Association between Traumatic Subarachnoid Hemorrhage and Acute Respiratory Failure in Moderate-to-Severe Traumatic Brain Injury Patients
Abstract
1. Introduction
2. Materials and Methods
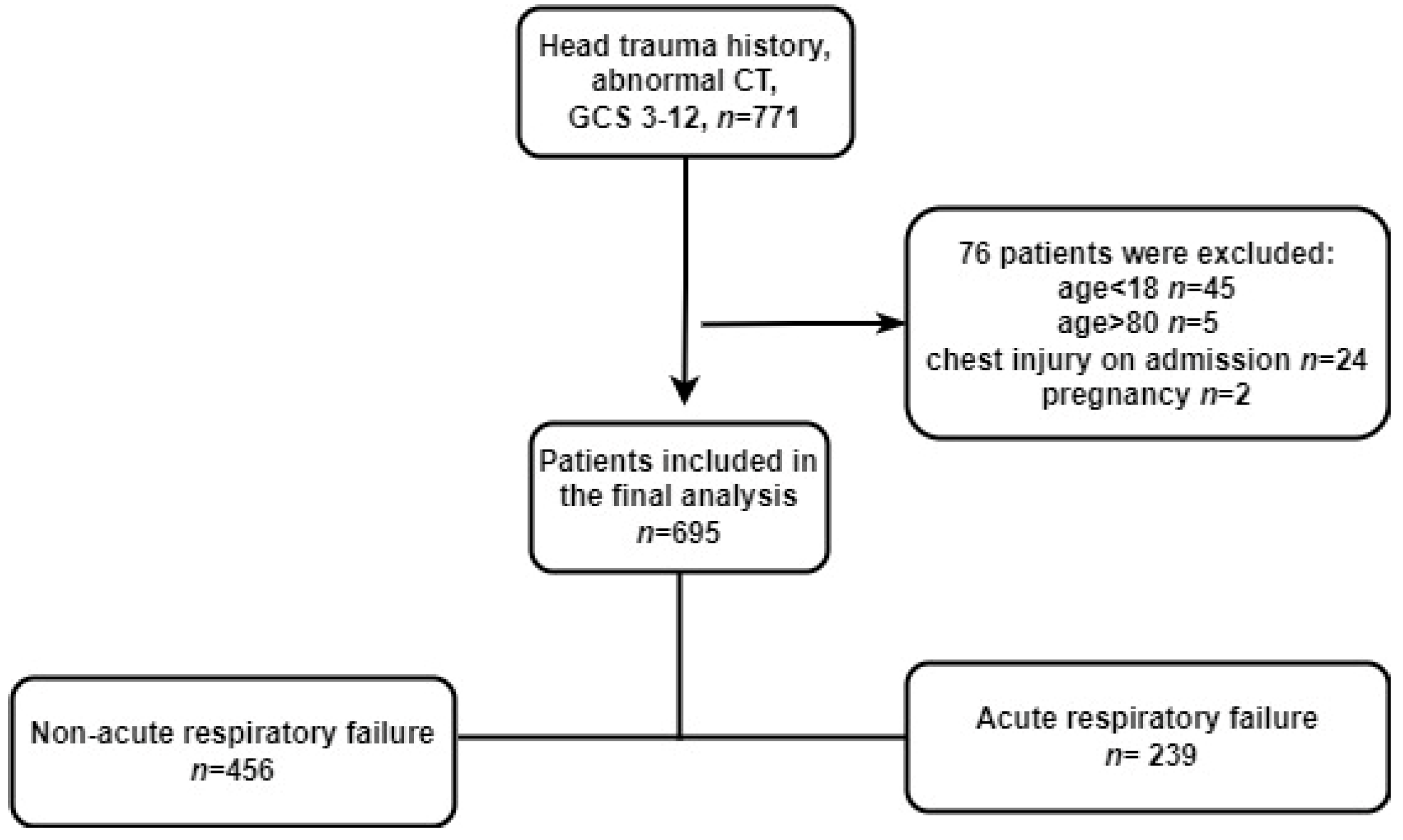
2.1. Study Design, Population, and Setting
2.2. Data Collection and Primary Outcome
2.3. Exposure and Covariates
2.4. Statistical Methods
2.5. Sensitivity Analysis
3. Results
3.1. Population
3.2. Baseline Characteristics
3.3. Primary Outcome
3.4. Propensity Score Matching for Outcomes
3.5. Sensitive Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, D.M.; Hochrieser, H.; Metnitz, P.G.H.; Mauritz, W. Tracheostomy is associated with decreased hospital mortality after moderate or severe isolated traumatic brain injury. Wien. Klin. Wochenschr. 2016, 128, 397–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kerr, N.A.; Vaccari, J.P.D.R.; Abbassi, S.; Kaur, H.; Zambrano, R.; Wu, S.; Dietrich, W.D.; Keane, R.W. Traumatic Brain Injury-Induced Acute Lung Injury: Evidence for Activation and Inhibition of a Neural-Respiratory-Inflammasome Axis. J. Neurotrauma 2018, 35, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.L.; Davis, R.L. Acute lung injury in isolated traumatic brain injury. Neurosurgery 1997, 40, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Frisvold, S.K.; Robba, C.; Guérin, C. What respiratory targets should be recommended in patients with brain injury and respiratory failure? Intensive Care Med. 2019, 45, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Ferbert, A.; Deinsberger, W.; Kleffmann, J.; Kästner, S.; Godau, J.; Schüler, M.; Tryba, M.; Gehling, M. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocritical Care 2014, 21, 186–191. [Google Scholar] [CrossRef]
- Whitaker-Lea, W.A.; Valadka, A.B. Acute Management of Moderate-Severe Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 227–243. [Google Scholar] [CrossRef]
- Quigley, M.R.; Chew, B.G.; Swartz, C.E.; Wilberger, J.E. The clinical significance of isolated traumatic subarachnoid hemorrhage. J. Trauma Acute Care Surg. 2013, 74, 581–584. [Google Scholar] [CrossRef]
- Armin, S.S.; Colohan, A.R.T.; Zhang, J.H. Traumatic subarachnoid hemorrhage: Our current understanding and its evolution over the past half century. Neurol. Res. 2006, 28, 445–452. [Google Scholar] [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Sheng, B.; Lai, N.-S.; Yao, Y.; Dong, J.; Li, Z.-B.; Zhao, X.-T.; Liu, J.-Q.; Li, X.-Q.; Fang, X.-G. Early serum miR-1297 is an indicator of poor neurological outcome in patients with aSAH. Biosci. Rep. 2018, 38, BSR20180646. [Google Scholar] [CrossRef]
- Mattioli, C.; Beretta, L.; Gerevini, S.; Veglia, F.; Citerio, G.; Cormio, M.; Stocchetti, N. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: A multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J. Neurosurg. 2003, 98, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Caldwell, E.C.; Deem, S.; Newell, D.W.; Heckbert, S.R.; Rubenfeld, G.D. Acute lung injury in patients with subarachnoid hemorrhage: Incidence, risk factors, and outcome. Crit. Care Med. 2006, 34, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Brihaye, J.; Frowein, R.A.; Lindgrén, S.; Loew, F.; Stroobandt, G. Report on the meeting of the W.F.N.S. neuro-traumatology committee, Brussels, 19–23 September 1976. Acta Neurochir. 1978, 40, 181–186. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Giannella, M.; Scudeller, L.; Tedeschi, S.; Rinaldi, M.; Bussini, L.; Fornaro, G.; Pascale, R.; Pancaldi, L.; Pasquini, Z.; et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: A multicentre cohort study (PREDI-CO study). Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1545–1553. [Google Scholar] [CrossRef]
- Schlenk, F.; Graetz, D.; Nagel, A.; Schmidt, M.; Sarrafzadeh, A.S. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit. Care 2008, 12, R9. [Google Scholar] [CrossRef]
- Mata-Mbemba, D.; Mugikura, S.; Nakagawa, A.; Murata, T.; Ishii, K.; Kushimoto, S.; Tominaga, T.; Takahashi, S.; Takase, K. Traumatic midline subarachnoid hemorrhage on initial computed tomography as a marker of severe diffuse axonal injury. J. Neurosurg. 2018, 129, 1317–1324. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Xu, C.; Ying, J.-C.; Lü, W.-B.; Hong, G.-L.; Li, M.-F.; Wu, B.; Yao, Y.-M.; Lu, Z.-Q. Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. Crit. Care 2020, 24, 75. [Google Scholar] [CrossRef]
- Feng, M.; McSparron, J.I.; Kien, D.T.; Stone, D.J.; Roberts, D.H.; Schwartzstein, R.M.; Vieillard-Baron, A.; Celi, L.A. Transthoracic echocardiography and mortality in sepsis: Analysis of the MIMIC-III database. Intensive Care Med. 2018, 44, 884–892. [Google Scholar] [CrossRef]
- Rincon, F.; Ghosh, S.; Dey, S.; Maltenfort, M.; Vibbert, M.; Urtecho, J.; McBride, W.; Moussouttas, M.; Bell, R.; Ratliff, J.K.; et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery 2012, 71, 795–803. [Google Scholar] [CrossRef]
- Shigemori, M.; Tokutomi, T.; Hirohata, M.; Maruiwa, H.; Kaku, N.; Kuramoto, S. Clinical significance of traumatic subarachnoid hemorrhage. Neurol. Med. Chir. 1990, 30, 396–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haghbayan, H.; Boutin, A.; Laflamme, M.; Lauzier, F.; Shemilt, M.; Moore, L.; Zarychanski, R.; Douville, V.; Fergusson, D.; Turgeon, A.F. The Prognostic Value of MRI in Moderate and Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, e1280–e1288. [Google Scholar] [CrossRef] [PubMed]
- Cicuendez, M.; Castaño-León, A.; Ramos, A.; Hilario, A.; Gómez, P.A.; Lagares, A. The added prognostic value of magnetic resonance imaging in traumatic brain injury: The importance of traumatic axonal injury when performing ordinal logistic regression. J. Neuroradiol. 2019, 46, 299–306. [Google Scholar] [CrossRef]
- Bai, W.; Li, P.; Ning, Y.-L.; Jiang, Y.-L.; Yang, N.; Chen, X.; Zhou, Y.-G. Reduction in Blood Glutamate Levels Combined with the Genetic Inactivation of A2AR Significantly Alleviate Traumatic Brain Injury-Induced Acute Lung Injury. Shock 2019, 51, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhu, W.-L.; Ning, Y.-L.; Li, P.; Zhao, Y.; Yang, N.; Chen, X.; Jiang, Y.-L.; Yang, W.-Q.; Jiang, N.-P.; et al. Dramatic increases in blood glutamate concentrations are closely related to traumatic brain injury-induced acute lung injury. Sci. Rep. 2017, 7, 5380. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-S.; Huang, K.-F.; Huang, C.-C.; Wang, J.-Y. Thaliporphine derivative improves acute lung injury after traumatic brain injury. BioMed Res. Int. 2015, 2015, 729831. [Google Scholar] [CrossRef]
- Hu, P.J.; Pittet, J.-F.; Kerby, J.D.; Bosarge, P.L.; Wagener, B.M. Acute brain trauma, lung injury, and pneumonia: More than just altered mental status and decreased airway protection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1–L115. [Google Scholar] [CrossRef]
- Kerr, N.; Vaccari, J.P.D.R.; Dietrich, W.D.; Keane, R.W. Neural-respiratory inflammasome axis in traumatic brain injury. Exp. Neurol. 2020, 323, 113080. [Google Scholar] [CrossRef]
- Shao, X.F.; Li, B.; Shen, J.; Wang, Q.F.; Chen, S.S.; Jiang, X.C.; Qiang, D. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int. Immunopharmacol. 2020, 79, 106175. [Google Scholar] [CrossRef]
- Xu, X.; Zhi, T.; Chao, H.; Jiang, K.; Liu, Y.; Bao, Z.; Fan, L.; Wang, D.; Li, Z.; Liu, N.; et al. ERK1/2/mTOR/Stat3 pathway-mediated autophagy alleviates traumatic brain injury-induced acute lung injury. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018, 1864, 1663–1674. [Google Scholar] [CrossRef]
- Zhang, C.-N.; Li, F.-J.; Zhao, Z.-L.; Zhang, J.-N. The role of extracellular vesicles in traumatic brain injury-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L885–L891. [Google Scholar] [CrossRef] [PubMed]

| Unmatched | Matched (1:1) | |||||
|---|---|---|---|---|---|---|
| Item | Low Grade | High Grade | p-Value | Low Grade | High Grade | p-Value |
| n | 481 | 214 | 166 | 166 | ||
| Age | 50 (39, 60) | 53 (43, 60) | 0.052 | 53 (44, 62) | 52 (43, 60) | 0.276 |
| Male | 373 (77.5) | 171 (79.9) | 0.551 | 124 (74.7) | 134 (80.7) | 0.235 |
| GCS | 6 (4, 9) | 7 (5, 9) | 0.284 | 7 (4, 9) | 6.5 (5.0, 9.0) | 0.595 |
| Multiple injury | 165 (34.3) | 95 (44.4) | 0.014 | 74 (44.6) | 63 (38.0) | 0.265 |
| Smoke | 175 (36.4) | 83 (38.8) | 0.603 | 56 (33.7) | 60 (36.1) | 0.730 |
| HTN | 52 (10.8) | 27 (12.6) | 0.573 | 20 (12.0) | 16 (9.6) | 0.596 |
| DM | 11 (2.3) | 5 (2.3) | 1.000 | 6 (3.6) | 4 (2.4) | 0.748 |
| COPD | 1 (0.2) | 0 (0.0) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 |
| CAD | 8 (1.7) | 4 (1.9) | 1.000 | 2 (1.2) | 4 (2.4) | 0.685 |
| EDH | 96 (20.0) | 56 (26.2) | 0.084 | 45 (27.1) | 36 (21.7) | 0.307 |
| SDH | 126 (26.2) | 114 (53.3) | <0.001 | 82 (49.4) | 78 (47.0) | 0.742 |
| Brain contusion | 294 (61.1) | 170 (79.4) | <0.001 | 121 (72.9) | 132 (79.5) | 0.197 |
| IVH | 56 (11.6) | 90 (42.1) | <0.001 | 49 (29.5) | 52 (31.3) | 0.811 |
| MLS | 0.0 (0.0, 0.5) | 0.4 (0.0, 0.8) | <0.001 | 0.50 (0.79) | 0.53 (0.66) | 0.76 |
| Basal cistern | <0.001 | 0.854 | ||||
| 0 | 364 (75.7) | 113 (52.8) | 103 (62.0) | 99 (59.6) | ||
| 1 | 77 (16.0) | 44 (20.6) | 32 (19.3) | 36 (21.7) | ||
| 2 | 40 (8.3) | 57 (26.6) | 31 (18.7) | 31 (18.7) | ||
| Aspiration | 338 (70.3) | 145 (67.8) | 0.565 | 112 (67.5) | 113 (68.1) | 1.000 |
| Intubation | 241 (50.1) | 119 (55.6) | 0.208 | 87 (52.4) | 91 (54.8) | 0.741 |
| Lung consolidation | 135 (28.1) | 63 (29.4) | 0.780 | 44 (26.5) | 45 (27.1) | 1.000 |
| Venous infusion > 3000 mL | 179 (37.2) | 107 (50.0) | 0.002 | 79 (47.6) | 79 (47.6) | 1.000 |
| General anesthesia and operation before ARF | 434 (90.2) | 199 (93.0) | 0.301 | 149 (89.8) | 153 (92.2) | 0.566 |
| Variable | n | ARF (n/%) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| No. of events/no. of patients at risk (%) | ||||
| Low Fisher grade | 481 | 142 (29.5) | ||
| High Fisher grade | 214 | 99 (46.3) | ||
| Crude analysis | 695 | 241 (34.7) | 2.06 (1.47~2.87) | <0.001 |
| Multivariable analysis a | 695 | 241 (34.7) | 1.78 (1.11~2.85) | 0.016 |
| With matching b | 332 | 124 (37.3) | 1.59 (1.02~2.49) | 0.042 |
| With inverse probability weighting c | 695 | 241 (34.7) | 1.48 (1.05~2.06) | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, R.; Fang, Q.-X.; He, Y.-X.; Shi, Y.-W.; Ge, S.-N.; Ma, R.-N.; Qu, Y. Association between Traumatic Subarachnoid Hemorrhage and Acute Respiratory Failure in Moderate-to-Severe Traumatic Brain Injury Patients. J. Clin. Med. 2022, 11, 3995. https://doi.org/10.3390/jcm11143995
Li M, Wang R, Fang Q-X, He Y-X, Shi Y-W, Ge S-N, Ma R-N, Qu Y. Association between Traumatic Subarachnoid Hemorrhage and Acute Respiratory Failure in Moderate-to-Severe Traumatic Brain Injury Patients. Journal of Clinical Medicine. 2022; 11(14):3995. https://doi.org/10.3390/jcm11143995
Chicago/Turabian StyleLi, Min, Rui Wang, Qi-Xing Fang, Yi-Xuan He, Ying-Wu Shi, Shun-Nan Ge, Rui-Na Ma, and Yan Qu. 2022. "Association between Traumatic Subarachnoid Hemorrhage and Acute Respiratory Failure in Moderate-to-Severe Traumatic Brain Injury Patients" Journal of Clinical Medicine 11, no. 14: 3995. https://doi.org/10.3390/jcm11143995
APA StyleLi, M., Wang, R., Fang, Q.-X., He, Y.-X., Shi, Y.-W., Ge, S.-N., Ma, R.-N., & Qu, Y. (2022). Association between Traumatic Subarachnoid Hemorrhage and Acute Respiratory Failure in Moderate-to-Severe Traumatic Brain Injury Patients. Journal of Clinical Medicine, 11(14), 3995. https://doi.org/10.3390/jcm11143995







