Abstract
(1) Background: There is a need for a brief assessment of cognitive function, both in patient care and scientific research, for which the Montreal Cognitive Assessment (MoCA) is a psychometrically reliable and valid tool. However, fine-grained normative data allowing for adjustment for age, education, and/or sex are lacking, especially for its Memory Index Score (MIS). (2) Methods: A total of 820 healthy individuals aged 18–91 (366 men) completed the Dutch MoCA (version 7.1), of whom 182 also completed the cued recall and recognition memory subtests enabling calculation of the MIS. Regression-based normative data were computed for the MoCA Total Score and MIS, following the data-handling procedure of the Advanced Neuropsychological Diagnostics Infrastructure (ANDI). (3) Results: Age, education level, and sex were significant predictors of the MoCA Total Score (Conditional R2 = 0.4, Marginal R2 = 0.12, restricted maximum likelihood (REML) criterion at convergence: 3470.1) and MIS (Marginal R2 = 0.14, REML criterion at convergence: 682.8). Percentile distributions are presented that allow for age, education and sex adjustment for the MoCA Total Score and the MIS. (4) Conclusions: We present normative data covering the full adult life span that can be used for the screening for overall cognitive deficits and memory impairment, not only in older people with or people at risk of neurodegenerative disease, but also in younger individuals with acquired brain injury, neurological disease, or non-neurological medical conditions.
1. Introduction
The assessment of cognitive function in clinical practice is crucial as part of the diagnostic work-up of patients with acquired brain injury, neurodegenerative disease, and other medical conditions and has become increasingly important as an outcome measure in clinical trials and experimental research. Neuropsychological assessment is the gold standard for measuring cognitive performance in various domains, such as memory, orientation, executive function, language, and visuoconstructive ability [1]. However, neuropsychological assessments, in general, are time-consuming, costly, and require expertise in administration, scoring, and interpretation. As a result, tools that enable a shorter examination of cognitive performance have been extensively studied over the years. This has resulted in cognitive screening instruments that have gained widespread use yet have poor psychometric properties, such as the Mini-Mental State Examination [2]. Other cognitive screening tools have been developed with acceptable psychometric properties, such as the Addenbrooke’s Cognitive Examination (currently in its third edition, the ACE-III) or the Revised Cambridge Cognitive Assessment (CAMCOG-R). However, these screeners have been predominantly developed for the detection of dementia, lack the sensitivity to detect more subtle cognitive impairments, and may not be available in all territories [3]. The Montreal Cognitive Assessment (MoCA) has been developed to overcome these shortcomings, with the aim to better detect individuals with mild cognitive impairment (MCI) [4]. In short, the MoCA consists of several items addressing the domains of visuoconstruction, executive function, memory, language, orientation, and attention that can be easily administered and scored (with a maximum score of 30) and that also comes with parallel versions to minimize test-specific practice effects (www.mocatest.org (accessed on 20 June 2022)).
Since its introduction, the MoCA has gained international popularity, as its developers have been very successful in introducing culture-sensitive and language-specific international versions. Also, strict harmonization guidelines have been maintained to enable international comparison. Moreover, its psychometric properties have been studied extensively, showing a good test–retest reliability [5,6]. However, results on the validity of the instrument’s cut-off scores are somewhat mixed. Traditionally, the MoCA employs a cut-off score for the detection of individuals with dementia or MCI, as compared to cognitively unimpaired older adults. Originally, a cut-off score of <26 was established for detecting both MCI and Alzheimer’s dementia in healthy older adults, with a sensitivity of 90–100% and a specificity of 87% [4]. However, with regard to this cut-off score, several studies demonstrated a lower sensitivity and specificity for detecting MCI (e.g., 72%/73% [7]; 85%/81% [8]; 96%/58% [9]). A low specificity has also been reported for the detection of dementia [10]. One explanation for these mixed findings may lie in the demographic variables of the participants, notably educational attainment and age, which may vary considerably across studies and geographical regions. In the original MoCA study [4], no effect of age on the MoCA score was reported, whereas individuals with 12 years of education or less performed worse on the MoCA, resulting in an education adjustment of 1 additional point for those with 12 years of education or less. However, this original study only included a relatively small sample of older adults, limiting the participants’ age range. Later studies using samples with a wider age range showed that age, sex, and/or education level were significant predictors of the MoCA total score [6,11].
The need for a more fine-grained adjustment for age, sex, and education in interpreting an individual’s performance of the MoCA has become even more important as the MoCA is increasingly being used in a variety of patient samples. While originally developed for the detection of MCI and dementia due to Alzheimer’s disease, the MoCA has been studied to assess cognitive deficits in patients with a variety of medical conditions [12], including HIV [13], Parkinson’s disease [14], multiple sclerosis [15], stroke [16], frontotemporal dementia [17], substance-related cognitive disorders [18,19], cardiac arrest [20], fibromyalgia [21], Huntington’s disease [22], syncope and unexpected falls [23], cerebellar disease [24], schizophrenia [25], sickle cell disease [26], type 2 diabetes [27], and COVID-19 [28]. Many of these conditions affect younger adults, who may perform above established cut-off scores based on samples of older individuals even when cognitive impairment is present. Alternatively, individuals with low education levels (or even poor literacy) may perform below the cut-off score in the absence of cognitive deficits. This highlights the importance of age- and education-adjusted normative data for the MoCA total score in individuals below the age of 65. So far, several studies have established demographically-adjusted normative data for different language versions of the MoCA (see Table 1 for an overview). However, many studies that present normative to date focused on older adults exclusively, some studies have included only small samples, and no studies have examined samples from Northwest Europe.

Table 1.
Overview of available normative data for international versions of the Montreal Cognitive Assessment (MoCA).
In addition, the Memory Index Score (MoCA-MIS) is a relatively recent addition to the standard MoCA (with a maximum score of 15), which extends the classic free-recall memory subtest with a cued-recall and multiple-choice recognition test [61]. The MoCA-MIS has been studied in individuals with MCI, showing that a MoCA-MIS cut-off of <7, in addition to a total MoCA cut-off score of < 20, resulted in a 90.5% conversion rate for Alzheimer’s dementia after 18 months. Others [62] have demonstrated that the MoCA-MIS was able to distinguish cognitively unimpaired older adults from MCI patients to the same extent as a story recall test. Additionally, relative to other MoCA indices, the MoCA-MIS demonstrated the largest group difference between patients with alcohol Korsakoff’s syndrome, patients with alcohol-related cognitive disorder other than Korsakoff’s syndrome, and uncomplicated alcoholics [63]. Although the study by Kaur et al. [62] administered the MoCA-MIS in 2205 older adults (mean age = 72.7), they did not provide age and education-adjusted scores for this large sample (apart from a non-informative group mean of 12.2, SD = 2.8). Apolinario et al. [30] presented regression-based normative data for 597 cognitively unimpaired Brazilians aged 50 to 90. However, 81% (n = 484) of the participants in their sample completed only 10 or fewer years of formal education, making this sample not representative of the population of many other countries. Moreover, data on the MoCA-MIS for individuals younger than 50 are lacking altogether.
The present study sets out to compute age- and education-based normative data for the Dutch language version of the MoCA and the MoCA-MIS for individuals aged 18–91 using a demographically-adjusted regression-based normative approach, the validity of which has long been established for neuropsychological tests [64,65]. Such normative data facilitate the use of the MoCA as a short but valid cognitive screen in patient samples other than older adults with MCI or dementia.
2. Materials and Methods
2.1. Participants
A total of 825 healthy participants were included in this study (Table 2 shows the demographics of all participants).

Table 2.
Number of individuals per age group, stratified for education (into three groups) and sex (for descriptive purposes).
All participants took part in research projects as healthy volunteers. Of these, 363 were volunteers in the ABRIM cohort who participated in aging research across the lifespan at the Donders Centre for Cognition, and 210 individuals took part as volunteers in a study on the psychometric properties of the MoCA [6]. In addition, healthy control samples from various other studies were included: 45 individuals participated in [68,69], 27 in [70], 60 people participated as controls in [71], 25 in a study by Sutter and colleagues [72], and 65 in studies by De Jonghe et al. (including [7,23]). Exclusion criteria included age younger than 18, the presence of any condition with a profound impact on the brain and cognitive health beyond normal aging, such as psychiatric disorders (e.g., major depressive disorder, bipolar disorder, schizophrenia), neurological conditions (e.g., dementia, history of stroke, epilepsy), substance use disorders, or a history of other major health conditions that could impact cognition (e.g., history of brain tumor). Care was taken to include individuals representative of all age groups and education levels, with a balanced sex distribution. Education level was scored in 7 categories, based on the Dutch educational system [66], which is similar to UNESCO’s ISCED scale [73], in which 1 reflects less than primary school and 7 an academic degree. These levels were then categorized as low (levels 1–4), average (level 5), or high (levels 6–7) for descriptive purposes only. All participants were fluent in Dutch.
2.2. Procedure
All participants were administered the authorized paper-and-pencil Dutch version of the Montreal Cognitive Assessment 7.1 (www.mocatest.org (accessed on 20 June 2022)). The optional cued-recall and multiple-choice memory test items were administered in n = 184, enabling the calculation of the MIS (maximum score = 15). Test administration and scoring were performed by trained psychologists in accordance with the test instructions and scoring manual. The MoCA Total Score reflects the education-uncorrected Total Score (maximum = 30). Higher scores reflect better performance.
2.3. Data Processing and Analyses
As the data are part of the larger Advanced Neuropsychological Diagnostics Infrastructure (ANDI), the standardized data-handling procedure for ANDI was followed. More details about this process can be found elsewhere [74], but we will provide a brief summary of all the data processing steps here.
First, all data from different sources were combined in a single data file. For each source, a unique identifier was added. After merging the data, we checked whether all recorded values were valid observations for healthy individuals. We defined ‘extreme borders’, with, on one side, the high border (set as the maximum possible score) and on the other, the low border, which reflected the lowest possible score that could be obtained from a person given they are indeed cognitively healthy. Scores exceeding the high border (coding errors) or low border (indicative of severe pathology) were removed from the dataset in order not to overestimate the variance. Age was coded as male = 0 and female = 1; education was coded in the aforementioned 7 categories.
Subsequently, we fitted a multi-level regression model to determine which scores were demographically-corrected outliers. Not all outliers can be found by looking at a single criterion value. Neuropsychological test scores depend, to some extent, on the demographic characteristics of the person. For this reason, we wanted to parse out the effects of age, sex, and level of education and determine which scores were abnormal given this demographic correction. Because the data from both the MoCA total score and MoCA MIS originated from different sources, a multi-level model was fit to consider the differences between studies [75]. Although level of education is an ordinal scale, we treated it as an interval scale and estimated the linear effect of education to avoid estimating separate parameters for all levels of education. To determine which demographic corrections were necessary, we used a backward selection procedure, thus removing effects if removal resulted in a lower Akaike Information Criterion (AIC) [76]. After fitting the multi-level model and selecting the appropriate demographic terms, we used the residuals (and not the raw scores) to decide whether the scores were abnormal. We used the median absolute deviation from the median (MAD) [77] instead of the more common approach of standard deviations because a few outlying scores can increase the standard deviation considerably. We used -3.5 MAD as a cut-off criterion, meaning all residuals exceeding -3.5 MAD were removed.
As the goal of this paper is to create regression formulas with which normative comparisons can be made, it is, therefore, necessary that the dependent variables are normally distributed [78]. Cognitive screening instruments such as the MoCA are not normally distributed and are usually left-skewed, also due to the effects of demographic variables [79]. One solution is to partial out the effects of demographic variables by means of using the residual scores from a regression analysis. However, these residual scores may still not be normal. We, therefore, chose to partial out the effects of the demographic variables and, as a next step, transformed all scores as this is recommended to meet the assumption of normality [80]. Instead of transforming the raw scores by common transformations, such as the square root of the raw scores, we used the Box–Cox procedure [81,82] to find the power transformation to best approximate the normal distribution. The Box–Cox procedure uses an algorithm that looks at several possible power transformations of the raw data (e.g., 0.501, 0.502, 0.503, etc.) and evaluates the distribution of the residuals with each power transformation. The transformation that results in the best approximation of normality for the residuals is saved (using an acceptable range of −2.0–+2.0 for skewness and −0.7–+0.7 for kurtosis [83]). By applying the Box–Cox-selected power transformation to the raw data, the residuals (from the previously selected models) are as normally distributed as possible [78]. The power transformations may result in very small or large values on the MoCA, which may be difficult to interpret. Therefore, we standardized all these transformed scores to the familiar z-scale with a mean of 0 and a standard deviation of 1. In the final step, the multi-level regression models were again fitted to obtain the parameter estimates for the clean, normalized dataset. As before, the AIC selects the demographic terms. The final model was then saved.
The resulting regression analyses were then used to compute the expected score (ES) for the transformed and standardized MoCA Total Score and MoCA-MIS and back-transformed to the original scoring scale of the MoCA Total Score and MoCA-MIS. Subsequently, residual scores (RS) were calculated for each individual by subtracting each individual’s expected score from the observed score (RS = OS − ES). The frequency distribution for the residue scores for the MoCA Total Score and MoCA-MIS was then converted into a percentile distribution [84].
All analyses were performed in RStudio [85].
3. Results
Figure 1 shows the flowchart of the data processing and the model fit, resulting in final samples of n = 820 for the MoCA total score and n = 182 for the MoCA-MIS, respectively. The Box–Cox power transformation with an exponent of 2.85 for the MoCA Total Score resulted in a skewness estimate of −0.091 and a kurtosis of 2.355. For the MoCA-MIS, Box–Cox power transformation with an exponent of 3.09 resulted in a skewness estimate of 0.157 and a kurtosis of 1.996, which are well within the acceptable range for normality.
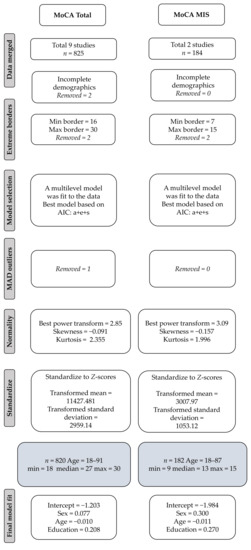
Figure 1.
Flowchart showing the included studies and their data processing.
For the MoCA Total Score, we used AIC model selection. The best-fit model included all transformed and standardized predictor variables, age, sex, and education, resulting in the following regression formula: −1.203 + 0.077 × sex − 0.01 × age + 0.208 × education (Conditional R2 = 0.4, Marginal R2 = 0.12, restricted maximum likelihood (REML) criterion at convergence: 3470.1). Table A1 in Appendix A shows the percentile distribution for the MoCA Total Score, stratified for age, education level, and sex.
For the MoCA-MIS, the same procedure was followed, and, in this case, also all predictor variables were included in the model, resulting in the following regression formula: −1.984 + 0.3 × sex − 0.011 × age + 0.270 × education (Marginal R2 = 0.14. REML criterion at convergence: 682.8). Random effect variances were not high enough to calculate R2 for random effects for the MoCA-MIS. Table A2 in Appendix A shows the regression-based percentile distributions for the MoCA MIS stratified for age group, education level, and sex.
4. Discussion
Here, we present regression-based normative data for the Dutch version of the MoCA and the MoCA-MIS based on a cognitively unimpaired sample of 820 individuals aged between 18 and 91. For both the MoCA Total Score and the MoCA-MIS, we found that age, educational attainment (using three categories: low, average, and high), and sex were significant predictors. The age-, education-, and sex-adjusted percentile distributions we present can be used for a fine-grained interpretation of MoCA(-MIS) scores, for instance, using recently published consensus criteria [86] (see Table 3). Such an approach is more valid than using the fixed cut-off score [4] that does not take age into account and which has only a limited adjustment for education level (i.e., one ‘bonus point’ awarded to individuals with 12 years of education or less). Furthermore, our wide age range also enables the administration and interpretation of MoCA scores in younger adults, which is relevant since the MoCA is increasingly being used in study samples or patients of a younger age and in disorders outside the field of mild cognitive impairment or dementia.

Table 3.
Consensus criteria for converting percentiles to diagnostically meaningful labels (based on [85]).
The finding that younger age and higher educational attainment were associated with a higher MoCA Total Score is in line with other international studies using the MoCA, most of which demonstrate considerable age and education effects (see Table 1). These results agree with evidence from most other neuropsychological tests, as the performance on those tests is consistently predicted by education level or years of education [87]. In addition, there is abundant evidence that cognitive function declines over time across the life span, especially in cognitive domains that represent fluent abilities such as executive function, working memory, and episodic memory [88], which are well represented in the MoCA. To date, sex differences on the MoCA Total Score have been reported, especially in larger samples (see also Table 1), in line with our results. In our sample, sex was also found to be a predictor for the MoCA-MIS, with women outperforming men. This corroborates large-scale studies showing sex differences in the memory domain, with women overall performing better on verbal memory tests, while men tend to perform better on spatial memory tasks [89]. However, as in our study sample, it should be noted that sex differences in cognitive functions are typically very small yet significant in large samples, while the variability in performance within each sex is overall substantially larger than the differences between the two sexes [90].
The results of our study also stress the problematic nature of applying a single cut-off score to determine whether an individual is cognitively impaired, thus ignoring the substantial effect of demographic variables on the test performance. This was also recently noted in a study by Engedal et al. [36]. In their study sample of 4780 cognitively unimpaired individuals over 70, a normal scoring range of 22–27 was found, with approximately half of their participants obtaining a score below the established cut-off score of 26 that is said to be indicative of cognitive impairment [4]. This is not only due to the design of the Engedal et al. [36] study, which consisted of older adults over the age of 70, as in our sample, we also found a substantial proportion of participants performing below the cut-off scores for cognitive impairment and dementia, even in the younger ages. Moreover, it is important to stress, as also outlined by Engedal et al. [36], that the original study by Nasreddine et al. [4] adopted a design fully different from ours, as their aim was not to publish demographically-adjusted normative data but to compare a group of healthy volunteers with individuals with MCI or Alzheimer’s dementia. It should also be noted that their sample of cognitively unimpaired was relatively small (n = 90, mean age 72.8, mean years of education 13.3) and that both the MCI and the dementia sample in that study had fewer years of education (12.3 and 10.0 respectively), possibly resulting in a too liberal cut-off score, resulting in poor specificity. In our study and those of others (also see [36] for a brief overview), a low education level may result in low MoCA scores in cognitively unimpaired individuals. This point is further illustrated by a recently published study [91] in a large and highly educated US sample (n = 3650), in which 26.8% of cognitively normal White participants and 57.9% of cognitively normal Black individuals were misclassified as being ‘cognitively impaired’ when applying the cut-off of 26. Clearly, the specificity of a single cut-off score for the MoCA is too low for validly substantiating clinical classifications, making it crucial to apply demographically-adjusted normative analyses when interpreting MoCA scores to minimize the risk of false-positive outcomes [56,91,92].
Our study is the first to present age-, education-, and sex-adjusted normative data for the MoCA-MIS in younger adults (i.e., <50 years of age). This score was introduced recently [61], demonstrating that the MoCA-MIS was promising in predicting the conversion from mild cognitive impairment to Alzheimer’s dementia. Others [63] demonstrated that the MoCA-MIS was able to distinguish between patients with Korsakoff’s amnesia, patients with other alcohol-related cognitive disorders, and individuals with alcohol use disorder without cognitive deficits. More research on the applicability of the MoCA-MIS is needed in other clinical groups. Furthermore, the psychometric properties of the MoCA-MIS, notably the test–retest reliability, are suboptimal, probably because of the skewed nature of this variable due to ceiling effects in cognitively unimpaired individuals [6]. Our analyses took the non-normality and skewness of this variable into account, making the presented normative data of the MoCA-MIS applicable for use in clinical samples. It should nevertheless be emphasized that the MoCA-MIS, which relies on the encoding, free and cued recall, and recognition of five words, is not a substitute for a more extensive memory assessment, which typically involves memory for word lists, pictures, spatial locations, or paired associates [79].
Our study also has some limitations. First, although our total sample size is large, our sample size is modest for the MoCA-MIS, as this optional part was administered only to a subset of participants. Furthermore, even larger data sets of cognitively unimpaired individuals for the MoCA exist. However, our regression-based statistical approach overcomes the problem of stratified norms that numbers tend to be small in some age and education strata, as the regression-based norm has been shown to require 2.5–5.5 times smaller samples compared to traditional norming [64]. Furthermore, most larger-scale data sets have a smaller age range, usually limited to older adults, rather than the full adult lifespan of our current sample. Additionally, all data were collected using the Dutch MoCA 7.1. Recently, MoCA 8.1 was introduced in the Netherlands, but the versions only differ slightly. That is, 7.1 uses the word madelief (daisy) in the memory subtest, while in 8.1, the word lelie (lily) is used, and in 7.1, the MIS cued recall and recognition tests are optional, while these are part of the standard administration procedure in 8.1. These minor alterations make our normative data set also applicable for the MoCA 8.1. Obviously, our data have been collected using the Dutch version of the MoCA in Dutch-speaking individuals from the Netherlands. Thus, caution is needed when applying normative data (or cut-off scores) obtained with one language version of the MoCA to other language versions altogether (which also pertains to applying the original Canadian French/English cut-off score [4] in other countries). Although the official available international versions of the MoCA were created with the goal of being equivalent, slight differences between MoCA performances across regions may be present. These may not only be due to variations between the respective translations but also related to the use of culture-specific items (for instance, different international MoCA versions use different animals for naming, related to the familiarity with these animals in a specific region of the world, also see [93]). Finally, although we applied exclusion criteria to exclude individuals with psychiatric disorders or brain diseases, we did not perform extensive neuropsychological assessments or obtain magnetic resonance images in our participants. As a result, there is the possibility that our sample of cognitively unimpaired people contains individuals with undetected or subclinical cognitive dysfunction or brain pathology. However, it should be stressed that only recruiting individuals without a history of any disease, pathology, symptom, or complaint will result in supernormal samples that are not representative of the general population [36].
5. Conclusions
In conclusion, our study results highlight the need for age-, education-, and sex-adjusted normative data for the MoCA. In addition to its Total Score, we also present normative data for the MIS, which is relevant for the screening of individuals with possible memory impairment. As the normative data presented here cover the full adult life span (from ages 18 to 91), they can be used for the screening of overall cognitive deficits and memory impairment, not only in older adults with (suspected) neurodegenerative disease but also in younger individuals with possible cognitive impairment.
Author Contributions
Conceptualization, R.P.C.K. and N.R.d.V.; methodology, N.R.d.V.; formal analysis, N.R.d.V.; investigation, C.J.W.H.B., M.G.J. and J.F.M.d.J.; resources, B.A.G.D. and J.M.O.; data curation, N.R.d.V.; writing—original draft preparation, R.P.C.K. and N.R.d.V.; writing—review and editing, C.J.W.H.B., M.G.J., J.F.M.d.J. and B.A.G.D.; visualization, R.P.C.K. and N.R.d.V.; supervision, R.P.C.K.; project administration, N.R.d.V. All authors have read and agreed to the published version of the manuscript.
Funding
The construction of the ANDI database was supported by a MaGW grant (#480-12-015), awarded by the Netherlands Organization for Scientific Research.
Institutional Review Board Statement
All studies that contributed data to the Advanced Neuropsychological Diagnostics Infrastructure (ANDI) were approved by the Institutional Review Boards of the institutions where the studies were conducted and conducted in accordance with the Declaration of Helsinki and compliant with the then-current EU Data Protection Directive 95/46/EC. Written informed consents were obtained from all participants (see also De Vent et al., 2016, for the ANDI ethical statement). All anonymous data sets were made available by the principal investigators of the studies. Data were collected, stored, and shared in accordance with the EU’s General Data Protection Regulation (EU GDPR 2016/679). As the ANDI database does not contain any personally identifiable information (PII) and no new data were collected (all data were already available), the Dutch Medical Research Involving Human Subjects Act (WMO) does not apply to the current study, making this study exempt from formal ethics review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The normative data reported in this study are part of the Advanced Neuropsychological Diagnostics Infrastructure (ANDI), available at www.andi.nl (accessed on 20 June 2022).
Acknowledgments
We thank Erik Oudman, Katrin Sutter, Marije Derks-Dijkman, and Ilse van Tilborg for providing their MoCA data sets for inclusion in ANDI, Serge Walvoort and Cor de Jong for their roles in the NISPA MoCA project, and Thomas Pronk for their statistical advice.
Conflicts of Interest
N.R.d.V. is currently an employee of the Advanced Neuropsychological Diagnostics Infrastructure Company. The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
The tables in this appendix show the percentile distribution that allows for age, education, and sex adjustment. Although the presentation of the tables is stratified according to age, education level, and sex, this was done for presentation purposes only, as the percentile distribution is based on the outcome of the multi-level regression analysis (taking into account the sometimes uneven distribution of individuals across the individual strata). These tables can be used to convert an individual’s MoCA Total Score (Table A1) or Memory Index Score (MIS) (Table A2) into an age-, education-, and sex-adjusted percentile equivalent. The individual’s percentile can be used for clinical interpretation, for example, using the nomenclature described in Table 3 [86]. For instance, a female patient aged 64 with a university degree obtains a MoCA Total Score of 22 and an MIS of 8. This results in a percentile equivalent of 4 for the MoCA Total Score and a percentile of 1 for the MIS, reflecting a ‘below average’ overall cognitive performance and an ‘exceptionally low’ memory performance.

Table A1.
Percentile scores for the MoCA total score stratified per age group, education level, and sex.
Table A1.
Percentile scores for the MoCA total score stratified per age group, education level, and sex.
| Age Groups 18–59 | ||||||||||||||||||||||||
| 18–29 | 30–39 | 40–49 | 50–59 | |||||||||||||||||||||
| Education | Low | Av | High | Low | Av | High | Low | Av | High | Low | Av | High | ||||||||||||
| Sex | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W |
| MoCA Score | ||||||||||||||||||||||||
| 30 | 98 | 98 | 91 | 91 | 86 | 86 | 99 | 99 | 93 | 93 | 89 | 89 | 99 | 99 | 94 | 94 | 91 | 91 | 99 | 99 | 96 | 95 | 93 | 93 |
| 29 | 94 | 94 | 80 | 80 | 72 | 72 | 96 | 96 | 83 | 83 | 77 | 76 | 97 | 97 | 86 | 86 | 80 | 80 | 97 | 97 | 89 | 89 | 83 | 83 |
| 28 | 86 | 86 | 64 | 64 | 54 | 54 | 89 | 89 | 69 | 69 | 60 | 60 | 91 | 91 | 73 | 73 | 65 | 64 | 93 | 93 | 77 | 77 | 69 | 69 |
| 27 | 75 | 75 | 47 | 47 | 37 | 37 | 79 | 79 | 52 | 52 | 43 | 42 | 82 | 82 | 57 | 57 | 47 | 47 | 85 | 85 | 62 | 62 | 52 | 52 |
| 26 | 59 | 59 | 31 | 31 | 23 | 23 | 65 | 65 | 36 | 36 | 27 | 27 | 69 | 69 | 41 | 40 | 31 | 31 | 74 | 73 | 45 | 45 | 36 | 35 |
| 25 | 44 | 44 | 18 | 18 | 13 | 13 | 50 | 49 | 23 | 22 | 16 | 16 | 54 | 54 | 26 | 26 | 19 | 19 | 59 | 59 | 30 | 30 | 22 | 22 |
| 24 | 30 | 30 | 10 | 10 | 6 | 6 | 35 | 35 | 13 | 13 | 8 | 8 | 40 | 39 | 16 | 15 | 11 | 10 | 44 | 44 | 19 | 19 | 13 | 13 |
| 23 | 19 | 19 | 5 | 5 | 3 | 3 | 23 | 23 | 7 | 7 | 4 | 4 | 27 | 27 | 9 | 9 | 5 | 5 | 31 | 31 | 11 | 11 | 7 | 7 |
| 22 | 11 | 11 | 3 | 3 | 1 | 1 | 14 | 14 | 4 | 3 | 2 | 2 | 17 | 17 | 5 | 5 | 3 | 3 | 21 | 20 | 6 | 6 | 4 | 3 |
| 21 | 7 | 7 | 1 | 1 | 1 | 1 | 9 | 8 | 2 | 2 | 1 | 1 | 11 | 10 | 2 | 2 | 1 | 1 | 13 | 13 | 3 | 3 | 2 | 2 |
| 20 | 4 | 4 | 1 | 1 | 0 | 0 | 5 | 5 | 1 | 1 | 0 | 0 | 6 | 6 | 1 | 1 | 1 | 1 | 8 | 8 | 2 | 2 | 1 | 1 |
| 19 | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 4 | 4 | 1 | 1 | 0 | 0 | 5 | 5 | 1 | 1 | 0 | 0 |
| 18 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 |
| 17 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| ≤16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Age Groups 60–91 | ||||||||||||||||||||||||
| 60–69 | 70–79 | ≥80 | ||||||||||||||||||||||
| Education | Low | Av | High | Low | Av | High | Low | Av | High | |||||||||||||||
| Sex | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | ||||||
| MoCA Score | ||||||||||||||||||||||||
| 30 | 99 | 99 | 97 | 97 | 94 | 94 | 100 | 100 | 97 | 97 | 95 | 95 | 100 | 100 | 98 | 98 | 96 | 96 | ||||||
| 29 | 98 | 98 | 91 | 91 | 86 | 86 | 98 | 99 | 93 | 93 | 88 | 88 | 99 | 99 | 94 | 94 | 91 | 91 | ||||||
| 28 | 95 | 95 | 80 | 80 | 73 | 73 | 95 | 96 | 84 | 84 | 77 | 77 | 96 | 97 | 86 | 86 | 80 | 80 | ||||||
| 27 | 88 | 88 | 66 | 66 | 57 | 57 | 87 | 90 | 70 | 70 | 61 | 61 | 89 | 92 | 72 | 74 | 66 | 66 | ||||||
| 26 | 77 | 77 | 50 | 50 | 40 | 40 | 76 | 80 | 55 | 55 | 45 | 45 | 79 | 84 | 56 | 59 | 50 | 50 | ||||||
| 25 | 63 | 63 | 34 | 34 | 26 | 26 | 62 | 68 | 39 | 39 | 30 | 30 | 66 | 72 | 40 | 44 | 34 | 34 | ||||||
| 24 | 49 | 49 | 22 | 22 | 15 | 15 | 47 | 54 | 26 | 26 | 18 | 18 | 51 | 58 | 27 | 30 | 22 | 22 | ||||||
| 23 | 35 | 35 | 13 | 13 | 9 | 9 | 33 | 40 | 16 | 16 | 11 | 11 | 38 | 44 | 17 | 19 | 13 | 13 | ||||||
| 22 | 24 | 24 | 7 | 7 | 4 | 4 | 23 | 28 | 9 | 9 | 6 | 6 | 26 | 32 | 10 | 11 | 7 | 7 | ||||||
| 21 | 15 | 15 | 4 | 4 | 2 | 2 | 15 | 19 | 5 | 5 | 3 | 3 | 18 | 22 | 6 | 6 | 4 | 4 | ||||||
| 20 | 10 | 10 | 2 | 2 | 1 | 1 | 9 | 12 | 3 | 3 | 2 | 2 | 11 | 14 | 3 | 4 | 2 | 2 | ||||||
| 19 | 6 | 6 | 1 | 1 | 1 | 1 | 6 | 7 | 1 | 1 | 1 | 1 | 7 | 9 | 2 | 2 | 1 | 1 | ||||||
| 18 | 4 | 4 | 1 | 1 | 0 | 0 | 4 | 5 | 1 | 1 | 0 | 0 | 5 | 6 | 1 | 1 | 1 | 1 | ||||||
| 17 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 3 | 4 | 1 | 1 | 0 | 0 | ||||||
| ≤16 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | ||||||

Table A2.
Percentile scores for the MoCA Memory Index Score (MIS) per age group, education level, and sex.
Table A2.
Percentile scores for the MoCA Memory Index Score (MIS) per age group, education level, and sex.
| Age Groups 18–59 | ||||||||||||||||||||||||
| 18–29 | 30–39 | 40–49 | 50–59 | |||||||||||||||||||||
| Education | Low | Av | High | Low | Av | High | Low | Av | High | Low | Av | High | ||||||||||||
| Sex | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W |
| MIS | ||||||||||||||||||||||||
| 15 | 99 | 99 | 94 | 89 | 88 | 81 | 100 | 99 | 95 | 92 | 91 | 85 | 100 | 99 | 96 | 93 | 93 | 88 | 100 | 100 | 97 | 95 | 94 | 90 |
| 14 | 95 | 92 | 74 | 64 | 62 | 51 | 97 | 94 | 79 | 70 | 68 | 57 | 97 | 95 | 82 | 74 | 72 | 62 | 98 | 96 | 86 | 78 | 77 | 67 |
| 13 | 83 | 75 | 47 | 36 | 34 | 24 | 86 | 79 | 53 | 41 | 39 | 29 | 89 | 83 | 58 | 47 | 44 | 33 | 91 | 86 | 63 | 52 | 50 | 38 |
| 12 | 63 | 52 | 24 | 16 | 15 | 9 | 69 | 58 | 29 | 20 | 19 | 12 | 73 | 63 | 34 | 24 | 23 | 15 | 77 | 68 | 39 | 29 | 27 | 18 |
| 11 | 43 | 33 | 12 | 7 | 6 | 3 | 49 | 38 | 15 | 9 | 8 | 5 | 55 | 43 | 18 | 12 | 11 | 6 | 60 | 49 | 22 | 14 | 13 | 8 |
| 10 | 28 | 20 | 6 | 3 | 3 | 1 | 34 | 24 | 7 | 4 | 4 | 2 | 39 | 28 | 9 | 6 | 5 | 3 | 44 | 33 | 12 | 7 | 6 | 4 |
| 9 | 19 | 12 | 3 | 1 | 1 | 1 | 23 | 15 | 4 | 2 | 2 | 1 | 27 | 19 | 5 | 3 | 2 | 1 | 32 | 22 | 7 | 4 | 3 | 2 |
| 8 | 13 | 8 | 2 | 1 | 1 | 0 | 16 | 10 | 2 | 1 | 1 | 0 | 20 | 13 | 3 | 2 | 1 | 1 | 24 | 16 | 4 | 2 | 2 | 1 |
| 7 | 10 | 6 | 1 | 0 | 0 | 0 | 12 | 7 | 1 | 1 | 1 | 0 | 15 | 9 | 2 | 1 | 1 | 0 | 19 | 12 | 3 | 1 | 1 | 1 |
| 6 | 8 | 4 | 1 | 0 | 0 | 0 | 10 | 6 | 1 | 0 | 0 | 0 | 12 | 7 | 1 | 1 | 1 | 0 | 15 | 10 | 2 | 1 | 1 | 0 |
| 5 | 6 | 4 | 1 | 0 | 0 | 0 | 8 | 5 | 1 | 0 | 0 | 0 | 11 | 6 | 1 | 1 | 0 | 0 | 13 | 8 | 2 | 1 | 1 | 0 |
| 4 | 6 | 3 | 0 | 0 | 0 | 0 | 8 | 4 | 1 | 0 | 0 | 0 | 10 | 6 | 1 | 0 | 0 | 0 | 12 | 7 | 1 | 1 | 1 | 0 |
| 3 | 5 | 3 | 0 | 0 | 0 | 0 | 7 | 4 | 1 | 0 | 0 | 0 | 9 | 5 | 1 | 0 | 0 | 0 | 12 | 7 | 1 | 1 | 1 | 0 |
| 2 | 5 | 3 | 0 | 0 | 0 | 0 | 7 | 4 | 1 | 0 | 0 | 0 | 9 | 5 | 1 | 0 | 0 | 0 | 11 | 7 | 1 | 1 | 1 | 0 |
| 1 | 5 | 3 | 0 | 0 | 0 | 0 | 7 | 4 | 1 | 0 | 0 | 0 | 9 | 5 | 1 | 0 | 0 | 0 | 11 | 7 | 1 | 1 | 1 | 0 |
| Age Groups 60–91 | ||||||||||||||||||||||||
| 60–69 | 70–79 | ≥80 | ||||||||||||||||||||||
| Education | Low | Av | High | Low | Av | High | Low | Av | High | |||||||||||||||
| Sex | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | ||||||
| MIS | ||||||||||||||||||||||||
| 15 | 100 | 100 | 98 | 96 | 96 | 92 | 100 | 100 | 99 | 97 | 97 | 94 | 100 | 100 | 99 | 98 | 98 | 96 | ||||||
| 14 | 99 | 97 | 88 | 82 | 80 | 72 | 99 | 98 | 91 | 85 | 84 | 76 | 99 | 99 | 93 | 88 | 87 | 80 | ||||||
| 13 | 93 | 89 | 68 | 57 | 55 | 44 | 95 | 91 | 73 | 62 | 50 | 49 | 96 | 93 | 77 | 67 | 65 | 54 | ||||||
| 12 | 81 | 73 | 44 | 33 | 31 | 22 | 85 | 77 | 50 | 38 | 36 | 26 | 88 | 81 | 55 | 44 | 41 | 31 | ||||||
| 11 | 65 | 54 | 26 | 18 | 16 | 10 | 70 | 59 | 30 | 21 | 20 | 13 | 74 | 64 | 35 | 25 | 24 | 16 | ||||||
| 10 | 49 | 38 | 15 | 9 | 8 | 5 | 55 | 43 | 18 | 12 | 10 | 6 | 60 | 49 | 22 | 14 | 13 | 8 | ||||||
| 9 | 37 | 27 | 9 | 5 | 4 | 2 | 42 | 31 | 11 | 7 | 6 | 3 | 47 | 36 | 14 | 8 | 8 | 4 | ||||||
| 8 | 28 | 19 | 5 | 3 | 3 | 1 | 33 | 23 | 7 | 4 | 4 | 2 | 38 | 27 | 9 | 5 | 5 | 2 | ||||||
| 7 | 22 | 15 | 4 | 2 | 2 | 1 | 26 | 18 | 5 | 3 | 2 | 1 | 31 | 22 | 6 | 4 | 3 | 2 | ||||||
| 6 | 19 | 12 | 3 | 1 | 1 | 1 | 22 | 15 | 4 | 2 | 2 | 1 | 27 | 18 | 5 | 3 | 2 | 1 | ||||||
| 5 | 16 | 10 | 3 | 1 | 1 | 0 | 20 | 13 | 3 | 2 | 1 | 1 | 24 | 16 | 4 | 2 | 2 | 1 | ||||||
| 4 | 15 | 9 | 2 | 1 | 1 | 0 | 18 | 12 | 3 | 1 | 1 | 1 | 22 | 15 | 4 | 2 | 2 | 1 | ||||||
| 3 | 14 | 9 | 2 | 1 | 1 | 0 | 18 | 11 | 3 | 1 | 1 | 1 | 21 | 14 | 3 | 2 | 2 | 1 | ||||||
| 2 | 14 | 9 | 2 | 1 | 1 | 0 | 17 | 11 | 2 | 1 | 1 | 1 | 21 | 14 | 3 | 2 | 2 | 1 | ||||||
| 1 | 14 | 9 | 2 | 1 | 1 | 0 | 17 | 11 | 2 | 1 | 1 | 0 | 21 | 14 | 3 | 2 | 2 | 1 | ||||||
References
- Kessels, R.P.C.; Hendriks, M.P.H. Neuropsychological assessment. In Encyclopedia of Mental Health, 3rd ed.; Elsevier: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Nieuwenhuis-Mark, R.E. The death knoll for the MMSE: Has it outlived its purpose? J. Geriatr. Psychiatry Neurol. 2010, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Appels, B.A.; Scherder, E. The diagnostic accuracy of dementia-screening instruments with an administration time of 10 to 45 minutes for use in secondary care: A systematic review. Am. J. Alzheimers Dis. Other Demen. 2010, 25, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Blanco-Campal, A.; Diaz-Orueta, U.; Navarro-Prados, A.B.; Burke, T.; Libon, D.J.; Lamar, M. Features and psychometric properties of the Montreal Cognitive Assessment: Review and proposal of a process-based approach version (MoCA-PA). Appl. Neuropsychol. Adult 2021, 28, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, C.J.W.H.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Budy, M.J.J.; Beurmanjer, H.; De Jong, C.A.J.; Kessels, R.P.C. Psychometric properties of the Montreal Cognitive Assessment (MoCA) in healthy participants aged 18–70. Int. J. Psychiatry Clin. Pract. 2020, 24, 293–300. [Google Scholar] [CrossRef]
- Thissen, A.J.A.M.; van Bergen, F.; de Jonghe, J.F.M.; Kessels, R.P.C.; Dautzenberg, P.L.J. Bruikbaarheid en validiteit van de Nederlandse versie van de Montreal Cognitive Assessment (MoCA-D) bij het diagnosticeren van Mild Cognitive Impairment. Tijdschr. Gerontol. Geriatr. 2010, 41, 231–240. [Google Scholar] [CrossRef]
- Serrano, C.M.; Sorbara, M.; Minond, A.; Finlay, J.B.; Arizaga, R.L.; Iturry, M.; Martinez, P.; Heinemann, G.; Gagliardi, C.; Serra, A.; et al. Validation of the Argentine version of the Montreal Cognitive Assessment Test (MOCA): A screening tool for mild cognitive impairment and mild dementia in elderly. Dement. Neuropsychol. 2020, 14, 145–152. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Screening for mild cognitive impairment: Comparison of “MCI specific” screening instruments. J. Alzheimers Dis. 2016, 51, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.H.; Creavin, S.T.; Yip, J.L.; Noel-Storr, A.H.; Brayne, C.; Cullum, S. Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementias. Cochrane Database Syst. Rev. 2015, 2015, CD010775. [Google Scholar] [CrossRef] [Green Version]
- Konstantopoulos, K.; Vogazianos, P.; Doskas, T. Normative data of the Montreal Cognitive Assessment in the Greek population and Parkinsonian dementia. Arch. Clin. Neuropsychol. 2016, 31, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Carlew, A.R.; Smith, E.E.; Goette, W.; Lippe, B.; Lacritz, L.; Rossetti, H. Montreal Cognitive Assessment (MoCA) scores in medically compromised patients: A scoping review. Health Psychol. 2021, 40, 717–726. [Google Scholar] [CrossRef]
- Janssen, M.A.M.; Meulenbroek, O.; Steens, S.C.; Góraj, B.; Bosch, M.; Koopmans, P.P.; Kessels, R.P.C. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS 2015, 29, 2139–2148. [Google Scholar] [CrossRef]
- Rosenblum, S.; Meyer, S.; Gemerman, N.; Mentzer, L.; Richardson, A.; Israeli-Korn, S.; Livneh, V.; Karmon, T.F.; Nevo, T.; Yahalom, G.; et al. The Montreal Cognitive Assessment: Is it suitable for identifying mild cognitive impairment in Parkinson’s Disease? Mov. Disord. Clin. Pract. 2020, 7, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Rosca, E.C.; Simu, M. Montreal Cognitive Assessment for evaluating cognitive impairment in multiple sclerosis: A systematic review. Acta Neurol. Belg. 2020, 120, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, J.; Ovcar Stante, K.; Rakusa, M. The validity of the Montreal Cognitive Assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke. Acta Neurol. Belg. 2020, 120, 681–685. [Google Scholar] [CrossRef]
- Coleman, K.K.; Coleman, B.L.; MacKinley, J.D.; Pasternak, S.H.; Finger, E.C. Detection and differentiation of frontotemporal dementia and related disorders from Alzheimer disease using the Montreal Cognitive Assessment. Alzheimer Dis. Assoc. Disord. 2016, 30, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, C.J.W.H.; Jansen, M.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Lugtmeijer, S.; Markus, W.; De Jong, C.A.J.; Kessels, R.P.C. The Montreal Cognitive Assessment as a cognitive screen in addiction health care: A validation study for clinical practice. J. Subst. Use 2018, 24, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Luque, B.; García, V.; Tabernero, C. Depression and cognitive impairment in a spanish sample of psychoactive substance users receiving mental health care. Healthcare 2022, 10, 887. [Google Scholar] [CrossRef]
- van Gils, P.; van Heugten, C.; Hofmeijer, J.; Keijzer, H.; Nutma, S.; Duits, A. The Montreal Cognitive Assessment is a valid cognitive screening tool for cardiac arrest survivors. Resuscitation 2022, 172, 130–136. [Google Scholar] [CrossRef]
- Murillo-Garcia, A.; Leon-Llamas, J.L.; Villafaina, S.; Rohlfs-Dominguez, P.; Gusi, N. MoCA vs. MMSE of fibromyalgia patients: The possible role of dual-task tests in detecting cognitive impairment. J. Clin. Med. 2021, 10, 125. [Google Scholar] [CrossRef]
- Rosca, E.C.; Simu, M. Montreal cognitive assessment for evaluating cognitive impairment in Huntington’s disease: A systematic review. CNS Spectr. 2022, 27, 27–45. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, S.C.; de Jonghe, J.; Germans, T.; Ruiter, J.H.; Jansen, R. Cognitive impairment is very common in elderly patients with syncope and unexplained falls. J Am. Med. Dir. Assoc. 2017, 18, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosca, E.C.; Cornea, A.; Simu, M. Montreal Cognitive Assessment for evaluating the cognitive impairment in patients with schizophrenia: A systematic review. Gen. Hosp. Psychiatry 2020, 65, 64–73. [Google Scholar] [CrossRef]
- Early, M.L.; Linton, E.; Bosch, A.; Campbell, T.; Hill-Briggs, F.; Pecker, L.H.; Lance, E.I.; Lanzkron, S. The Montreal cognitive assessment as a cognitive screening tool in sickle cell disease: Associations with clinically significant cognitive domains. Br. J. Haematol. 2022, in press. [CrossRef]
- Machii, N.; Kudo, A.; Saito, H.; Tanabe, H.; Iwasaki, M.; Hirai, H.; Masuzaki, H.; Shimabukuro, M. Walking speed is the sole determinant criterion of sarcopenia of mild cognitive impairment in Japanese elderly patients with type 2 diabetes mellitus. J. Clin. Med. 2020, 9, 2133. [Google Scholar] [CrossRef]
- Aiello, E.N.; Gramegna, C.; Esposito, A.; Gazzaniga, V.; Zago, S.; Difonzo, T.; Maddaluno, O.; Appollonio, I.; Bolognini, N. The Montreal Cognitive Assessment (MoCA): Updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin. Exp. Res. 2022, 34, 375–382. [Google Scholar] [CrossRef]
- Aiello, E.N.; Fiabane, E.; Manera, M.R.; Radici, A.; Grossi, F.; Ottonello, M.; Pain, D.; Pistarini, C. Screening for cognitive sequelae of SARS-CoV-2 infection: A comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Neurol. Sci. 2022, 43, 81–84. [Google Scholar] [CrossRef]
- Apolinario, D.; Dos Santos, M.F.; Sassaki, E.; Pegoraro, F.; Pedrini, A.; Cestari, B.; Amaral, A.H.; Mitt, M.; Müller, M.B.; Suemoto, C.K.; et al. Normative data for the Montreal Cognitive Assessment (MoCA) and the Memory Index Score (MoCA-MIS) in Brazil: Adjusting the nonlinear effects of education with fractional polynomials. Int. J. Geriatr. Psychiatry 2018, 33, 893–899. [Google Scholar] [CrossRef]
- Bartos, A.; Fayette, D. Validation of the Czech Montreal Cognitive Assessment for Mild Cognitive Impairment due to Alzheimer disease and Czech norms in 1,552 Elderly persons. Dement. Geriatr. Cogn. Disord. 2018, 46, 335–345. [Google Scholar] [CrossRef]
- Borland, E.; Nagga, K.; Nilsson, P.M.; Minthon, L.; Nilsson, E.D.; Palmqvist, S. The Montreal Cognitive Assessment: Normative data from a large Swedish population-based cohort. J. Alzheimers Dis. 2017, 59, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesar, K.G.; Yassuda, M.S.; Porto, F.H.G.; Brucki, S.M.D.; Nitrini, R. MoCA Test: Normative and diagnostic accuracy data for seniors with heterogeneous educational levels in Brazil. Arq. Neuropsiquiatr. 2019, 77, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classon, E.; van den Hurk, W.; Lyth, J.; Johansson, M.M. Montreal Cognitive Assessment: Normative data for cognitively healthy Swedish 80- to 94-year-olds. J. Alzheimers Dis. 2022, 87, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef]
- Engedal, K.; Gjøra, L.; Benth, J.Š.; Wagle, J.; Rønqvist, T.K.; Selbæk, G. The Montreal Cognitive Assessment: Normative data from a large, population-based sample of cognitive healthy older adults in Norway: The HUNT study. J. Alzheimers Dis. 2022, 86, 589–599. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Santana, I. Montreal Cognitive Assessment (MoCA): Normative study for the Portuguese population. J. Clin. Exp. Neuropsychol. 2011, 33, 989–996. [Google Scholar] [CrossRef]
- Gaete, M.; Jorquera, S.; Bello-Lepe, S.; Mendoza, Y.M.; Véliz, M.; Alonso-Sanchez, M.F.; Lira, J. Standardized results of the Montreal Cognitive Assessment (MoCA) for neurocognitive screening in a Chilean population. Neurologia, 2020, in press. [CrossRef]
- Gluhm, S.; Goldstein, J.; Loc, K.; Colt, A.; Liew, C.V.; Corey-Bloom, J. Cognitive performance on the Mini-Mental State Examination and the Montreal Cognitive Assessment across the healthy adult lifespan. Cogn. Behav. Neurol. 2013, 26, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hayek, M.; Tarabey, L.; Abou-Mrad, T.; Fadel, P.; Abou-Mrad, F. Normative data for the Montreal Cognitive Assessment in a Lebanese older adult population. J. Clin. Neurosci. 2020, 74, 81–86. [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Vigen, T.; Berge, T.; Einvik, G.; Aarsland, D.; Rønning, O.M.; Thommessen, B.; Røsjø, H.; Tveit, A.; Ihle-Hansen, H. Montreal Cognitive Assessment in a 63- to 65-year-old Norwegian cohort from the general population: Data from the Akershus Cardiac Examination 1950 Study. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.M.; Cho, Y.S.; Park, S.; Lee, B.H.; Sohn, B.K.; Choi, C.H.; Choi, J.S.; Jeong, H.Y.; Cho, S.J.; Lee, J.H.; et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.A.; Coen, R.F.; Frewen, J.; Donoghue, O.A.; Cronin, H.; Savva, G.M. Normative values of cognitive and physical function in older adults: Findings from the Irish Longitudinal Study on Ageing. J. Am. Geriatrr. Soc. 2013, 61 (Suppl. 2), S279–S290. [Google Scholar] [CrossRef]
- Kopecek, M.; Stepankova, H.; Lukavsky, J.; Ripova, D.; Nikolai, T.; Bezdicek, O. Montreal cognitive assessment (MoCA): Normative data for old and very old Czech adults. Appl. Neuropsychol. Adult 2017, 24, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Larouche, E.; Tremblay, M.P.; Potvin, O.; Laforest, S.; Bergeron, D.; Laforce, R.; Monetta, L.; Boucher, L.; Tremblay, P.; Belleville, S.; et al. Normative data for the Montreal Cognitive Assessment in middle-aged and elderly Quebec-French people. Arch. Clin. Neuropsychol. 2016, 31, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Lee, D.W.; Cho, S.J.; Na, D.L.; Jeon, H.J.; Kim, S.K.; Lee, Y.R.; Youn, J.H.; Kwon, M.; Lee, J.H.; et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: Validation of the Korean version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Li, F.; Zhou, A.; Wang, F.; Zuo, X.; Jia, X.F.; Song, H.; Jia, J. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: A population-based study. J. Geriatr. Psychiatry Neurol. 2011, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Malek-Ahmadi, M.; Powell, J.J.; Belden, C.M.; O’Connor, K.; Evans, L.; Coon, D.W.; Nieri, W. Age- and education-adjusted normative data for the Montreal Cognitive Assessment (MoCA) in older adults age 70–99. Aging Neuropsychol. Cogn. 2015, 22, 755–761. [Google Scholar] [CrossRef]
- Muayqil, T.A.; Alamri, N.K.; Alqahtani, A.M.; Julaidan, S.S.; Alsuhaibani, R.; Nafisah, I.; Alkeridy, W.A.; Aljafen, B.N.; Alanazy, M.H. Normative and equated data of the original and basic versions of the Montreal Cognitive Assessment among community dwelling Saudi Arabians. Behav. Neurol. 2021, 2021, 5395627. [Google Scholar] [CrossRef]
- Narazaki, K.; Nofuji, Y.; Honda, T.; Matsuo, E.; Yonemoto, K.; Kumagai, S. Normative data for the Montreal Cognitive Assessment in a Japanese community-dwelling older population. Neuroepidemiology 2013, 40, 23–29. [Google Scholar] [CrossRef]
- Ojeda, N.; Del Pino, R.; Ibarretxe-Bilbao, N.; Schretlen, D.J.; Pena, J. Test de evaluacion cognitiva de Montreal: Normalizacion y estandarizacion de la prueba en poblacion española [Montreal Cognitive Assessment Test: Normalization and standardization for Spanish population]. Rev. Neurol. 2016, 63, 488–496. [Google Scholar] [CrossRef]
- Pereiro, A.X.; Ramos-Lema, S.; Lojo-Seoane, C.; Guàrdia-Olmos, J.; Facal-Mayo, D.; Juncos-Rabadán, O. Normative data for the Montreal Cognitive Assessment (MOCA) in a Spanish sample of community-dweller adults. Eur. Geriatr. Med. 2017, 8, 240–244. [Google Scholar] [CrossRef]
- Pinto, T.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.; Ximenes, R.; Sougey, E.B. Influence of age and education on the performance of elderly in the Brazilian version of the Montreal Cognitive Assessment battery. Dement. Geriatr. Cogn. Disord. 2018, 45, 290–299. [Google Scholar] [CrossRef]
- Rossetti, H.C.; Lacritz, L.H.; Cullum, C.M.; Weiner, M.F. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 2011, 77, 1272–1275. [Google Scholar] [CrossRef]
- Rossetti, H.C.; Lacritz, L.H.; Hynan, L.S.; Cullum, C.M.; Van Wright, A.; Weiner, M.F. Montreal Cognitive Assessment performance among community-dwelling African Americans. Arch. Clin. Neuropsychol. 2017, 32, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Sachs, B.C.; Chelune, G.J.; Rapp, S.R.; Couto, A.M.; Willard, J.J.; Williamson, J.D.; Sink, K.M.; Coker, L.H.; Gaussoin, S.A.; Gure, T.R.; et al. Robust demographically-adjusted normative data for the Montreal Cognitive Assessment (MoCA): Results from the systolic blood pressure intervention trial. Clin. Neuropsychol. 2022, in press. [CrossRef] [PubMed]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, M.; Chiorri, C.; Passaniti, C.; Sant’Elia, V.; Trojano, L.; Santangelo, G. Comparison of alternate and original forms of the Montreal Cognitive Assessment (MoCA): An Italian normative study. Neurol. Sci. 2019, 40, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sink, K.M.; Craft, S.; Smith, S.C.; Maldjian, J.A.; Bowden, D.W.; Xu, J.; Freedman, B.I.; Divers, J. Montreal Cognitive Assessment and Modified Mini Mental State Examination in African Americans. J. Aging Res. 2015, 2015, 872018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomann, A.E.; Goettel, N.; Monsch, R.J.; Berres, M.; Jahn, T.; Steiner, L.A.; Monsch, A.U. The Montreal Cognitive Assessment: Normative data from a German-speaking cohort and comparison with international normative samples. J. Alzheimers Dis. 2018, 64, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Julayanont, P.; Brousseau, M.; Chertkow, H.; Phillips, N.; Nasreddine, Z.S. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2014, 62, 679–684. [Google Scholar] [CrossRef]
- Kaur, A.; Edland, S.D.; Peavy, G.M. The MoCA-Memory Index Score: An efficient alternative to paragraph recall for the detection of amnestic mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2018, 32, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, C.J.W.H.; Walvoort, S.J.W.; Dijkstra, B.A.G.; de Jong, C.A.J.; Kessels, R.P.C. The course of cognitive performance during inpatient treatment in patients with alcohol use disorder with no, mild or major neurocognitive disorders. Alcohol Alcohol. 2021, 56, 89–100. [Google Scholar] [CrossRef]
- Oosterhuis, H.E.; van der Ark, L.A.; Sijtsma, K. Sample size requirements for traditional and regression-based norms. Assessment 2016, 23, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Matthews, C.G.; Grant, I.; Avitable, N. Demographic Corrections with Comprehensive Norms: An Overzealous Attempt or a Good Start? J. Clin. Exp. Neuropsychol. 1996, 18, 449–458. [Google Scholar] [CrossRef]
- Duits, A.; Kessels, R. Schatten van het premorbide functioneren. In Neuropsychologische Diagnostiek: De Klinische Praktijk; Hendriks, M., Kessels, R., Gorissen, M., Schmand, B., Duits, A., Eds.; Boom: Amsterdam, The Netherlands, 2014; pp. 173–186. [Google Scholar]
- Hochstenbach, J.; Mulder, T.; van Limbeek, J.; Donders, R.; Schoonderwaldt, H. Cognitive decline following stroke: A comprehensive study of cognitive decline following stroke. J. Clin. Exp. Neuropsychol. 1998, 20, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kasse, E.; Oudman, E.; Olivier, M.; Wijnia, J.W.; Postma, A. Subtle object location perception deficits in Korsakoff’s syndrome. J. Exp. Clin. Neuropsychol. 2019, 41, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Oudman, E.; Postma, A.; Van der Stigchel, S.; Appelhof, B.; Wijnia, J.W.; Nijboer, T.C. The Montreal Cognitive Assessment (MoCA) is superior to the Mini Mental State Examination (MMSE) in detection of Korsakoff’s syndrome. Clin. Neuropsychol. 2014, 28, 1123–1132. [Google Scholar] [CrossRef]
- Derks-Dijkman, M.W.; Schaefer, R.S.; Stegeman, M.; Van Tilborg, I.D.A.; Kessels, R.P.C. Effects of musical mnemonics on working memory performance in cognitively unimpaired young and older adults. Exp. Aging Res. 2022; revision submitted. [Google Scholar]
- Janssen, M.A.M.; Bosch, M.; Koopmans, P.P.; Kessels, R.P.C. Validity of the Montreal Cognitive Assessment and the HIV Dementia Scale in the assessment of cognitive impairment in HIV-1 infected patients. J. Neurovirol. 2015, 21, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Sutter, K.; Oostwoud Wijdenes, L.; Van Beers, R.J.; Claassen, J.A.H.R.; Kessels, R.P.C.; Medendorp, W.P. Does early-stage Alzheimer’s disease affect the dynamics of motor adaptation? In 2022 Neuroscience Meeting Planner (Online Abstracts); Society for Neuroscience: Chicago, IL, USA, 2022. [Google Scholar]
- UNESCO. International Standard Classification of Education (ISCED 2011); UNESCO Institute for Statistics: Montreal, QC, Canada, 2011. [Google Scholar]
- de Vent, N.R.; Agelink van Rentergem, J.A.; Schmand, B.A.; Murre, J.M.; ANDI Consortium; Huizenga, H.M. Advanced Neuropsychological Diagnostics Infrastructure (ANDI): A Normative database created from control datasets. Front. Psychol. 2016, 7, 1601. [Google Scholar] [CrossRef] [Green Version]
- Curran, P.J.; Hussong, A.M. Integrative data analysis: The simultaneous analysis of multiple data sets. Psychol. Methods 2009, 14, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2003. [Google Scholar]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.R.; Howell, D.C. Comparing an individual’s test score against norms derived from small samples. Clin. Neuropsychol. 1998, 12, 482–486. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Crawford, J.R.; Garthwaite, P.H.; Azzalini, A.; Howell, D.C.; Laws, K.R. Testing for a deficit in single-case studies: Effects of departures from normality. Neuropsychologia 2006, 44, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Sakia, R.M. The Box-Cox transformation technique: A review. Statistician 1992, 41, 169–178. [Google Scholar] [CrossRef]
- Curran, P.J.; West, S.G.; Finch, J.F. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol. Methods 1996, 1, 16–29. [Google Scholar] [CrossRef]
- Van den Berg, E.; Nys, G.M.S.; Brands, A.M.A.; Ruis, C.; Van Zandvoort, M.J.E.; Kessels, R.P.C. The Brixton Spatial Anticipation Test as a test for executive function: Validity in patient groups and norms for older adults. J. Int. Neuropsychol. Soc. 2009, 15, 695–703. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 20 June 2022).
- Guilmette, T.J.; Sweet, J.J.; Hebben, N.; Koltai, D.; Mahone, E.M.; Spiegler, B.J.; Stucky, K.; Westerveld, M.; Conference Participants. American Academy of Clinical Neuropsychology consensus conference statement on uniform labeling of performance test scores. Clin. Neuropsychol. 2020, 34, 437–453. [Google Scholar] [CrossRef] [Green Version]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Eikelboom, W.S.; Bertens, D.; Kessels, R.P.C. Cognitive rehabilitation in normal aging and individuals with subjective cognitive decline. In Cognitive Rehabilitation and Neuroimaging; Chiaravalloti, N., Weber, E., DeLuca, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 37–67. [Google Scholar] [CrossRef]
- Asperholm, M.; Högman, N.; Rafi, J.; Herlitz, A. What did you do yesterday? a meta-analysis of sex differences in episodic memory. Psychol. Bull. 2019, 145, 785–821. [Google Scholar] [CrossRef]
- Asperholm, M.; van Leuven, L.; Herlitz, A. Sex differences in episodic memory variance. Front. Psychol. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, L.N.; McDonald, T.; Robinson, B.; Sass, J.R.; Loring, D.W.; Hewitt, K.C. Classification statistics of the Montreal Cognitive Assessment (MoCA): Are we interpreting the MoCA correctly? Clin. Neuropsychol. 2022; ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Thomann, A.E.; Berres, M.; Goettel, N.; Steiner, L.A.; Monsch, A.U. Enhanced diagnostic accuracy for neurocognitive disorders: A revised cut-off approach for the Montreal Cognitive Assessment. Alzheimers Res. Ther. 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Mirza, N.; Waheed, W. Developing guidelines for the translation and cultural adaptation of the Montreal Cognitive Assessment: Scoping review and qualitative synthesis. Br. J. Psychiatry Open 2022, 8, e21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
