Serum Concentration of Selected Angiogenesis-Related Molecules Differs among Molecular Subtypes, Body Mass Index and Menopausal Status in Breast Cancer Patients
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Patient Selection and Data
2.3. Sample Preparation
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feig, W.B.; Ching, D.C. (Eds.) The MD Anderson Surgical Oncology Handbook, 6th ed.; University of Texas MD Anderson Cancer Center: Houston, TX, USA, 2019; ISBN 978-1-4963-5815-8. [Google Scholar]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, K.N. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [PubMed]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Chen, W.L.; Zhang, F.; Wei, X.L.; Zeng, D.; Liang, Y.K.; Wu, J.D.; Zhang, L.Y.; Guo, C.P.; Zeng, H.C.; et al. Over-expression of both VEGF-C and Twist predicts poor prognosis in human breast cancer. Clin. Transl. Oncol. 2019, 21, 1250–1259. [Google Scholar] [CrossRef]
- Yotsumoto, F.; Tokunaga, E.; Oki, E.; Maehara, Y.; Yamada, H.; Nakajima, K.; Nam, S.O.; Miyata, K.; Koyanagi, M.; Doi, K.; et al. Molecular hierarchy of heparin-binding EGF-like growth factor-regulated angiogenesis in triple-negative breast cancer. Mol. Cancer Res. 2013, 11, 506–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yotsumoto, F.; Oki, E.; Tokunaga, E.; Maehara, Y.; Kuroki, M.; Miyamoto, S. HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. Int. J. Cancer 2010, 127, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Ruan, L.; Shang, D.; Wu, Y.; Lu, P.; Lü, P.; Yang, Y.; Wei, Y.; Dong, X.; Ren, D.; et al. Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Potent Target for Breast Cancer Therapy. Cancer Biother. Radiopharm. 2016, 31, 85–90. [Google Scholar] [CrossRef]
- Jansson, S.; Aaltonen, K.; Bendahl, P.O.; Falck, A.K.; Karlsson, M.; Pietras, K.; Rydén, L. The PDGF pathway in breast cancer is linked to tumour aggressiveness, triple-negative subtype and early recurrence. Breast Cancer Res. Treat. 2018, 169, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Mori, S.; Hamada, Y.; Hieda, M.; Kawaguchi, N.; Shaker, M.; Tao, Y.; Yoshidome, K.; Tsujimoto, M.; Matsuura, N. Platelet-derived growth factor regulates breast cancer progression via β-catenin expression. Pathobiology 2011, 78, 253–260. [Google Scholar] [CrossRef]
- Carvalho, I.; Milanezi, F.; Martins, A.; Reis, M.R.; Schmitt, F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005, 7, 788–795. [Google Scholar] [CrossRef] [Green Version]
- Jansson, S.; Bendahl, P.O.; Grabau, D.A.; Falck, A.K.; Fernö, M.; Aaltonen, K.; Rydén, L. The three receptor tyrosine kinases c-KIT, VEGFR2 and PDGFRα, closely spaced at 4q12, show increased protein expression in triple-negative breast cancer. PLoS ONE 2014, 9, e102176. [Google Scholar] [CrossRef]
- Ghosh, S.; Sullivan, A.C.; Zerkowski, P.M.; Molinaro, M.A.; Rimm, L.D.; Camp, L.R.; Chung, G.G. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum. Pathol. 2008, 39, 1835–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adhes. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Hirakata, T.; Kurozumi, S.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; Oyama, T.; Shirabe, K. VEGF-A Is Associated with the Degree of TILs and PD-L1 Expression in Primary Breast Cancer. Vivo 2020, 34, 2641–2646. [Google Scholar] [CrossRef]
- Dumond, A.; Pagès, G. Neuropilins, as Relevant Oncology Target: Their Role in the Tumoral Microenvironment. Front. Cell Dev. Biol. 2020, 8, 662. [Google Scholar] [CrossRef]
- Yasuoka, H.; Kodama, R.; Tsujimoto, M.; Yoshidome, K.; Akamatsu, H.; Nakahara, M.; Inagaki, M.; Sanke, T.; Nakamura, Y. Neuropilin-2 expression in breast cancer: Correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer 2009, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Arpel, A.; Gamper, C.; Spenlé, C.; Fernandez, A.; Jacob, L.; Baumlin, N.; Laquerriere, P.; Orend, G.; Crémel, G.; Bagnard, D. Inhibition of primary breast tumor growth and metastasis using a neuropilin-1 transmembrane domain interfering peptide. Oncotarget 2016, 7, 54723–54732. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Schneider, P.B.; Miller, D.K. Angiogenesis of Breast Cancer. J. Clin. Oncol. 2005, 23, 1782–1790. [Google Scholar] [CrossRef]
- Barron, A.G.; Goua, M.; Wahle, W.J.K.; Bermano, G. Circulating levels of angiogenesis-related growth factors in breast cancer: A study to profile proteins responsible for tubule formation. Oncol. Rep. 2017, 38, 1886–1894. [Google Scholar] [CrossRef] [Green Version]
- Bottrell, A.; Meng, Y.H.; Najy, J.A.; Hurst, N., Jr.; Kim, S.; Kim, J.C.; Kim, E.S.; Moon, A.; Kim, E.J.; Park, S.Y.; et al. An oncogenic activity of PDGF-C and its splice variant in human breast cancer. Growth Factors 2019, 37, 131–145. [Google Scholar] [CrossRef]
- Kim, S.; You, D.; Jeong, Y.; Yoon, S.Y.; Kim, S.; Lee, J.E. Inhibition of platelet-derived growth factor C and their receptors additionally increases doxorubicin effects in triple-negative breast cancer cells. Eur. J. Pharmacol. 2021, 895, 173868. [Google Scholar] [CrossRef] [PubMed]
- Crawford, Y.; Kasman, I.; Yu, L.; Zhong, C.; Wu, X.; Modrusan, Z.; Kaminker, J.; Ferrara, N. PDGF-C Mediates the Angiogenic and Tumorigenic Properties of Fibroblasts Associated with Tumors Refractory to Anti-VEGF Treatment. Cancer Cell 2009, 15, 21–34. [Google Scholar] [PubMed] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, J.M. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, F.; Soares, R. Obesity and cancer phenotype: Is angiogenesis a missed link? Life Sci. 2015, 139, 16–23. [Google Scholar]
- Gu, J.W.; Young, E.; Patterson, G.S.; Makey, L.K.; Wells, J.; Huang, M.; Tucker, B.K.; Miele, L. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol. Ther. 2011, 11, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kishida, K.; Shimomura, I.; Maeda, N.; Nagaretani, H.; Matsuda, M.; Nishizawa, H.; Kihara, S.; Funahashi, T.; Matsuzawa, Y. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem. Biophys. Res. Commun. 2002, 292, 781–786. [Google Scholar] [CrossRef]
- Trédan, O.; Lacroix-Triki, M.; Guiu, S.; Mouret-Reynier, M.A.; Barrière, J.; Bidard, F.C.; Braccini, A.L.; Mir, O.; Villanueva, C.; Barthélémy, P. Angiogenesis and tumor microenvironment: Bevacizumabin the breast cancer model. Target. Oncol. 2015, 10, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tamimi, M.R.; Collins, C.L.; Schnitt, J.S.; Gilmore, L.H.; Connolly, L.J.; Colditz, A.G. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: Results from the Nurses’ Health Study. Breast Cancer Res. Treat. 2011, 129, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Dent, S.F. The role of VEGF in triple-negative breast cancer: Where do we go from here? Ann. Oncol. 2009, 20, 1615–1617. [Google Scholar] [CrossRef]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtio, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef]


| Healthy | Breast Cancer Patients | ||
|---|---|---|---|
| Median (Interquartile Range) | Median (Interquartile Range) | p-Value | |
| Total | N = 31 | N = 205 | |
| VEGF (pg/mL) | 242.8 (113–437.4) | 270.8 (144.3–407) | 0.652 |
| HB-EGF (pg/mL) | 142.3 (118.8–173.7) | 128.9 (100.5–172.6) | 0.152 |
| PDGF-CC (pg/mL) | 985.8 (752.9–1203) | 1032.5 (824–1222.5) | 0.333 |
| NRP-1 (pg/mL) | 264.9 (194.2–311.5) | 257.7 (218.3–301.1) | 0.698 |
| VEGF (pg/mL) | HB-EGF (pg/mL) | PDGF-CC (pg/mL) | NEUROPILIN-1 (pg/mL) | ||
|---|---|---|---|---|---|
| Ν | Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | |
| Total | |||||
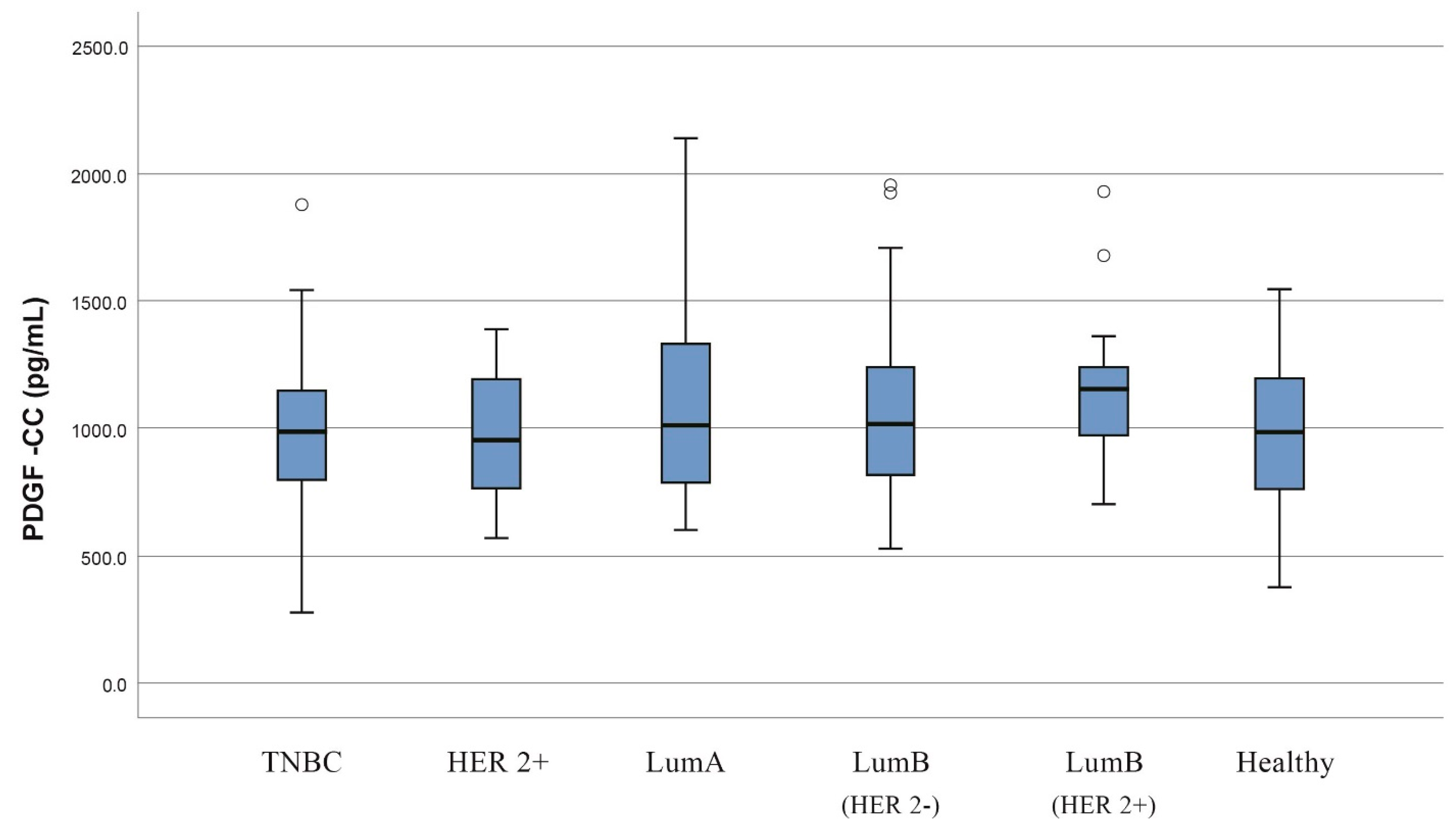
| Luminal A | 60 | 249.3 (159.9–377.7) | 137.7 (102.8–169.9) | 1004 (776.7–1350.3) | 261.1 (214.1–312.3) |
| Luminal B | 75 | 316.5 (145.4–449) | 128.4 (99–158.6) | 1060 (836.9–1263) | 255 (221.2–305.8) |
| Luminal B (HER2−) | 51 | 337.2 (166.4–449) | 121.2 (98.9–157.8) | 1018 (793.3–1263) | 245.1 (221.2–294) |
| Luminal B (HER2+) | 24 | 261.6 (133.5–521.9) | 138.2 (102.4–166.6) | 1156 (968.1–1254.5) | 274.3 (225.1–333.4) |
| Triple Negative | 33 | 337.9 (198.9–478.8) | 121.8 (89.3–189.4) | 984.7 (768.8–1161.5) | 242.3 (199.2–288.5) |
| HER2+ | 19 | 273.6 (114.3–380.7) | 117.9 (102.4–158.8) | 951.7 (736.7–1220) | 250.1 (231.7–323) |
| Healthy | 31 | 242.8 (113–437.4) | 142.3 (118.8–173.7) | 985.8 (752.9–1203) | 264.9 (194.2–311.5) |
| VEGF | HB-EGF | |||
| Median (Interquartile Range) | p-Value | Median (Interquartile Range) | p-Value | |
| Grade | 0.757 | 0.551 | ||
| 1 | 303.0 (114.4–437.7) | 125.5 (87.1–152.3) | ||
| 2 | 266.2 (145.4–398.8) | 129.3 (101.1–165.4) | ||
| 3 | 311.5 (143.5–416.4) | 127.7 (99.4–178.2) | ||
| Stage | 0.365 | 0.219 | ||
| Ι | 310 (166–431) | 129 (87–160) | ||
| ΙΙ | 229 (100–374) | 118 (101–158) | ||
| ΙΙΙ | 337 (216–398) | 142 (124–210) | ||
| ΙV | 267 (130–472) | 119 (91–172) | ||
| PDGF-CC | NEUROPILIN-1 | |||
| Median (Interquartile Range) | p-Value | Median (Interquartile Range) | p-Value | |
| Grade | 0.731 | 0.585 | ||
| 1 | 948.7 (719.8–1265.0) | 271.3 (249.3–284.3) | ||
| 2 | 1001.0 (793.3–1215.0) | 247.3 (213.7–300.5) | ||
| 3 | 1041.0 (850.8–1201.0) | 264.1 (223.8–321.9) | ||
| Stage | 0.169 | 0.947 | ||
| Ι | 965 (762–1.189) | 264 (215–295) | ||
| ΙΙ | 1.009 (905–1.188) | 260 (234–290) | ||
| ΙΙΙ | 1.171 (934–1.354) | 250 (206–322) | ||
| ΙV | 1.007 (720–1.143) | 242 (218–350) |
| BMI | |||
|---|---|---|---|
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | ||
| Median (Interquartile Range) | Median (Interquartile Range) | p-Value | |
| Healthy | |||
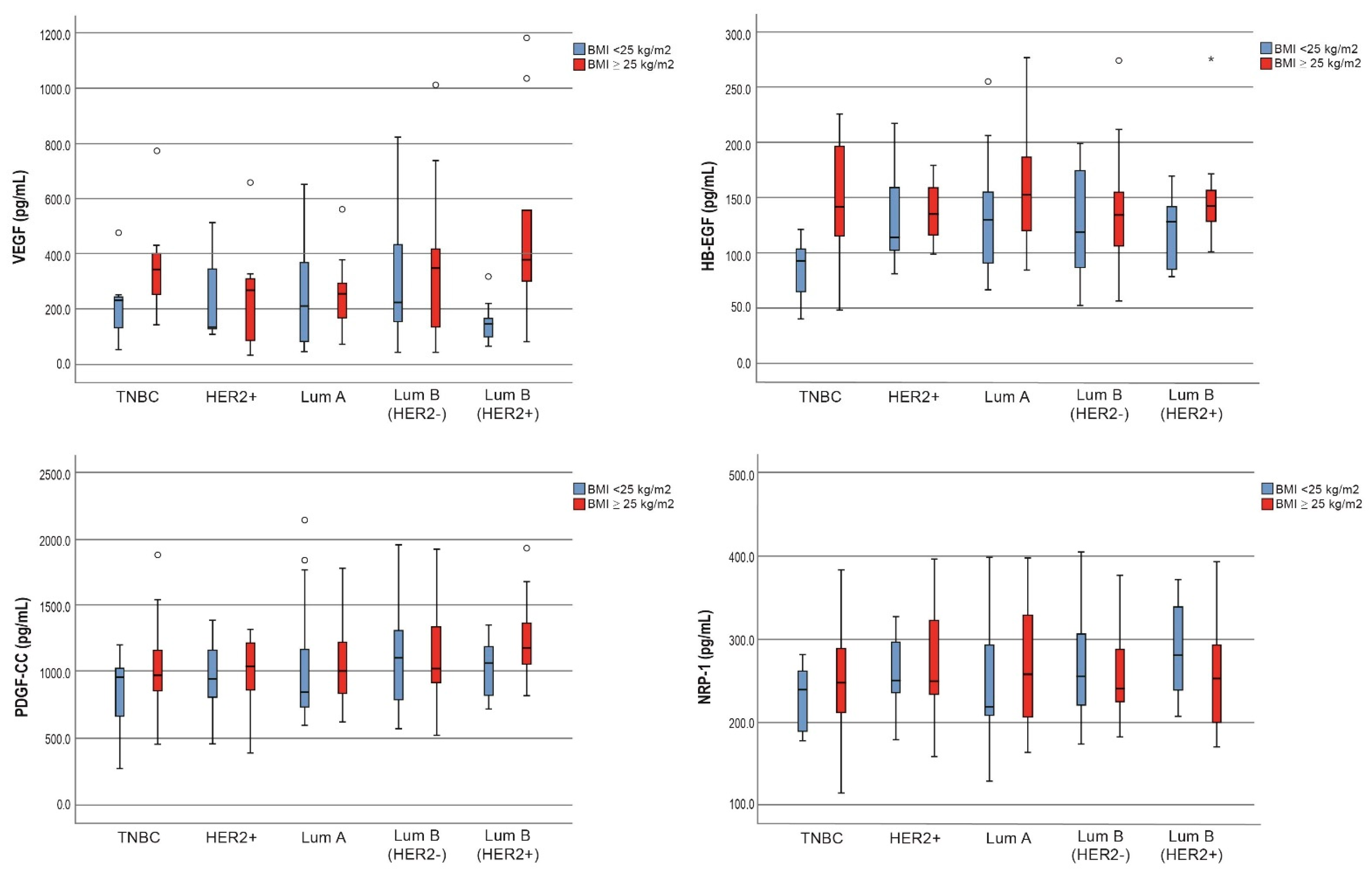
| VEGF | 211.1 (91.6–315.3) | 292.9 (156.3–502.1) | 0.224 |
| HB-EGF | 130.4 (108.8–151.1) | 155.2 (125.9–190.0) | 0.077 |
| PDGF-CC | 1026.5 (631.3–1240.8) | 938.9 (759.3–1126.0) | 0.790 |
| NEUROPILIN-1 | 303.8 (193.3–332.7) | 233.8 (190.4–270.2) | 0.142 |
| Breast Cancer | |||
| VEGF | 212.2 (104.2–375.2) | 280.5 (168.7–374.2) | 0.049 |
| HB-EGF | 111.3 (88.1–149.2) | 142.6 (112.2–179.8) | <0.001 |
| PDGF-CC | 1009.0 (758.1–1211.0) | 1034.0 (885.0–1315.0) | 0.275 |
| NEUROPILIN-1 | 254.5 (214.4–313.6) | 249.8 (213.7–300.5) | 0.670 |
| IDC | |||
| VEGF | 212.9 (123.3–385.7) | 306.7 (185.3–402.5) | 0.084 |
| HB-EGF | 116.4 (87.4–152.2) | 141.3 (109.2–181.5) | 0.004 |
| PDGF-CC | 1018.5 (742.8–1206.0) | 1027.5 (874.0–1244.0) | 0.493 |
| NEUROPILIN-1 | 251.5 (214.4–303.1) | 248.3 (220.5–301.8) | 0.908 |
| ILC | |||
| VEGF | 174.6 (72.3–271.8) | 270.2 (227.8–371.4) | 0.035 |
| HB-EGF | 110.2 (95.7–173.6) | 129.3 (113.7–162.2) | 0.376 |
| PDGF-CC | 985.4 (739.5–1267.8) | 1034.0 (867.7–1560.5) | 0.376 |
| NEUROPILIN-1 | 238.7 (213.8–321.9) | 213.7 (185.3–321.8) | 0.295 |
| Lum B (HER2+) | |||
| VEGF | 145.4 (91.2–193.9) | 379.4 (256.9–795.5) | 0.008 |
| HB-EGF | 128.2 (92.6–144.7) | 142.6 (117.4–163.8) | 0.094 |
| PDGF-CC | 1065.0 (808.4–1229.0) | 1183.0 (988.4–1522.5) | 0.161 |
| NEUROPILIN-1 | 279.6 (229.4–351.9) | 252.5 (185.4–305.5) | 0.297 |
| Lum B (HER2−) | |||
| VEGF | 224.4 (128.4–471.5) | 348.4 (126.1–424.8) | 0.626 |
| HB-EGF | 118.7 (86.4–174.2) | 134.4 (105.3–159.5) | 0.516 |
| PDGF-CC | 1108.0 (790.5–1315.0) | 1025.0 (905.9–1346.8) | 0.850 |
| NEUROPILIN-1 | 255.0 (214.6–329.6) | 240.5 (223.2–288.8) | 0.588 |
| HER2+ | |||
| VEGF | 135.8 (128.0–380.7) | 268.9 (74.4–319.4) | 0.536 |
| HB-EGF | 114.0 (102.4–189.6) | 134.8 (110.7–161.7) | 0.536 |
| PDGF-CC | 951.7 (685.5–1171.0) | 1041.0 (799.6–1256.0) | 0.999 |
| NEUROPILIN-1 | 250.1 (230.5–323.0) | 249.8 (232.7–332.2) | 0.837 |
| TN | |||
| VEGF | 232.4 (82.7–252.1) | 343.7 (240.6–412.9) | 0.083 |
| HB-EGF | 92.6 (56.0–111.1) | 141.4 (110.1–198.9) | 0.010 |
| PDGF-CC | 960.8 (596.0–1043.0) | 974.3 (827.4–1164.8) | 0.182 |
| NEUROPILIN-1 | 238.6 (183.2–267.9) | 247.1 (205.3–290.8) | 0.299 |
| Healthy | Breast Cancer Patients | ||
|---|---|---|---|
| Median (Interquartile Range) | Median (Interquartile Range) | p-Value | |
| Premenopause | |||
| VEGF (pg/mL) | 239.2 (123.3–413.4) | 240 (128.4–317.6) | 0.988 |
| HB-EGF (pg/mL) | 144.4 (137.1–176.5) | 125.2 (94.7–171.2) | 0.039 |
| PDGF-CC (pg/mL) | 1048 (920.3–1228) | 1077.5 (885–1265) | 0.981 |
| NRP-1 (pg/mL) | 271.1 (207.1–324.1) | 254.8 (210.6–293.4) | 0.278 |
| Postmenopause | |||
| VEGF (pg/mL) | 259.9 (98–523.6) | 287.5 (161.8–409.2) | 0.906 |
| HB-EGF (pg/mL) | 120.7 (114–162.1) | 129.3 (102.4–174.2) | 0.995 |
| PDGF-CC (pg/mL) | 835.8 (622.6–1105) | 984.7 (800.6–1199) | 0.040 |
| NRP-1 (pg/mL) | 237.7 (189.1–291.7) | 260.4 (221.2–305.8) | 0.129 |
| VEGF | HB-EGF | PDGF-CC | NRP-1 | |
|---|---|---|---|---|
| Premenopause | p-Value | p-Value | p-Value | p-Value |
| IDC vs. Healthy | 0.674 | 0.023 | 0.769 | 0.355 |
| ILC vs. Healthy | 0.634 | 0.396 | 0.711 | 0.220 |
| DCIS vs. Healthy | 0.493 | 0.543 | 0.543 | 0.880 |
| IDC vs. ILC | 0.485 | 0.566 | 0.816 | 0.545 |
| IDC vs. DCIS | 0.328 | 0.682 | 0.350 | 0.620 |
| ILC vs. DCIS | 0.438 | 0.999 | 0.898 | 0.438 |
| Postmenopause | ||||
| IDC vs. Healthy | 0.690 | 0.956 | 0.045 | 0.144 |
| ILC vs. Healthy | 0.575 | 0.360 | 0.227 | 0.227 |
| DCIS vs. Healthy | 0.462 | 0.432 | 0.076 | 0.145 |
| IDC vs. ILC | 0.136 | 0.202 | 0.390 | 0.830 |
| IDC vs. DCIS | 0.107 | 0.319 | 0.619 | 0.340 |
| ILC vs. DCIS | 0.657 | 0.094 | 0.363 | 0.511 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balalis, D.; Tsakogiannis, D.; Kalogera, E.; Kokkali, S.; Tripodaki, E.; Ardavanis, A.; Manatakis, D.; Dimas, D.; Koufopoulos, N.; Fostira, F.; et al. Serum Concentration of Selected Angiogenesis-Related Molecules Differs among Molecular Subtypes, Body Mass Index and Menopausal Status in Breast Cancer Patients. J. Clin. Med. 2022, 11, 4079. https://doi.org/10.3390/jcm11144079
Balalis D, Tsakogiannis D, Kalogera E, Kokkali S, Tripodaki E, Ardavanis A, Manatakis D, Dimas D, Koufopoulos N, Fostira F, et al. Serum Concentration of Selected Angiogenesis-Related Molecules Differs among Molecular Subtypes, Body Mass Index and Menopausal Status in Breast Cancer Patients. Journal of Clinical Medicine. 2022; 11(14):4079. https://doi.org/10.3390/jcm11144079
Chicago/Turabian StyleBalalis, Dimitrios, Dimitrios Tsakogiannis, Eleni Kalogera, Stefania Kokkali, Elli Tripodaki, Alexandros Ardavanis, Dimitrios Manatakis, Dionysios Dimas, Nektarios Koufopoulos, Florentia Fostira, and et al. 2022. "Serum Concentration of Selected Angiogenesis-Related Molecules Differs among Molecular Subtypes, Body Mass Index and Menopausal Status in Breast Cancer Patients" Journal of Clinical Medicine 11, no. 14: 4079. https://doi.org/10.3390/jcm11144079
APA StyleBalalis, D., Tsakogiannis, D., Kalogera, E., Kokkali, S., Tripodaki, E., Ardavanis, A., Manatakis, D., Dimas, D., Koufopoulos, N., Fostira, F., Korkolis, D., Misitzis, I., Vassos, N., Spiliopoulou, C., Vlachodimitropoulos, D., Bletsa, G., & Arkadopoulos, N. (2022). Serum Concentration of Selected Angiogenesis-Related Molecules Differs among Molecular Subtypes, Body Mass Index and Menopausal Status in Breast Cancer Patients. Journal of Clinical Medicine, 11(14), 4079. https://doi.org/10.3390/jcm11144079







