Kushikata, T.; Hirota, K. Availability of preoperative neutrophil-lymphocyte ratio to predict postoperative delirium after head and neck free-flap reconstruction: A retrospective study. PLoS ONE 2021, 16, e0254654. [Google Scholar] [CrossRef]
- He, R.; Wang, F.; Shen, H.; Zeng, Y.; Zhang, L. Association between increased neutrophil-to-lymphocyte ratio and postoperative delirium in elderly patients with total hip arthroplasty for hip fracture. BMC Psychiatry 2020, 20, 496. [Google Scholar] [CrossRef]
- Kotfis, K.; Ślozowska, J.; Safranow, K.; Szylińska, A.; Listewnik, M. The Practical Use of White Cell Inflammatory Biomarkers in Prediction of Postoperative Delirium after Cardiac Surgery. Brain Sci. 2019, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, S.J.; Nandyala, S.V.; Marquez-Lara, A.; Oglesby, M.; Patel, A.A.; Singh, K. Incidence and Risk Factors for Postoperative Delirium after Lumbar Spine Surgery. Spine 2013, 38, 1790–1796. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seo, S.W.; Kim, J.Y. Comprehensive risk factor evaluation of postoperative delirium following major surgery: Clinical data warehouse analysis. Neurol. Sci. 2019, 40, 793–800. [Google Scholar] [CrossRef]
- Kim, K.-N.; Kim, C.-H.; Kim, K.-I.; Yoo, H.-J.; Park, S.-Y.; Park, Y.-H. Development and validation of the Korean Nursing Delirium Scale. J. Korean Acad. Nurs. 2012, 42, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Wang, B.; Yin, J.; Xue, Q.; Gao, S.; Xing, L.; Wang, H.; Liu, W.; Liu, X. Risk factors for postoperative delirium after spinal surgery: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 32, 1417–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Ma, X.H.; Ye, J.B.; Liu, C.Z.; Zhou, Z.Y. Systematic review and meta-analysis of risk factor for postoperative delirium following spinal surgery. J. Orthop. Surg. Res. 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, D.; Lou, Y.; Li, Z. Risk factors for postoperative delirium after spine surgery in middle- and old-aged patients. Aging Clin. Exp. Res. 2017, 29, 1039–1044. [Google Scholar] [CrossRef]
- Susano, M.J.; Scheetz, S.D.; Grasfield, R.H.; Cheung, D.; Xu, X.; Kang, J.D.; Smith, T.R.; Lu, Y.; Groff, M.W.; Chi, J.H.; et al. Retrospective analysis of perioperative variables associated with postoperative delirium and other adverse outcomes in older patients after spine surgery. J. Neurosurg. Anesthesiol. 2019, 31, 385–391. [Google Scholar] [CrossRef]
- Kassie, G.M.; Nguyen, T.A.; Ellett, L.M.K.; Pratt, N.L.; Roughead, E.E. Preoperative medication use and postoperative delirium: A systematic review. BMC Geriatr. 2017, 17, 298. [Google Scholar] [CrossRef] [Green Version]
- Bilotta, F.; Lauretta, M.P.; Borozdina, A.; Mizikov, V.M.; Rosa, G. Postoperative delirium: Risk factors, diagnosis and perioperative care. Minerva Anestesiol. 2013, 79, 1066–1076. [Google Scholar]
- Clegg, A.; Young, J.B. Which medications to avoid in people at risk of delirium: A systematic review. Age Ageing 2011, 40, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Vacas, S.; Degos, V.; Tracey, K.J.; Maze, M. High-mobility Group Box 1 Protein Initiates Postoperative Cognitive Decline by Engaging Bone Marrow–derived Macrophages. Anesthesiology 2014, 120, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Kawano, T.; Yamanaka, D.; Aoyama, B.; Tateiwa, H.; Shigematsu-Locatelli, M.; Nishigaki, A.; Iwata, H.; Locatelli, F.M.; Yokoyama, M. Involvement of acute neuroinflammation in postoperative delirium-like cognitive deficits in rats. J. Anesth. 2018, 32, 506–517. [Google Scholar] [CrossRef]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef]
- Neerland, B.E.; Hall, R.J.; Seljeflot, I.; Frihagen, F.; MacLullich, A.M.J.; Raeder, J.; Wyller, T.B.; Watne, L.O. Associations Between Delirium and Preoperative Cerebrospinal Fluid C-Reactive Protein, Interleukin-6, and Interleukin-6 Receptor in Individuals with Acute Hip Fracture. J. Am. Geriatr. Soc. 2016, 64, 1456–1463. [Google Scholar] [CrossRef]
- Beloosesky, Y.; Hendel, D.; Weiss, A.; Hershkovitz, A.; Grinblat, J.; Pirotsky, A.; Barak, V. Cytokines and C-Reactive Protein Production in Hip-Fracture-Operated Elderly Patients. J. Gerontol. Ser. A 2007, 62, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Simone, M.J.; Tan, Z.S. The Role of Inflammation in the Pathogenesis of Delirium and Dementia in Older Adults: A Review. CNS Neurosci. Ther. 2011, 17, 506–513. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, Y.; Fang, Q.; Zhang, W.; Cheng, X. Platelet-to-lymphocyte ratio as a predictive index for delirium in critically ill patients: A retrospective observational study. Medicine 2020, 99, e22884. [Google Scholar] [CrossRef]
- Song, Q.; Pan, R.; Jin, Y.; Wang, Y.; Cheng, Y.; Liu, J.; Wu, B.; Liu, M. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol. Sci. 2020, 41, 2511–2520. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, Y.; Chen, Y.; Li, S.; Huang, X.; Zhang, H.; Jiang, F.; Chen, Q. Association of lymphocyte to monocyte ratio and risk of in-hospital mortality in patients with acute type A aortic dissection. Biomark. Med. 2019, 13, 1263–1272. [Google Scholar] [CrossRef]
- Hemond, C.C.; Glanz, B.I.; Bakshi, R.; Chitnis, T.; Healy, B.C. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with neurological disability and brain atrophy in multiple sclerosis. BMC Neurol. 2019, 19, 23. [Google Scholar] [CrossRef] [Green Version]
- Mano, Y.; Yoshizumi, T.; Yugawa, K.; Ohira, M.; Motomura, T.; Toshima, T.; Itoh, S.; Harada, N.; Ikegami, T.; Soejima, Y.; et al. Lymphocyte-to-Monocyte Ratio Is a Predictor of Survival after Liver Transplantation for Hepatocellular Carcinoma. Liver Transplant. 2018, 24, 1603–1611. [Google Scholar] [CrossRef]
- Kwon, B.S.; Jeong, D.H.; Byun, J.M.; Lee, T.H.; Choi, K.U.; Song, Y.J.; Suh, D.S.; Kim, K.H. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J. Cancer 2018, 9, 1127–1134. [Google Scholar] [CrossRef]
- Fan, Z.; Li, Y.; Ji, H.; Jian, X. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: A retrospective cohort study. BMJ Open 2018, 8, e023459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Li, T.; Zhuang, D.; Cai, S.; Yang, J.; Ding, F.; Chen, X.; Tian, F.; Huang, M.; Li, L.; et al. The Monocyte-to-Lymphocyte Ratio at Hospital Admission Is a Novel Predictor for Acute Traumatic Intraparenchymal Hemorrhage Expansion after Cerebral Contusion. Mediat. Inflamm. 2020, 2020, 5483981. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.P.; Park, M. C-Reactive Protein/Albumin Ratio Predicts 90-Day Mortality of Septic Patients. PLoS ONE 2013, 8, e59321. [Google Scholar] [CrossRef]
- Fairclough, E.; Cairns, E.; Hamilton, J.; Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009, 9, 30–33. [Google Scholar] [CrossRef]
- Park, J.E.; Chung, K.S.; Song, J.H.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; et al. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. J. Clin. Med. 2018, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Çağdaş, M.; Rencüzoğullari, I.; Karakoyun, S.; Karabağ, Y.; Yesin, M.; Artaç, I.; Iliş, D.; Çağdaş, S.; Tezcan, A.H.; Tanboğa, H.I. Assessment of Relationship Between C-Reactive Protein to Albumin Ratio and Coronary Artery Disease Severity in Patients with Acute Coronary Syndrome. Angiology 2019, 70, 361–368. [Google Scholar] [CrossRef]
- Rencuzogullari, I.; Karabağ, Y.; Çağdaş, M.; Karakoyun, S.; Seyis, S.; Gürsoy, M.O.; Yesin, M.; Artaç, I.; İliş, D.; Tanboğa, I.H. Assessment of the relationship between preprocedural C-reactive protein/albumin ratio and stent restenosis in patients with ST-segment elevation myocardial infarction. Rev. Port. Cardiol. 2019, 38, 269–277. [Google Scholar] [CrossRef]
- Peng, J.; Wu, G.; Chen, J.; Chen, H. Preoperative C-Reactive Protein/Albumin Ratio, a Risk Factor for Postoperative Delirium in Elderly Patients after Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 2601–2605. [Google Scholar] [CrossRef]
- Kalyoncuoğlu, M.; Biter, H.I.; Durmuş, G.; Baştan, B.; Can, M.M. C-reactive Protein to Albumin Ratio as A Novel Inflammatory Biomarker for Postoperative Delirium in Patients Undergoing Transcatheter Aortic Valve Replacement. Med. Bull. Haseki 2020, 58, 183–192. [Google Scholar] [CrossRef]
| Variable | non-POD (n = 2331) | POD (n = 187) | p Value |
|---|---|---|---|
| Age, n (median, IQR) | 65 (56, 72) | 74 (67, 78) | <0.001 |
| Male, n (%) | 1011 (43.4) | 96 (51.3) | 0.035 |
| Obesity (BMI > 29.9), n (%) | 195 (8.4) | 9 (4.8) | 0.087 |
| ASA physical status > 2, n (%) | 715 (30.7) | 112 (59.9) | <0.001 |
| Emergency surgery, n (%) | 126 (5.4) | 7 (3.7) | 0.328 |
| HTN, n (%) | 1197 (51.4) | 106 (56.7) | 0.16 |
| DM, n (%) | 571 (24.5) | 56 (29.9) | 0.097 |
| Heart disease, n (%) | 267 (11.5) | 35 (18.7) | 0.003 |
| Stroke, n (%) | 135 (5.8) | 20 (10.7) | 0.007 |
| Cancer, n (%) | 182 (7.8) | 13 (7.0) | 0.674 |
| Dyslipidemia, n (%) | 430 (18.4) | 21 (11.2) | 0.013 |
| Parkinson’s disease, n (%) | 19 (0.8) | 9 (4.8) | <0.001 |
| Dementia, n (%) | 21 (0.9) | 7 (3.7) | <0.001 |
| Depression, n (%) | 60 (2.6) | 8 (4.3) | 0.167 |
| Kidney disease, n (%) | 106 (4.5) | 11 (5.9) | 0.404 |
| Liver disease, n (%) | 79 (3.4) | 5 (2.7) | 0.6 |
| Insomnia, n (%) | 144 (6.2) | 17 (9.1) | 0.117 |
| Sleep disorder, n (%) | 137 (5.9) | 16 (8.6) | 0.14 |
| Alcohol, n (%) | 585 (25.1) | 32 (17.1) | 0.015 |
| Smoking, n (%) | 384 (16.5) | 32 (17.1) | 0.821 |
| Preoperative used drugs | |||
| Calcium channel blockers, n (%) | 1014 (43.5) | 81 (43.3) | 0.961 |
| Diuretics, n (%) | 230 (9.9) | 22 (11.8) | 0.405 |
| Beta blockers, n (%) | 192 (8.2) | 13 (7.0) | 0.536 |
| ACE inhibitors, n (%) | 10 (0.4) | 1 (0.5) | 0.833 |
| Angiotensin receptor blockers, n (%) | 139 (6.0) | 11 (5.9) | 0.964 |
| Other antihypertensives, n (%) | 29 (1.2) | 1 (0.5) | 0.39 |
| Miscellaneous CV drugs, n (%) | 127 (5.4) | 13 (7.0) | 0.388 |
| Anti-depressants, n (%) | 77 (3.3) | 4 (2.1) | 0.385 |
| Hypnotics, n (%) | 718 (30.8) | 67 (35.8) | 0.153 |
| Anti-psychotics, n (%) | 168 (7.2) | 33 (17.6) | <0.001 |
| Opioids, n (%) | 2317 (99.4) | 186 (99.5) | 0.91 |
| Corticosteroids, n (%) | 648 (27.8) | 68 (36.4) | 0.012 |
| Antihistamines, n (%) | 815 (35.0) | 95 (50.8) | <0.001 |
| H2 blockers, n (%) | 689 (29.6) | 54 (28.9) | 0.844 |
| Intra and postoperative factors | |||
| Postop. ICU care, n (%) | 339 (14.5) | 90 (48.1) | <0.001 |
| Patient-controlled analgesia, n (%) | 2236 (95.9) | 180 (96.3) | 0.825 |
| Operation time, hours (median, IQR) | 3.6 (2.8, 4.6) | 3.9 (2.9, 5.1) | 0.004 |
| Surgical range, level (median, IQR) | 2 (1, 2) | 2 (1, 3) | 0.002 |
| Fluid balance (input–output), mL (median, IQR) | 1.0 (0.6, 1.6) | 1.1 (0.6, 1.8) | 0.074 |
| non-POD (n = 2331) | POD (n = 187) | p Value | |
|---|---|---|---|
| NLR, median (IQR) | 2.17 (1.54, 3.29) | 2.68 (1.90, 4.19) | <0.001 |
| MLR, median (IQR) | 0.23 (0.17, 0.33) | 0.29 (0.21, 0.39) | <0.001 |
| PLR, median (IQR) | 137.88 (105.04, 182.98) | 140.98 (103.86, 185.35) | 0.465 |
| CAR, median (IQR) | 0.26 (0.22, 0.67) | 0.47 (0.25, 1.89) | <0.001 |
| IQR of Inflammatory Markers | ||||
|---|---|---|---|---|
| NLR Q1 | NLR Q2 | NLR Q3 | NLR Q4 | |
| uOR | reference | 2.38 | 2.73 | 3.28 |
| 95% CI | 1.41–4.0 | 1.63–4.58 | 1.98–5.45 | |
| p value | 0.001 | <0.001 | <0.001 | |
| MLR Q1 | MLR Q2 | MLR Q3 | MLR Q4 | |
| uOR | reference | 1.21 | 1.88 | 3.0 |
| 95% CI | 0.72–2.02 | 1.17–3.04 | 1.92–4.7 | |
| p value | 0.472 | 0.009 | <0.001 | |
| PLR Q1 | PLR Q2 | PLR Q3 | PLR Q4 | |
| uOR | reference | 0.8 | 1.09 | 1.0 |
| 95% CI | 0.52–1.24 | 0.72–1.64 | 0.66–1.52 | |
| p value | 0.314 | 0.683 | >0.999 | |
| CAR Q1 | CAR Q2 | CAR Q3 | CAR Q4 | |
| uOR | reference | 1.78 | 2.51 | 3.38 |
| 95% CI | 1.06–2.97 | 1.56–4.04 | 2.14–5.33 | |
| p value | 0.029 | <0.001 | <0.001 | |
| Variable | aOR (95% CI) | p Value |
|---|---|---|
| IQR of Inflammatory Markers | ||
| IQR NLR Q2 | 2.26 (1.24–4.14) | 0.008 |
| IQR NLR Q3 | 2.47 (1.3–4.72) | 0.006 |
| IQR NLR Q4 | 2.86 (1.38–5.92) | 0.005 |
| IQR MLR Q2 | 0.87 (0.48–1.56) | 0.636 |
| IQR MLR Q3 | 1.07 (0.6–1.91) | 0.819 |
| IQR MLR Q4 | 1.46 (0.77–2.75) | 0.241 |
| IQR PLR Q2 | 0.5 (0.3–0.84) | 0.008 |
| IQR PLR Q3 | 0.7 (0.42–1.16) | 0.168 |
| IQR PLR Q4 | 0.48 (0.27–0.85) | 0.011 |
| IQR CAR Q2 | 0.92 (0.52–1.63) | 0.782 |
| IQR CAR Q3 | 1.53 (0.9–2.59) | 0.116 |
| IQR CAR Q4 | 1.6 (0.94–2.72) | 0.085 |
| Other variables | ||
| Age | 1.05 (1.03–1.07) | <0.001 |
| Male | 1.8 (1.22–2.65) | 0.003 |
| Obesity (BMI > 29.9) | 0.72 (0.33–1.53) | 0.39 |
| ASA physical status > 2 | 2.07 (1.37–3.13) | <0.001 |
| Emergency surgery | 0.92 (0.38–2.23) | 0.852 |
| HTN | 0.77 (0.53–1.12) | 0.174 |
| DM | 0.95 (0.64–1.4) | 0.779 |
| Heart disease | 0.71 (0.44–1.16) | 0.171 |
| Stroke | 1.04 (0.58–1.88) | 0.885 |
| Cancer | 0.52 (0.28–0.99) | 0.047 |
| Dyslipidemia | 0.66 (0.4–1.11) | 0.12 |
| Parkinson’s disease | 4.46 (1.76–11.29) | 0.002 |
| Dementia | 3.8 (1.4–10.31) | 0.009 |
| Depression | 2.64 (1.14–6.16) | 0.024 |
| Kidney disease | 0.78 (0.37–1.61) | 0.496 |
| Liver disease | 0.48 (0.17–1.4) | 0.179 |
| Insomnia | 0.9 (0.07–12.27) | 0.937 |
| Sleep disorder | 1.64 (0.11–23.51) | 0.717 |
| Alcohol | 0.66 (0.4–1.08) | 0.097 |
| Smoking | 1.1 (0.66–1.85) | 0.714 |
| Calcium channel blockers | 0.85 (0.6–1.21) | 0.37 |
| Diuretics | 0.76 (0.43–1.32) | 0.328 |
| Beta blockers | 0.79 (0.4–1.55) | 0.486 |
| ACE inhibitors | 0.95 (0.08–11.84) | 0.97 |
| Angiotensin receptor blockers | 1.13 (0.54–2.38) | 0.741 |
| Other antihypertensives | 0.25 (0.03–2.31) | 0.222 |
| Miscellaneous CV drugs | 1.51 (0.75–3.03) | 0.247 |
| Antidepressants | 0.64 (0.21–1.93) | 0.424 |
| Hypnotic sedatives | 1.07 (0.71–1.61) | 0.749 |
| Antipsychotics | 3.52 (2.08–5.97) | <0.001 |
| Opioids | 0.69 (0.08–6.09) | 0.734 |
| Corticosteroids | 1.16 (0.8–1.68) | 0.43 |
| Antihistamine antiallergics | 1.41 (0.95–2.11) | 0.089 |
| H2 receptor antagonist | 1.08 (0.71–1.62) | 0.729 |
| Operation time | 1.11 (0.97–1.26) | 0.118 |
| Surgical range | 1.13 (0.96–1.32) | 0.146 |
| Fluid balance (input–output) | 0.91 (0.75–1.12) | 0.383 |
| Postop. ICU care | 3.83 (2.59–5.65) | <0.001 |
| Patient-controlled analgesia | 1.13 (0.45–2.81) | 0.796 |
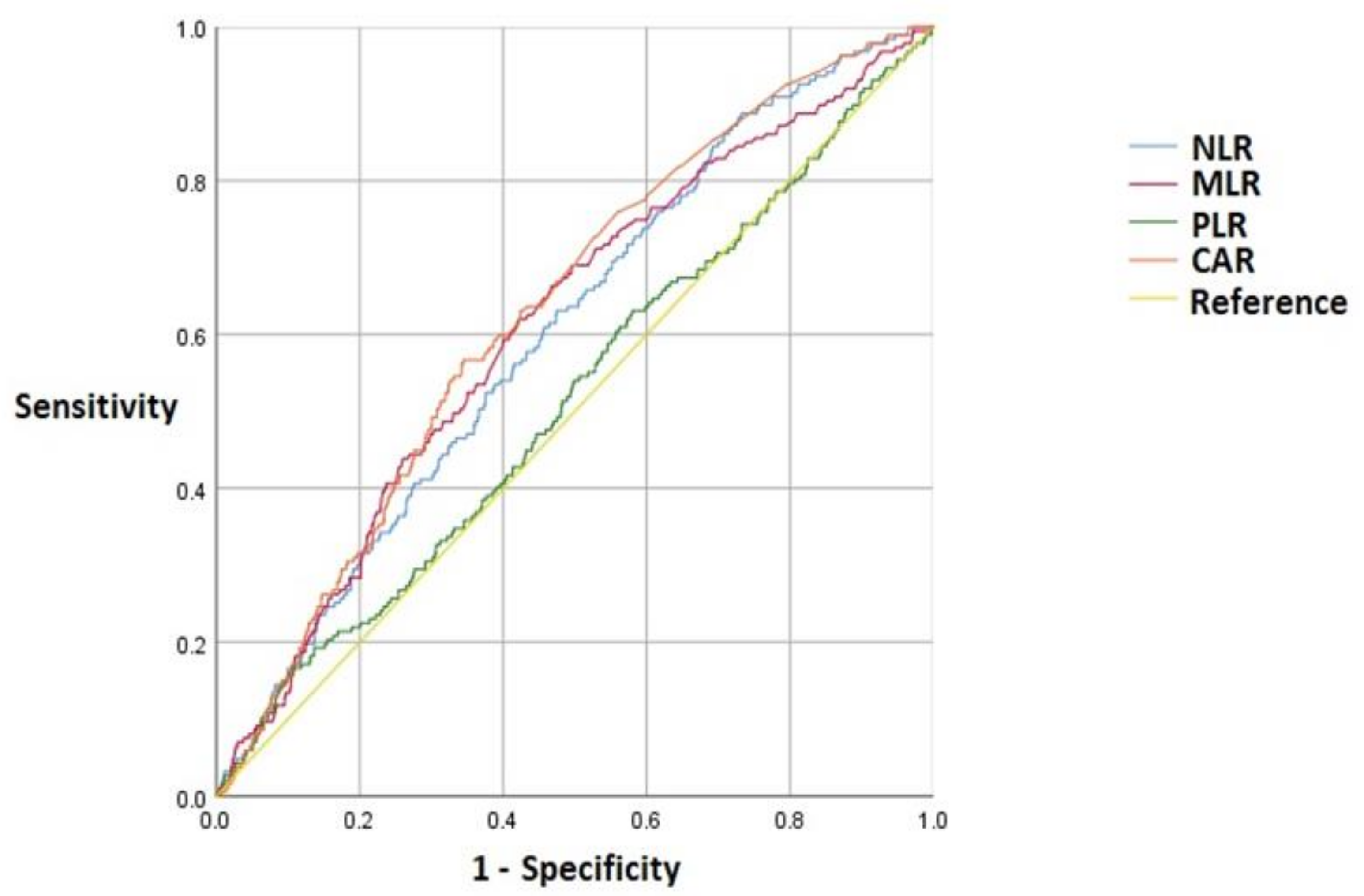
| Inflammatory Markers | Cutoff Value | AUC (95% CI) | Sensitivity | Specificity | p Value |
|---|---|---|---|---|---|
| NLR | 2.26 | 0.60 (0.56–0.64) | 0.63 | 0.53 | <0.001 |
| MLR | 0.26 | 0.61 (0.57–0.65) | 0.62 | 0.58 | <0.001 |
| PLR | 233.90 | 0.52 (0.47–0.56) | 0.16 | 0.90 | 0.464 |
| CAR | 0.38 | 0.63 (0.59–0.67) | 0.57 | 0.66 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.S.; Lee, J.J.; Kwon, Y.-S.; Kim, J.-H.; Sohn, J.-H. Preoperative Inflammatory Markers and the Risk of Postoperative Delirium in Patients Undergoing Lumbar Spinal Fusion Surgery. J. Clin. Med. 2022, 11, 4085. https://doi.org/10.3390/jcm11144085
Yang JS, Lee JJ, Kwon Y-S, Kim J-H, Sohn J-H. Preoperative Inflammatory Markers and the Risk of Postoperative Delirium in Patients Undergoing Lumbar Spinal Fusion Surgery. Journal of Clinical Medicine. 2022; 11(14):4085. https://doi.org/10.3390/jcm11144085
Chicago/Turabian StyleYang, Jin Seo, Jae Jun Lee, Young-Suk Kwon, Jong-Ho Kim, and Jong-Hee Sohn. 2022. "Preoperative Inflammatory Markers and the Risk of Postoperative Delirium in Patients Undergoing Lumbar Spinal Fusion Surgery" Journal of Clinical Medicine 11, no. 14: 4085. https://doi.org/10.3390/jcm11144085
APA StyleYang, J. S., Lee, J. J., Kwon, Y.-S., Kim, J.-H., & Sohn, J.-H. (2022). Preoperative Inflammatory Markers and the Risk of Postoperative Delirium in Patients Undergoing Lumbar Spinal Fusion Surgery. Journal of Clinical Medicine, 11(14), 4085. https://doi.org/10.3390/jcm11144085






