Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Design and Selection Criteria
2.3. Statistical Analysis
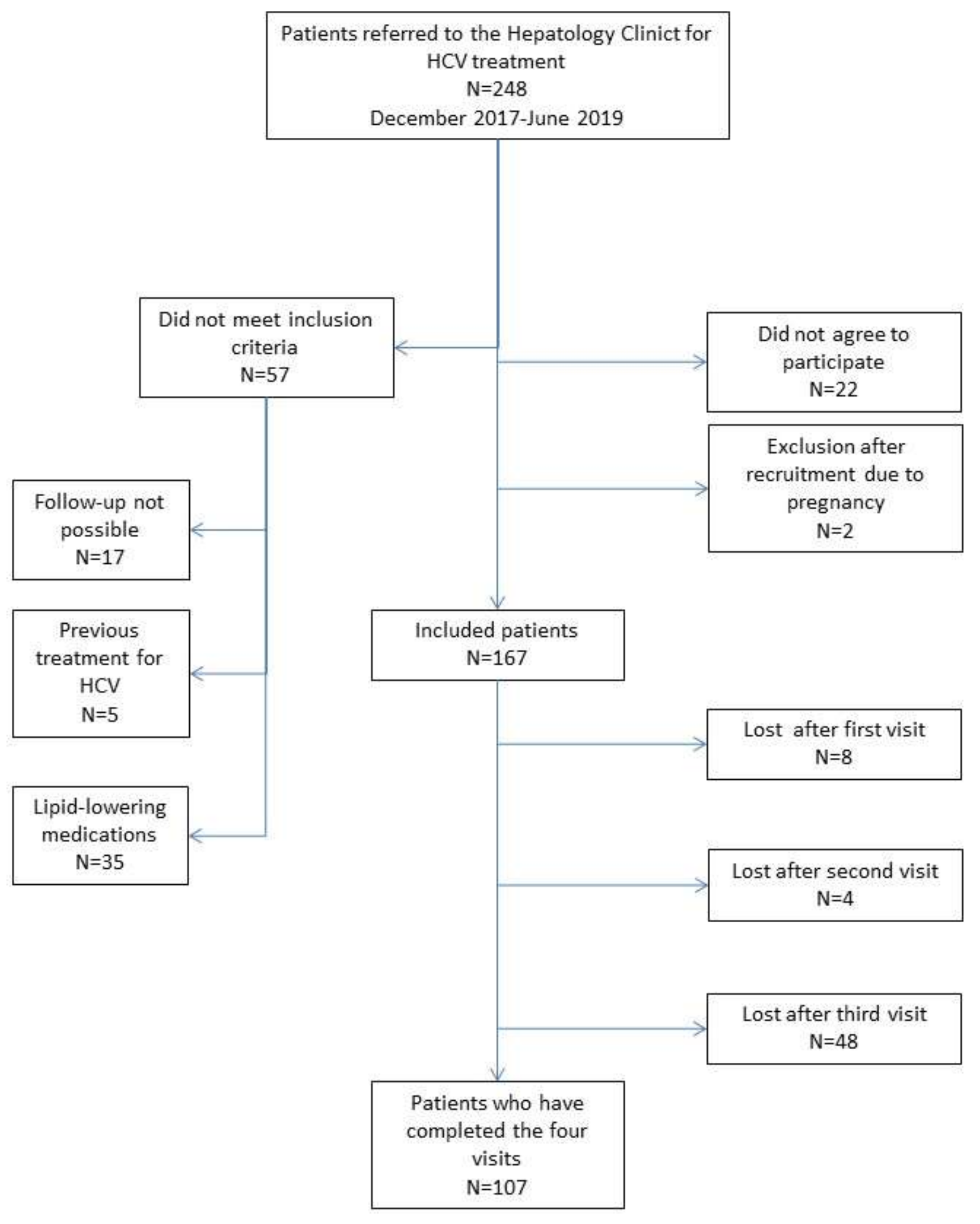
3. Results
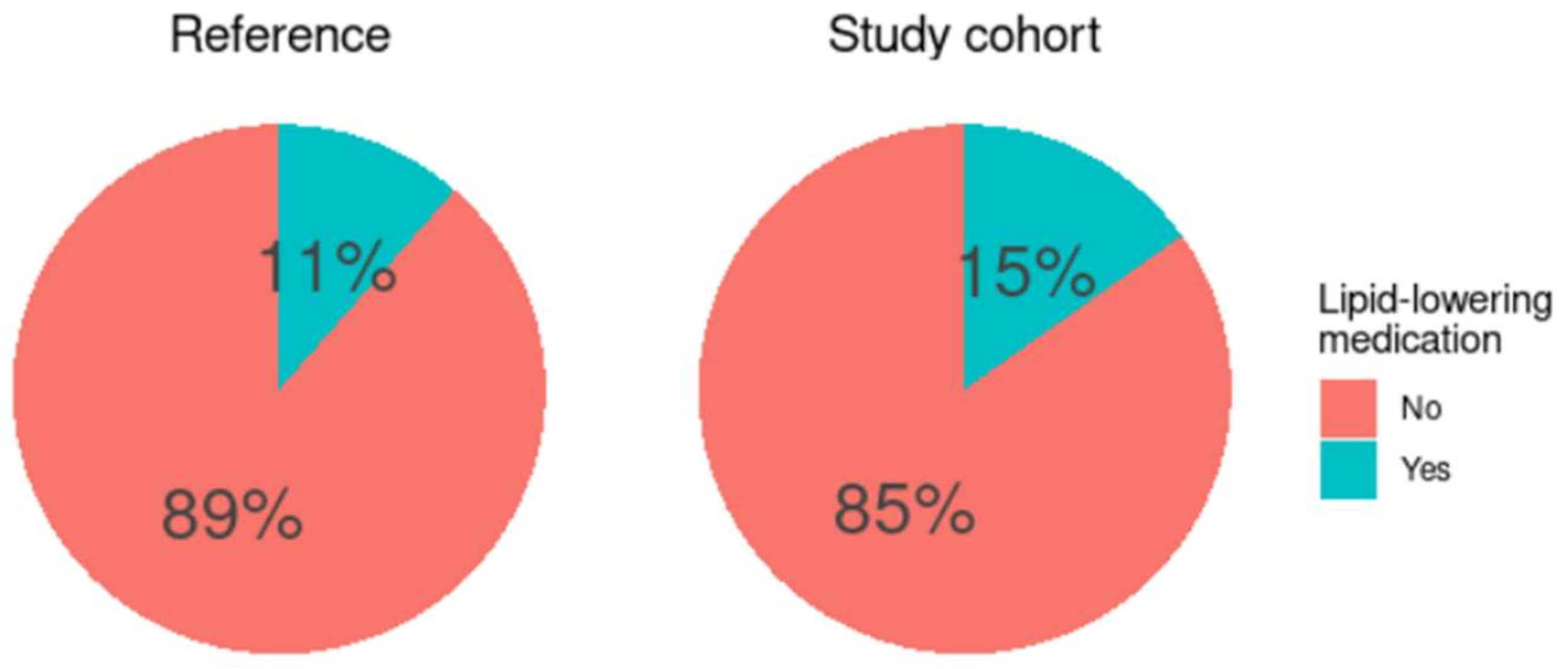
3.1. Baseline Characteristics
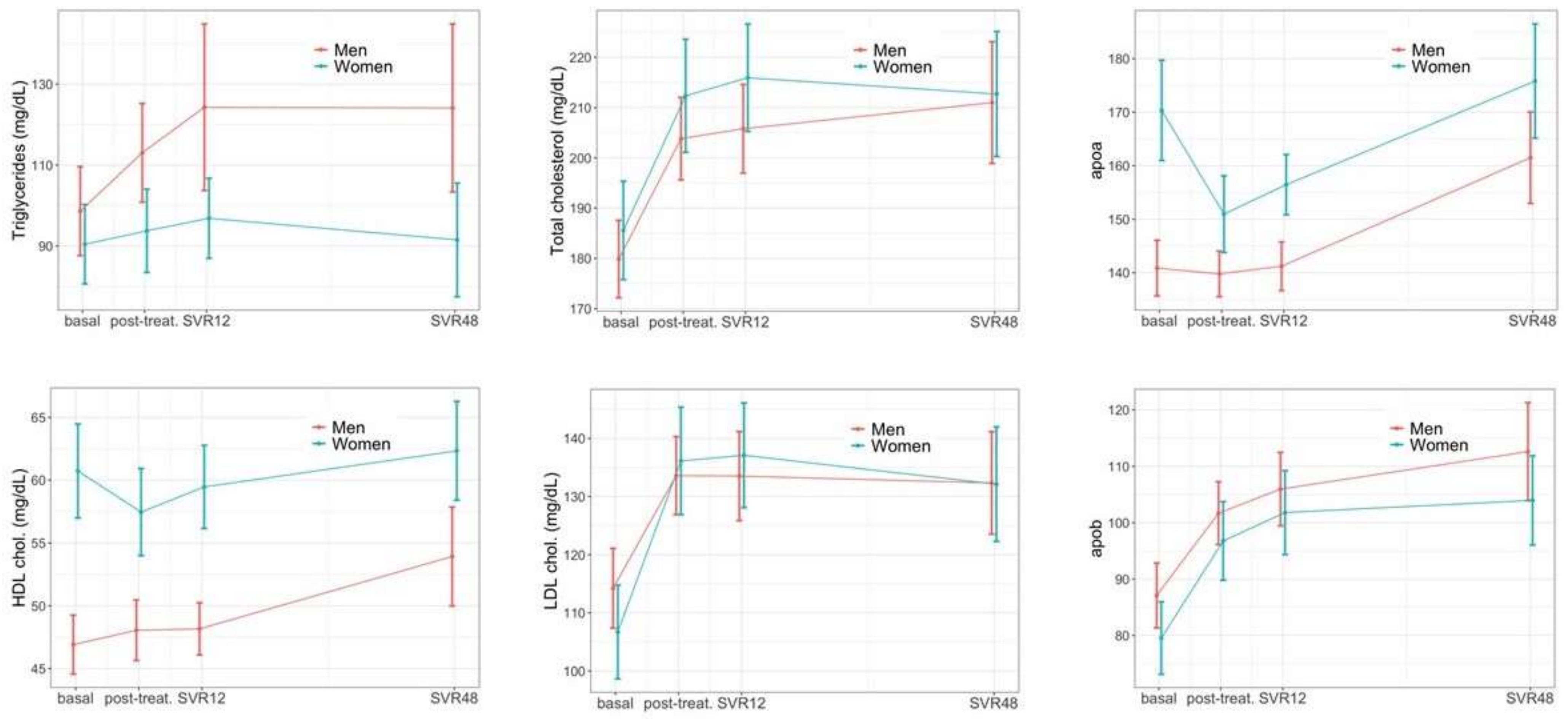
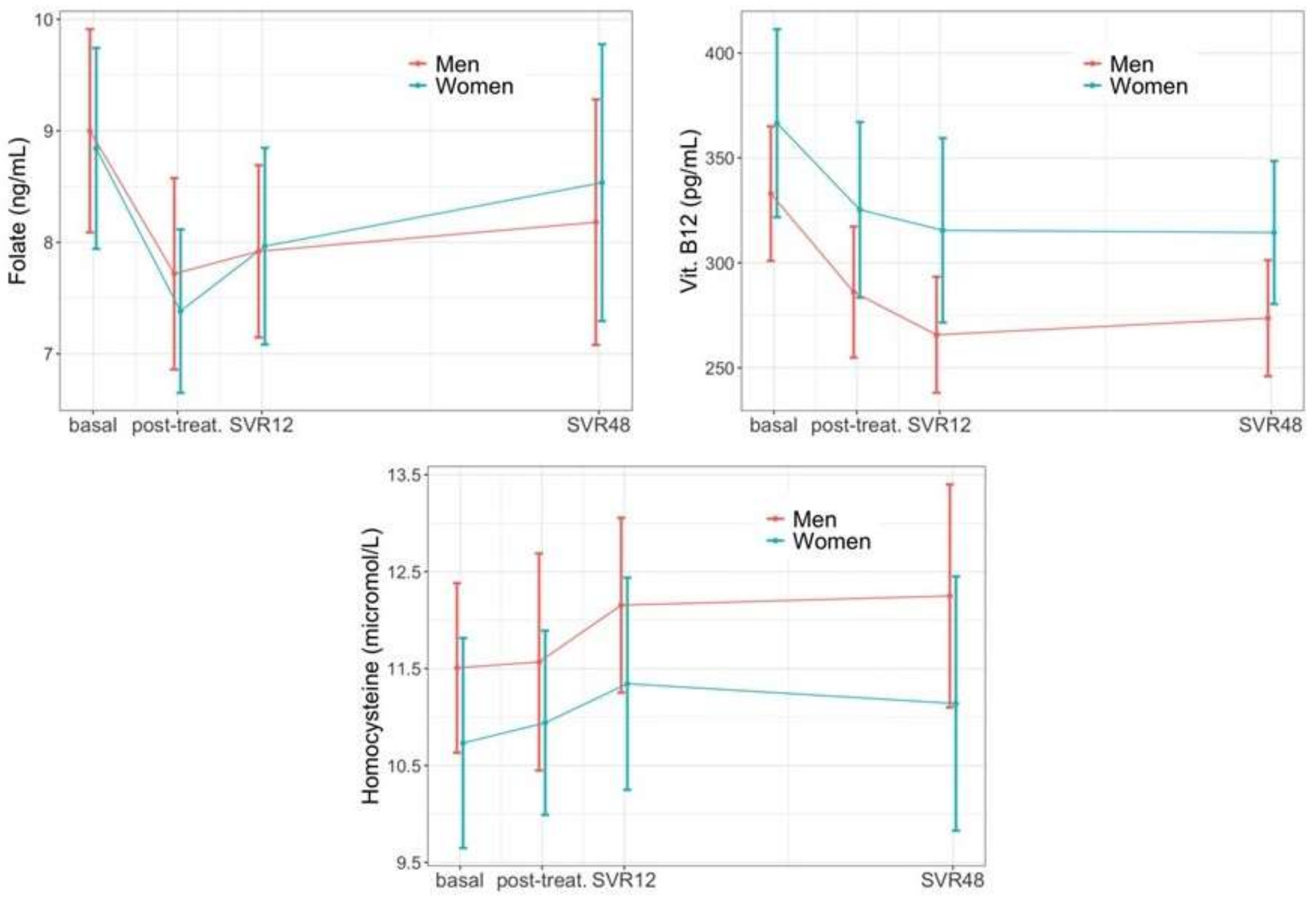
3.2. Longitudinal Hepatic Changes
3.3. Longitudinal Extra-Hepatic Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Global Hepatitis Report; WHO, World Health Organization: Geneva, Switzerland, 2017; 83p. [Google Scholar]
- Chang, M.-L. Metabolic alterations and hepatitis C: From bench to bedside. World J. Gastroenterol. 2016, 22, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Craxì, A.; Sulkowski, M.S.; Feld, J.J.; Manns, M.P. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 2015, 149, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Tamura, T. Homocysteine, Iron and Cardiovascular Disease: A Hypothesis. Nutrients 2015, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Bellia, C.; Mazzola, A.; Cabibi, D.; Cammà, C.; Caruso, A.; Di Marco, V.; Craxì, A.; Ciaccio, M. Methylenetetrahydrofolate reductase homozygosis and low-density lipoproteins in patients with genotype 1 chronic hepatitis C. J. Viral Hepat. 2011, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lott, W.B.; Takyar, S.S.; Tuppen, J.; Crawford, D.H.G.; Harrison, M.; Sloots, T.P.; Gowans, E.J. Vitamin B12 and hepatitis C: Molecular biology and human pathology. Proc. Natl. Acad. Sci. USA 2001, 98, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M.; Axiotis, C.A.; Hoofnagle, J.H.; Bacon, B.R. Measurements of iron status in patients with chronic hepatitis. Gastroenterology 1992, 102, 2108–2113. [Google Scholar] [CrossRef]
- González-Reimers, E.; Quintero-Platt, G.; Martín-González, C.; Pérez-Hernández, O.; Romero-Acevedo, L.; Santolaria-Fernández, F. Thrombin activation and liver inflammation in advanced hepatitis C virus infection. World J. Gastroenterol. 2016, 22, 4427–4437. [Google Scholar] [CrossRef]
- Borroni, G.; Andreoletti, M.; Casiraghi, M.A.; Ceriani, R.; Guerzoni, P.; Omazzi, B.; Terreni, N.; Salerno, F. Effectiveness of pegylated interferon/ribavirin combination in ‘real world’ patients with chronic hepatitis C virus infection. Aliment. Pharmacol. Ther. 2008, 27, 790–797. [Google Scholar] [CrossRef]
- Asselah, T.; Marcellin, P.; Schinazi, R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018, 38, 7–13. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.-P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Rinaldi, L.; Perrella, A.; Guarino, M.; De Luca, M.; Piai, G.; Coppola, N.; Pafundi, P.C.; Ciardiello, F.; Fasano, M.; Martinelli, E.; et al. Incidence and risk factors of early HCC occurrence in HCV patients treated with direct acting antivirals: A prospective multicentre study. J. Transl. Med. 2019, 17, 292. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology 2019, 156, 446–460. [Google Scholar] [CrossRef]
- Meissner, E.G.; Lee, Y.-J.; Osinusi, A.; Sims, Z.; Qin, J.; Sturdevant, D.; McHutchison, J.; Subramanian, M.; Sampson, M.; Naggie, S.; et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1–infected patients. Hepatology 2015, 61, 790–801. [Google Scholar] [CrossRef]
- Chida, T.; Kawata, K.; Ohta, K.; Matsunaga, E.; Ito, J.; Shimoyama, S.; Yamazaki, S.; Noritake, H.; Suzuki, T.; Suda, T.; et al. Rapid Changes in Serum Lipid Profiles during Combination Therapy with Daclatasvir and Asunaprevir in Patients Infected with Hepatitis C Virus Genotype 1b. Gut Liver 2018, 12, 201–207. [Google Scholar] [CrossRef]
- Endo, D.; Satoh, K.; Shimada, N.; Hokari, A.; Aizawa, Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J. Gastroenterol. 2017, 23, 2355–2364. [Google Scholar] [CrossRef][Green Version]
- Inoue, T.; Goto, T.; Iio, E.; Matsunami, K.; Fujiwara, K.; Shinkai, N.; Matsuura, K.; Matsui, T.; Nojiri, S.; Tanaka, Y. Changes in serum lipid profiles caused by three regimens of interferon-free direct-acting antivirals for patients infected with hepatitis C virus. Hepatol. Res. 2018, 48, E203–E212. [Google Scholar] [CrossRef]
- Graf, C.; Welzel, T.; Bogdanou, D.; Vermehren, J.; Beckel, A.; Bojunga, J.; Friedrich-Rust, M.; Dietz, J.; Kubesch, A.; Mondorf, A.; et al. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J. Clin. Med. 2020, 9, 2702. [Google Scholar] [CrossRef]
- Townsend, K.; Meissner, E.G.; Sidharthan, S.; Sampson, M.; Remaley, A.T.; Tang, L.; Kohli, A.; Osinusi, A.; Masur, H.; Kottilil, S. Interferon-Free Treatment of Hepatitis C Virus in HIV/Hepatitis C Virus-Coinfected Subjects Results in Increased Serum Low-Density Lipoprotein Concentration. AIDS Res. Hum. Retrovir. 2016, 32, 456–462. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Cheng, P.-N.; Tseng, C.-Y.; Tsai, W.-J.; Chiu, Y.-C.; Young, K.-C. Favouring modulation of circulating lipoproteins and lipid loading capacity by direct antiviral agents grazoprevir/elbasvir or ledipasvir/sofosbuvir treatment against chronic HCV infection. Gut 2017, 67, 1342–1350. [Google Scholar] [CrossRef]
- Mauss, S.; Berger, F.; Wehmeyer, M.H.; Ingiliz, P.; Hueppe, D.; Lutz, T.; Simon, K.G.; Schewe, K.; Rockstroh, J.K.; Baumgarten, A.; et al. Effect of Antiviral Therapy for HCV on Lipid Levels. Antivir. Ther. 2017, 22, 81–88. [Google Scholar] [CrossRef]
- González-Colominas, E.; Batlle, M.; Monge-Escartín, I.; Duran, X.; Viu, A.; de Antonio-Cuscó, M.; Grau, S.; Bessa, X.; Carrión, J.A. Impact of HCV cure with drug-acting antivirals in the use of concomitant medication and lipid profile: Follow-up data 2 years after the sustained virological response. Eur. J. Gastroenterol. Hepatol. 2020, 32, 214–222. [Google Scholar] [CrossRef]
- El Sagheer, G.; Soliman, E.; Ahmad, A.; Hamdy, L. Study of changes in lipid profile and insulin resistance in Egyptian patients with chronic hepatitis C genotype 4 in the era of DAAs. Libyan J. Med. 2018, 13, 1435124. [Google Scholar] [CrossRef]
- Drazilova, S.; Janicko, M.; Skladany, L.; Kristian, P.; Oltman, M.; Szantova, M.; Krkoska, D.; Mazuchova, E.; Piesecka, L.; Vahalova, V.; et al. Glucose Metabolism Changes in Patients with Chronic Hepatitis C Treated with Direct Acting Antivirals. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6095097. [Google Scholar] [CrossRef]
- Li, J.; Gordon, S.C.; Rupp, L.B.; Zhang, T.; Trudeau, S.; Holmberg, S.D.; Moorman, A.C.; Spradling, P.R.; Teshale, E.H.; Boscarino, J.A.; et al. Sustained virological response does not improve long-term glycaemic control in patients with type 2 diabetes and chronic hepatitis C. Liver Int. 2018, 39, 1027–1032. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Velosa, J.; Serejo, F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication–comparison of the new direct-acting antiviral agents with the old regimens. Scand. J. Gastroenterol. 2018, 53, 857–863. [Google Scholar] [CrossRef]
- Russo, F.P.; Zanetto, A.; Campello, E.; Bulato, C.; Shalaby, S.; Spiezia, L.; Gavasso, S.; Franceschet, E.; Radu, C.; Senzolo, M.; et al. Reversal of hypercoagulability in patients with HCV-related cirrhosis after treatment with direct-acting antivirals. Liver Int. 2018, 38, 2210–2218. [Google Scholar] [CrossRef]
- Tripodi, A.; D’Ambrosio, R.; Padovan, L.; Tosetti, G.; Aghemo, A.; Primignani, M.; Chantarangkul, V.; Peyvandi, F.; Colombo, M. Evaluation of coagulation during treatment with directly acting antivirals in patients with hepatitis C virus related cirrhosis. Liver Int. 2017, 37, 1295–1303. [Google Scholar] [CrossRef]
- Morales, A.L.; Junga, Z.; Singla, M.B.; Sjogren, M.; Torres, D. Hepatitis C eradication with sofosbuvir leads to significant metabolic changes. World J. Hepatol. 2016, 8, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Cicero, A.F.; Loggi, E.; Giovannini, M.; Conti, F.; Grandini, E.; Guarneri, V.; Scuteri, A.; Vitale, G.; Cursaro, C.; et al. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann. Hepatol. 2018, 17, 64–75. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Límites de Consumo de Bajo Riesgo de Alcohol. Actualización del Riesgo Relacionado con los Niveles de Consumo de Alcohol, el Patrón de Consumo y el Tipo de Bebida; Ministerio de Sanidad: Madrid, Spain, 2020. [Google Scholar]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.-T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Thi, V.L.D.; Granier, C.; Zeisel, M.B.; Guérin, M.; Mancip, J.; Granio, O.; Penin, F.; Lavillette, D.; Bartenschlager, R.; Baumert, T.F.; et al. Characterization of Hepatitis C Virus Particle Subpopulations Reveals Multiple Usage of the Scavenger Receptor BI for Entry Steps. J. Biol. Chem. 2012, 287, 31242–31257. [Google Scholar] [CrossRef]
- Diamond, D.L.; Syder, A.J.; Jacobs, J.M.; Sorensen, C.M.; Walters, K.-A.; Proll, S.C.; McDermott, J.E.; Gritsenko, M.A.; Zhang, Q.; Zhao, R.; et al. Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics. PLoS Pathog. 2010, 6, e1000719. [Google Scholar] [CrossRef]
- Voulgaris, T.; Sevastianos, V.A. Atherosclerosis as Extrahepatic Manifestation of Chronic Infection with Hepatitis C Virus. Hepat. Res. Treat. 2016, 2016, 7629318. [Google Scholar] [CrossRef]
- Williams-Nguyen, J.; Hawes, S.E.; Nance, R.M.; Lindström, S.; Heckbert, S.R.; Kim, H.N.; Mathews, W.C.; Cachay, E.R.; Budoff, M.; Hurt, C.B.; et al. Association between chronic Hepatitis C virus infection and myocardial infarction among people living with HIV in the United States. Am. J. Epidemiol. 2020, 189, 554–563. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Caturano, A.; Galiero, R.; Vetrano, E.; Nevola, R.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Di Marco, V.; et al. Impact of direct acting antivirals (DAAs) on cardiovascular events in HCV cohort with pre-diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2345–2353. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Coppola, C.; Narciso, V.; Nevola, R.; Rinaldi, L.; Calvaruso, V.; Staiano, L.; Di Marco, V.; et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis 2020, 296, 40–47. [Google Scholar] [CrossRef]
- Shimizu, K.; Soroida, Y.; Sato, M.; Hikita, H.; Kobayashi, T.; Endo, M.; Sato, M.; Gotoh, H.; Iwai, T.; Tateishi, R.; et al. Eradication of hepatitis C virus is associated with the attenuation of steatosis as evaluated using a controlled attenuation parameter. Sci. Rep. 2018, 8, 7845. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Chen, J.-Y.; Chiu, Y.-C.; Chiu, H.-C.; Tsai, L.-M. Augmenting central arterial stiffness following eradication of HCV by direct acting antivirals in advanced fibrosis patients. Sci. Rep. 2019, 9, 11584. [Google Scholar] [CrossRef]
- Jain, A.; Kalra, B.S.; Srivastava, S.; Chawla, S. Effect of sofosbuvir and daclatasvir on lipid profile, glycemic control and quality of life index in chronic hepatitis C, genotype 3 patients. Indian J. Gastroenterol. 2019, 38, 39–43. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Taura, N.; Motoyoshi, Y.; Akahoshi, H.; Nakamura, J.; Takahashi, Y.; Honda, T.; Yajima, H.; et al. Changes in serum LDL, PCSK9 and microRNA 122 in patients with chronic HCV infection receiving Daclatasvir/Asunaprevir. Biomed. Rep. 2019, 10, 156–164. [Google Scholar] [CrossRef]
- Doyle, M.-A.; Galanakis, C.; Mulvihill, E.; Crawley, A.; Cooper, C.L. Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters. Cells 2019, 8, 252. [Google Scholar] [CrossRef]
- Chaudhury, C.S.; Sheehan, J.; Chairez, C.; Akoth, E.; Gross, C.; Silk, R.; Kattakuzhy, S.; Rosenthal, E.; Kottilil, S.; Masur, H.; et al. No Improvement in Hemoglobin A1c Following Hepatitis C Viral Clearance in Patients with and without HIV. J. Infect. Dis. 2017, 217, 47–50. [Google Scholar] [CrossRef]
- Beig, J.; Orr, D.; Harrison, B.; Gane, E. Hepatitis C Virus Eradication with New Interferon-Free Treatment Improves Metabolic Profile in Hepatitis C Virus-Related Liver Transplant Recipients. Liver Transplant. 2018, 24, 1031–1039. [Google Scholar] [CrossRef]
- Ikeda, A.; Ikeda, K.; Takai, A.; Takahashi, K.; Ueda, Y.; Marusawa, H.; Seno, H.; Inagaki, N.; Kokuryu, H. Hepatitis C Treatment with Sofosbuvir and Ledipasvir Accompanied by Immediate Improvement in Hemoglobin A1c. Digestion 2017, 96, 228–230. [Google Scholar] [CrossRef][Green Version]
- Ciancio, A.; Bosio, R.; Bo, S.; Pellegrini, M.; Sacco, M.; Vogliotti, E.; Fassio, G.; Degerfeld, A.G.F.B.M.; Gallo, M.; Giordanino, C.; et al. Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J. Med. Virol. 2017, 90, 320–327. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef]
- Thompson, A.J.; Patel, K.; Chuang, W.-L.; Lawitz, E.J.; Rodriguez-Torres, M.; Rustgi, V.K.; Flisiak, R.; Pianko, S.; Diago, M.; Arora, S.; et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut 2012, 61, 128–134. [Google Scholar] [CrossRef]
- Czul, F.; Bhamidimarri, K.R. Noninvasive markers to assess liver fibrosis. J. Clin. Gastroenterol. 2016, 50, 445–457. [Google Scholar] [CrossRef]
- Ermens, A.; Vlasveld, L.; Lindemans, J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Mahamid, M.; Mahroum, N.; Bragazzi, N.L.; Shalaata, K.; Yavne, Y.; Adawi, M.; Amital, H.; Watad, A. Folate and B12 Levels Correlate with Histological Severity in NASH Patients. Nutrients 2018, 10, 440. [Google Scholar] [CrossRef]
- Frémont, S.; Champigneulle, B.; Gérard, P.; Felden, F.; Lambert, D.; Guéant, J.; Nicolas, J. Blood Transcobalamin Levels in Malignant Hepatoma. Tumor Biol. 1991, 12, 353–359. [Google Scholar] [CrossRef]
- Kane, S.P.; Murray-Lyon, I.M.; Paradinas, F.J.; Johnson, P.J.; Williams, R.; Orr, A.H.; Kohn, J. Vitamin B12 binding protein as a tumour marker for hepatocellular carcinoma. Gut 1978, 19, 1105–1109. [Google Scholar] [CrossRef]
- Przekop, D.; Klapaczynski, J.; Grytczuk, A.; Gruszewska, E.; Gietka, A.; Panasiuk, A.; Golaszewski, S.; Cylwik, B.; Chrostek, L. Non-Invasive Indirect Markers of Liver Fibrosis after Interferon-Free Treatment for Hepatitis C. J. Clin. Med. 2021, 10, 3951. [Google Scholar] [CrossRef]
- Bartolomei, G.; Cevik, R.E.; Marcello, A. Modulation of hepatitis C virus replication by iron and hepcidin in Huh7 hepatocytes. J. Gen. Virol. 2011, 92, 2072–2081. [Google Scholar] [CrossRef]
- Nishina, S.; Hino, K.; Korenaga, M.; Vecchi, C.; Pietrangelo, A.; Mizukami, Y.; Furutani, T.; Sakai, A.; Okuda, M.; Hidaka, I.; et al. Hepatitis C Virus–Induced Reactive Oxygen Species Raise Hepatic Iron Level in Mice by Reducing Hepcidin Transcription. Gastroenterology 2008, 134, 226–238. [Google Scholar] [CrossRef]
- Metwally, M.A.; Zein, C.O.; Zein, N.N. Clinical Significance of Hepatic Iron Deposition and Serum Iron Values in Patients with Chronic Hepatitis C Infection. Am. J. Gastroenterol. 2004, 99, 286–291. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Gil-Montero, M.; León-Muñoz, L.M.; Graciani, A.; Bayán-Bravo, A.; Taboada, J.M.; Banegas, J.R.; Rodríguez-Artalejo, F. Magnitude and Management of Hypercholesterolemia in the Adult Population of Spain, 2008–2010: The ENRICA Study. Rev. Esp. Cardiol. 2012, 65, 551–558. [Google Scholar] [CrossRef]


| Variable | All n = 167 | Men n = 88 | Women n = 79 | p-Value |
|---|---|---|---|---|
| Age | 55.2 (12.4) | 53.3 (10.8) | 57.3 (13.6) | 0.039 |
| Personal history of CVD | 3 (1.80%) | 2 (2.28%) | 1 (1.27%) | 1.000 |
| Stroke | 2 (1.20%) | 1 (1.14%) | 1 (1.27%) | 1.000 |
| Ischemic cardiopathy | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Peripheral vasculopathy | 1 (0.60%) | 1 (1.14%) | 0 (0.00%) | 1.000 |
| Family history of CVD | 0.091 | |||
| None | 122 (73.1%) | 69 (78.4%) | 53 (67.1%) | |
| Stroke | 2 (1.20%) | 1 (1.14%) | 1 (1.27%) | |
| Ischemic cardiopathy | 30 (18.0%) | 10 (11.4%) | 20 (25.3%) | |
| Peripheral vasculopathy | 13 (7.78%) | 8 (9.09%) | 5 (6.33%) | |
| CV risk factors | 51 (30.5%) | 26 (29.5%) | 25 (31.6%) | 0.900 |
| Hypertension | 37 (22.2%) | 19 (21.6%) | 18 (22.8%) | 1.000 |
| Diabetes Mellitus | 7 (4.19%) | 5 (5.68%) | 2 (2.53%) | 0.448 |
| Smoking | 0.077 | |||
| Never | 84 (50.3%) | 37 (42.0%) | 47 (59.5%) | |
| Previously | 30 (18.0%) | 19 (21.6%) | 11 (13.9%) | |
| Current | 53 (31.7%) | 32 (36.4%) | 21 (26.6%) | |
| Alcohol use | 23 (13.8%) | 16 (18.2%) | 7 (8.86%) | 0.128 |
| Body mass index (kg/m2) | 25.0 [22.6;27.1] | 25.0 [23.4;27.1] | 24.9 [22.0;27.3] | 0.748 |
| Abdominal perimeter (cm) | 92.3 (11.2) | 93.3 (10.1) | 91.3 (12.3) | 0.446 |
| Liver fibrosis (kPa) | 6.60 [5.40;10.2] | 7.00 [5.50;11.3] | 6.35 [5.15;8.80] | 0.141 |
| Liver fibrosis (Metavir) | 0.384 | |||
| 1 | 87 (53.4%) | 41 (48.2%) | 46 (59.0%) | |
| 2 | 26 (16.0%) | 13 (15.3%) | 13 (16.7%) | |
| 3 | 27 (16.6%) | 16 (18.8%) | 11 (14.1%) | |
| 4 | 23 (14.1%) | 15 (17.6%) | 8 (10.3%) | |
| Liver cirrhosis | 0.670 | |||
| No | 151 (90.4%) | 79 (89.8%) | 72 (91.1%) | |
| Yes, without PHT | 9 (5.39%) | 6 (6.82%) | 3 (3.80%) | |
| Yes, with PHT | 7 (4.19%) | 3 (3.41%) | 4 (5.06%) | |
| Esophageal varices | 7 (4.19%) | 4 (3.41%) | 3 (3.80%) | 1.000 |
| Ascites | 1 (0.60%) | 1 (1.14%) | 0 (0.00%) | 1.000 |
| Variable | All n = 167 | Men n = 88 | Women n = 79 | p-Value |
|---|---|---|---|---|
| Treatment | 0.909 | |||
| Sofosbuvir/Velpatasvir | 66 (39.5%) | 36 (40.9%) | 30 (38.0%) | |
| Ledipasvir/Sofosbuvir | 6 (3.59%) | 3 (3.41%) | 3 (3.80%) | |
| Glecaprevir/Pibrentasvir | 65 (38.9%) | 35 (39.8%) | 30 (38.0%) | |
| Elbasvir/Grazoprevir | 30 (18.0%) | 14 (15.9%) | 16 (20.3%) | |
| Treatment length | 0.848 | |||
| 12 weeks | 96 (57.8%) | 52 (59.1%) | 44 (56.4%) | |
| 8 weeks | 70 (42.2%) | 36 (40.9%) | 34 (43.6%) | |
| Viral genotype | 0.352 | |||
| 1 | 5 (3.03%) | 4 (4.65%) | 1 (1.27%) | |
| 1a | 50 (30.3%) | 29 (33.7%) | 21 (26.6%) | |
| 1b | 62 (37.6%) | 31 (36.0%) | 31 (39.2%) | |
| 2 | 4 (2.42%) | 1 (1.16%) | 3 (3.80%) | |
| 2a/c | 2 (1.21%) | 0 (0.00%) | 2 (2.53%) | |
| 3 | 16 (9.70%) | 8 (9.30%) | 8 (10.1%) | |
| 3a | 2 (1.21%) | 0 (0.00%) | 2 (2.53%) | |
| 4 | 21 (12.7%) | 10 (11.6%) | 11 (13.9%) | |
| 4c/d | 2 (1.21%) | 2 (2.33%) | 0 (0.00%) | |
| 5 | 1 (0.61%) | 1 (1.16%) | 0 (0.00%) | |
| Viral load (log) | 6.05 [5.36;6.54] | 6.11 [5.62;6.53] | 5.89 [5.16;6.55] | 0.199 |
| Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Basal n = 87 | post-T n = 81 | SVR12 n = 80 | SVR48 n = 51 | Basal n = 79 | post-T n = 78 | SVR12 n = 75 | SVR48 n = 56 | plong | psex | plong*sex |
| Bilirubin (mg/dL) | 0.74 [0.68;0.79] | 0.65 [0.59;0.70] | 0.63 [0.58;0.69] | 0.60 [0.53;0.67] | 0.65 [0.58;0.73] | 0.59 [0.54;0.65] | 0.58 [0.52;0.65] | 0.62 [0.54;0.71] | <0.001 | 0.006 | 0.032 |
| Albumin (g/dL) | 4.25 [4.17;4.32] | 4.28 [4.21;4.35] | 4.34 [4.28;4.41] | 4.34 [4.22;4.46] | 4.11 [4.04;4.18] | 4.12 [4.06;4.18] | 4.17 [4.10;4.23] | 4.26 [4.19;4.34] | <0.001 | 0.002 | 0.611 |
| Pre-albumin (mg/dL) | 22.3 [21.0;23.7] | 26.2 [24.7;27.6] | 27.1 [25.7;28.5] | 28.1 [26.0;30.1] | 18.4 [17.2;19.7] | 20.4 [19.3;21.5] | 21.7 [20.6;22.9] | 22.0 [20.5;23.5] | <0.001 | <0.001 | 0.002 |
| Alkaline phosphatase (U/L) | 84.7 [79.7;89.6] | 85.0 [79.7;90.4] | 82.3 [77.3;87.3] | 79.2 [74.2;84.2] | 93.0 [85.9;100] | 91.5 [84.8;98.2] | 88.8 [81.8;95.8] | 89.8 [82.2;97.4] | <0.001 | 0.175 | 0.523 |
| GGT (U/L) | 91.9 [66.6;117] | 29.1 [24.1;34.2] | 30.5 [24.9;36.2] | 36.4 [27.0;45.7] | 60.3 [46.5;74.1] | 26.3 [20.0;32.6] | 25.8 [20.9;30.7] | 23.9 [19.7;28.2] | <0.001 | 0.023 | 0.255 |
| AST (U/L) | 63.0 [51.7;74.3] | 26.9 [24.3;29.4] | 26.1 [23.7;28.4] | 27.2 [23.1;31.2] | 62.6 [43.1;82.2] | 25.1 [22.1;28.1] | 24.5 [19.7;29.4] | 22.7 [20.8;24.7] | <0.001 | 0.379 | 0.786 |
| ALT (U/L) | 81.7 [65.8;97.5] | 23.9 [20.3;27.5] | 21.5 [18.7;24.4] | 24.5 [17.7;31.4] | 62.7 [45.7;79.7] | 20.9 [16.3;25.6] | 19.3 [12.4;26.2] | 15.8 [14.0;17.5] | <0.001 | 0.036 | 0.447 |
| APRI | 1.08 [0.73;1.42] | 0.44 [0.36;0.51] | 0.41 [0.33;0.49] | 0.39 [0.30;0.47] | 1.07 [0.59;1.55] | 0.41 [0.31;0.50] | 0.37 [0.27;0.47] | 0.33 [0.27;0.38] | <0.001 | 0.465 | 0.772 |
| FIB-4 | 2.54 [2.03;3.06] | 1.92 [1.67;2.17] | 1.92 [1.66;2.17] | 1.76 [1.52;2.01] | 2.99 [2.23;3.76] | 2.13 [1.73;2.54] | 2.07 [1.72;2.43] | 2.06 [1.66;2.46] | <0.001 | 0.922 | 0.636 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Deza, D.; Martínez-Sapiña, A.; Espina, S.; Garcia-Rodriguez, B.; Fernandez-Bonilla, E.M.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease. J. Clin. Med. 2022, 11, 4049. https://doi.org/10.3390/jcm11144049
Casas-Deza D, Martínez-Sapiña A, Espina S, Garcia-Rodriguez B, Fernandez-Bonilla EM, Sanz-Paris A, Gonzalez-Irazabal Y, Bernal-Monterde V, Arbones-Mainar JM. Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease. Journal of Clinical Medicine. 2022; 11(14):4049. https://doi.org/10.3390/jcm11144049
Chicago/Turabian StyleCasas-Deza, Diego, Ana Martínez-Sapiña, Silvia Espina, Beatriz Garcia-Rodriguez, Eva M. Fernandez-Bonilla, Alejandro Sanz-Paris, Yolanda Gonzalez-Irazabal, Vanesa Bernal-Monterde, and Jose M. Arbones-Mainar. 2022. "Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease" Journal of Clinical Medicine 11, no. 14: 4049. https://doi.org/10.3390/jcm11144049
APA StyleCasas-Deza, D., Martínez-Sapiña, A., Espina, S., Garcia-Rodriguez, B., Fernandez-Bonilla, E. M., Sanz-Paris, A., Gonzalez-Irazabal, Y., Bernal-Monterde, V., & Arbones-Mainar, J. M. (2022). Evaluation of Cardiovascular Risk Factors after Hepatitis C Virus Eradication with Direct-Acting Antivirals in a Cohort of Treatment-Naïve Patients without History of Cardiovascular Disease. Journal of Clinical Medicine, 11(14), 4049. https://doi.org/10.3390/jcm11144049







