Misleading Meta-Analyses during COVID-19 Pandemic: Examples of Methodological Biases in Evidence Synthesis
Abstract
:1. Introduction
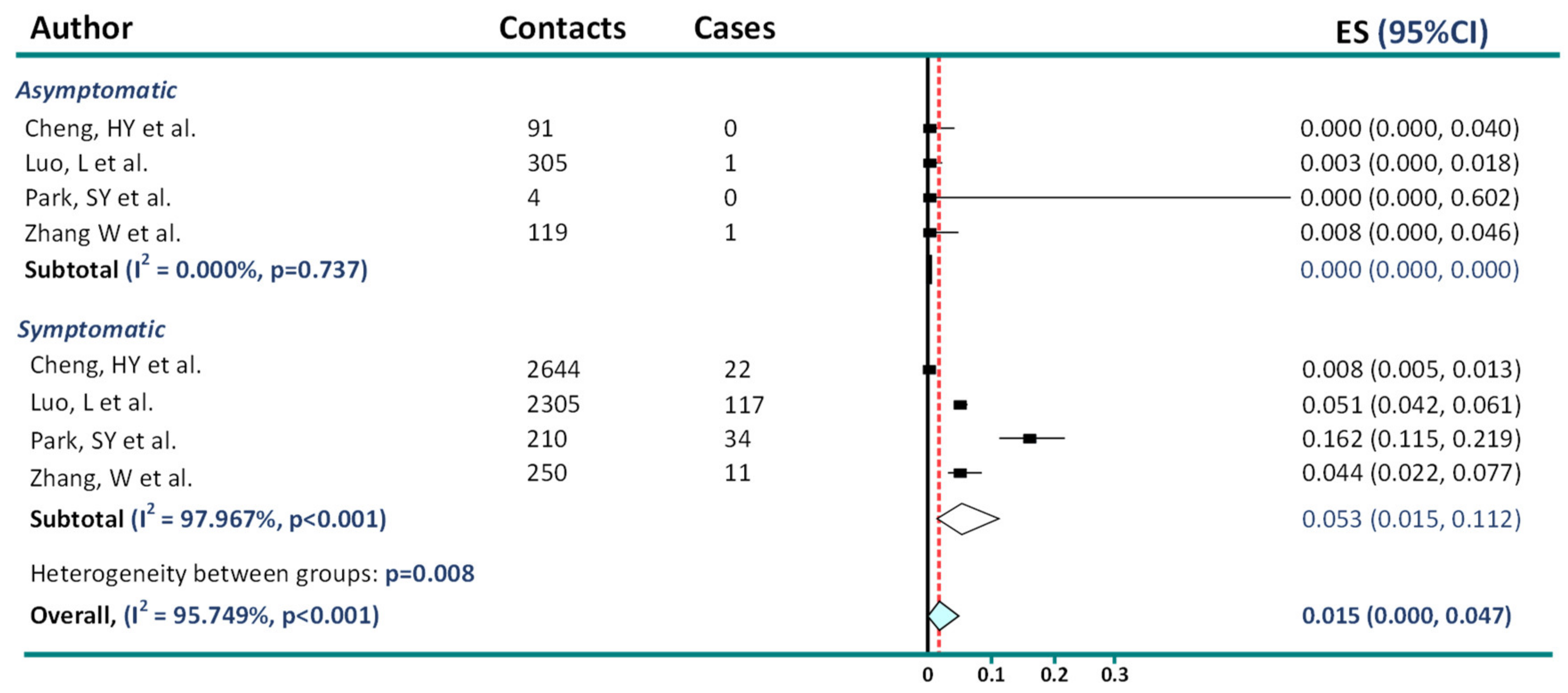
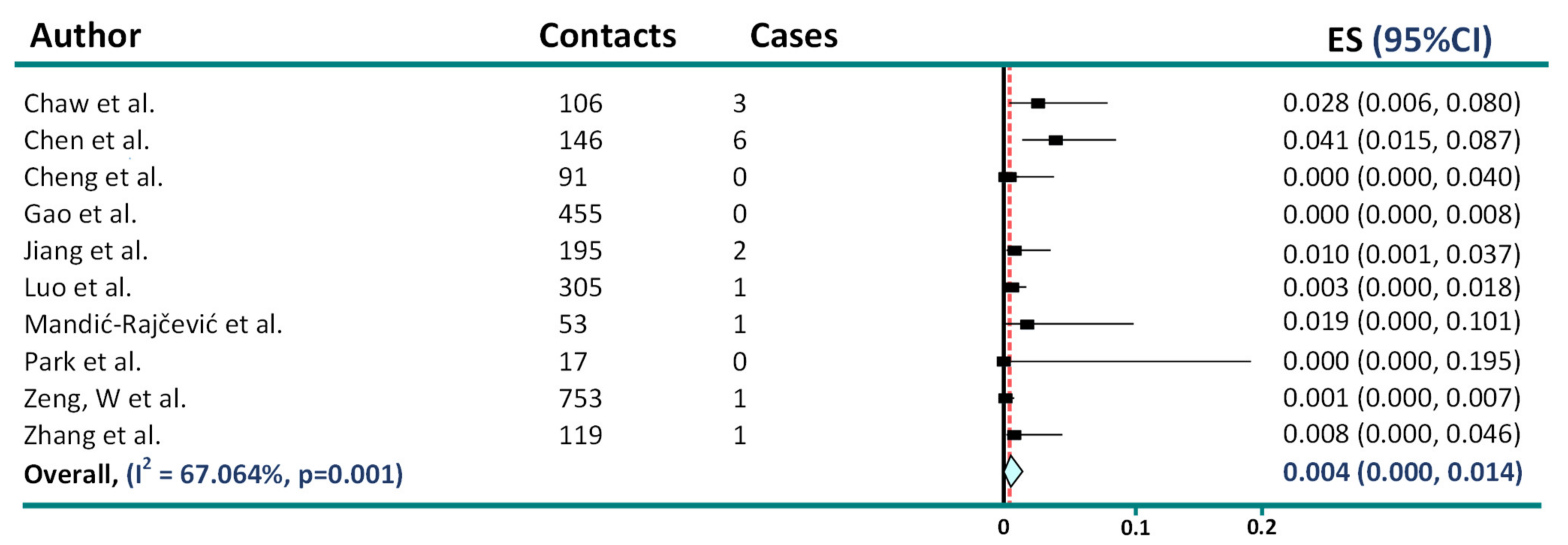
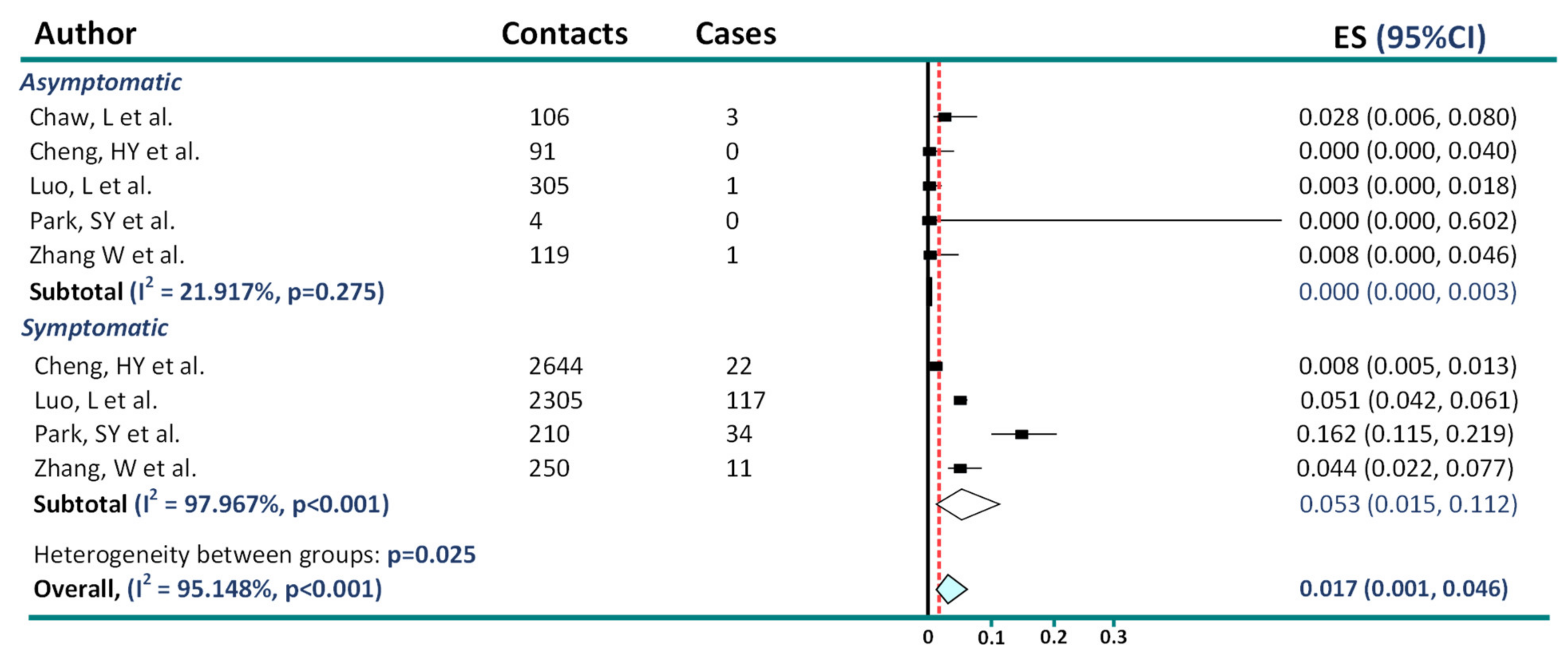
2. Asymptomatic Transmission in COVID-19
3. Wearing Facemasks and COVID-19
4. Ivermectin: An Example of How Critical Appraisal of Evidence Aids Good Science
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howick, J.; Koletsi, D.; Ioannidis, J.P.A.; Madigan, C.; Pandis, N.; Loef, M.; Walach, H.; Sauer, S.; Kleijnen, J.; Seehra, J.; et al. Most healthcare interventions tested in Cochrane Reviews are not effective according to high quality evidence: A systematic review and meta-analysis. J. Clin. Epidemiol. 2022, 148, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q. 2016, 94, 485–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef] [PubMed]
- Chaw, L.; Koh, W.C.; Jamaludin, S.A.; Naing, L.; Alikhan, M.F.; Wong, J. Analysis of SARS-CoV-2 transmission in different settings, among cases and close contacts from the Tablighi cluster in Brunei Darussalam. medRxiv 2020. [Google Scholar] [CrossRef]
- Chaw, L.; Koh, W.C.; Jamaludin, S.A.; Naing, L.; Alikhan, M.F.; Wong, J. Analysis of SARS-CoV-2 Transmission in Different Settings, Brunei. Emerg. Infect. Dis. 2020, 26, 2598–2606. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Jian, S.W.; Liu, D.P.; Ng, T.C.; Huang, W.T.; Lin, H.H. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern. Med. 2020, 180, 1156–1163. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.M.; Yi, S.; Lee, S.; Na, B.J.; Kim, C.B.; Kim, J.I.; Kim, H.S.; Kim, Y.B.; Park, Y.; et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg. Infect. Dis 2020, 26, 1666–1670. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Liu, D.; Liao, X.; Wu, X.; Jing, Q.; Zheng, J.; Liu, F.; Yang, S.; Bi, H.; Li, Z.; et al. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China. Ann. Intern. Med. 2020, 173, 879–887. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Luo, L.; Ma, Y.; Xu, C.; Qin, P.; Zhang, Z. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg. Infect. Dis. 2020, 26, 1924. [Google Scholar] [CrossRef]
- Qiu, X.; Nergiz, A.I.; Maraolo, A.E.; Bogoch, I.I.; Low, N.; Cevik, M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission-a living systematic review. Clin. Microbiol. Infect. 2021, 27, 511–519. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.H.; Yi, B.; Ding, K.Q.; Wang, H.B.; Wang, J.M.; Shi, H.B.; Wang, S.J.; Xu, G.Z. Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city. Chung Hua Liu Hsing Ping Hsueh Tsa Chih. 2020, 41, 667–671. [Google Scholar]
- Gao, M.; Yang, L.; Chen, X.; Deng, Y.; Yang, S.; Xu, H.; Chen, Z.; Gao, X. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir. Med. 2020, 169, 106026. [Google Scholar] [CrossRef]
- Mandić-Rajčević, S.; Masci, F.; Crespi, E.; Franchetti, S.; Longo, A.; Bollina, I.; Velocci, S.; Amorosi, A.; Baldelli, R.; Boselli, L.; et al. Contact tracing and isolation of asymptomatic spreaders to successfully control the COVID-19 epidemic among healthcare workers in Milan (Italy). medRxiv 2020. [Google Scholar] [CrossRef]
- Zeng, J.; Qiu, L.P.; Zou, Y. Epidemiological outcome of close contacts of coronavirus disease 2019 cases in Sichuan province. Chin. J. Public Health 2020, 36, 503–506. [Google Scholar] [CrossRef]
- Jiang, X.L.; Zhang, X.L.; Zhao, X.N.; Li, C.B.; Lei, J.; Kou, Z.Q.; Sun, W.K.; Hang, Y.; Gao, F.; Ji, S.X.; et al. Transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A three-family cluster study in China. J. Infect. Dis. 2020, 22, 22. [Google Scholar]
- Ma, Q.-X.; Shan, H.; Zhang, H.-L.; Li, G.-M.; Yang, R.-M.; Chen, J.-M. Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2. J. Med. Virol. 2020, 92, 1567–1571. [Google Scholar] [CrossRef]
- Asadi, S.; Cappa, C.D.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci. Rep. 2020, 10, 15665. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Nanda, A.; Hung, I.; Kwong, A.; Man, V.C.-M.; Roy, P.; Davies, L.; Douek, M. Efficacy of surgical masks or cloth masks in the prevention of viral transmission: Systematic review, meta-analysis, and proposal for future trial. J. Evid. Based Med. Healthc. 2021, 14, 97–111. [Google Scholar] [CrossRef]
- Hetemäki, I.; Kääriäinen, S.; Alho, P.; Mikkola, J.; Savolainen-Kopra, C.; Ikonen, N.; Nohynek, H.; Lyytikäinen, O. An outbreak caused by the SARS-CoV-2 Delta variant (B.1.617.2) in a secondary care hospital in Finland, May 2021. Eurosurveillance 2021, 26, 2100636. [Google Scholar] [CrossRef]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef]
- Krishnamachari, B.; Morris, A.; Zastrow, D.; Dsida, A.; Harper, B.; Santella, A.J. The role of mask mandates, stay at home orders and school closure in curbing the COVID-19 pandemic prior to vaccination. Am. J. Infect. Control 2021, 49, 1036–1042. [Google Scholar] [CrossRef]
- Xu, H.; Gan, Y.; Zheng, D.; Wu, B.; Zhu, X.; Xu, C.; Liu, C.; Tao, Z.; Hu, Y.; Chen, M.; et al. Relationship Between COVID-19 Infection and Risk Perception, Knowledge, Attitude, and Four Nonpharmaceutical Interventions During the Late Period of the COVID-19 Epidemic in China: Online Cross-Sectional Survey of 8158 Adults. J. Med. Internet Res. 2020, 22, e21372. [Google Scholar] [CrossRef]
- Lio, C.F.; Cheong, H.H.; Lei, C.I.; Lo, I.L.; Yao, L.; Lam, C.; Leong, I.H. Effectiveness of personal protective health behaviour against COVID-19. BMC Public Health 2021, 21, 827. [Google Scholar] [CrossRef]
- Doung-Ngern, P.; Suphanchaimat, R.; Panjangampatthana, A.; Janekrongtham, C.; Ruampoom, D.; Daochaeng, N.; Eungkanit, N.; Pisitpayat, N.; Srisong, N.; Yasopa, O.; et al. Case-Control Study of Use of Personal Protective Measures and Risk for SARS-CoV 2 Infection, Thailand. Emerg. Infect. Dis. 2020, 26, 2607–2616. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Zhang, L.; Zhang, M.; Guo, D.; Wu, W.; Zhang, X.; Kan, G.L.; Jia, L.; Huo, D.; et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: A cohort study in Beijing, China. BMJ Glob. Health 2020, 5, e002794. [Google Scholar] [CrossRef]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winsløw, U.C.; et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers: A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 335–343. [Google Scholar] [CrossRef]
- Saunders, C.; Abel, G. Ecological studies: Use with caution. Br. J. Gen. Pract. 2014, 64, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Millat-Martinez, P.; Ouchi, D.; Roberts, C.h.; Alemany, A.; Corbacho-Monné, M.; Ubals, M.; Tobias, A.; Tebé, C.; Ballana, E.; et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect. Dis. 2021, 21, 629–636. [Google Scholar] [CrossRef]
- Dupraz, J.; Butty, A.; Duperrex, O.; Estoppey, S.; Faivre, V.; Thabard, J.; Zuppinger, C.; Greub, G.; Pantaleo, G.; Pasquier, J.; et al. Prevalence of SARS-CoV-2 in Household Members and Other Close Contacts of COVID-19 Cases: A Serologic Study in Canton of Vaud, Switzerland. Open Forum Infect. Dis. 2021, 8, ofab149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Ochoa, S.A.; Muka, T. Meta-analysis on facemask use in community settings to prevent respiratory infection transmission shows no effect. Int. J. Infect. Dis 2021, 103, 257–259. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, W.A. A five-day course of ivermectin may reduce the duration of COVID-19 illness. Int. J. Infect. Dis. 2021, 110, 93–94. [Google Scholar] [CrossRef]
- Hashim, H.A.; Maulood, M.F.; Rasheed, A.M.; Fatak, D.F.; Kabah, K.K.; Abdulamir, A.S. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv 2020. [Google Scholar] [CrossRef]
- Rajter, J.C.; Sherman, M.S.; Fatteh, N.; Vogel, F.; Sacks, J.; Rajter, J.J. Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study. Chest 2021, 159, 85–92. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Meyerowitz-Katz, G.; Heathers, J.A.J.; Brown, N.J.L.; Sheldrick, K.A. The lesson of ivermectin: Meta-analyses based on summary data alone are inherently unreliable. Nat. Med. 2021, 27, 1853–1854. [Google Scholar] [CrossRef]
- Hill, A.; Mirchandani, M.; Ellis, L.; Pilkington, V. Ivermectin for the prevention of COVID-19: Addressing potential bias and medical fraud. J. Antimicrob. Chemother. 2022, 77, 1413–1416. [Google Scholar] [CrossRef]
- O’Mathuna, D.P. Ivermectin and the Integrity of Healthcare Evidence During COVID-19. Front. Public Health 2022, 10, 788972. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanaj, E.; Muka, T. Misleading Meta-Analyses during COVID-19 Pandemic: Examples of Methodological Biases in Evidence Synthesis. J. Clin. Med. 2022, 11, 4084. https://doi.org/10.3390/jcm11144084
Llanaj E, Muka T. Misleading Meta-Analyses during COVID-19 Pandemic: Examples of Methodological Biases in Evidence Synthesis. Journal of Clinical Medicine. 2022; 11(14):4084. https://doi.org/10.3390/jcm11144084
Chicago/Turabian StyleLlanaj, Erand, and Taulant Muka. 2022. "Misleading Meta-Analyses during COVID-19 Pandemic: Examples of Methodological Biases in Evidence Synthesis" Journal of Clinical Medicine 11, no. 14: 4084. https://doi.org/10.3390/jcm11144084
APA StyleLlanaj, E., & Muka, T. (2022). Misleading Meta-Analyses during COVID-19 Pandemic: Examples of Methodological Biases in Evidence Synthesis. Journal of Clinical Medicine, 11(14), 4084. https://doi.org/10.3390/jcm11144084