Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging
2.3. Systemic Glycemic Indices
2.4. Statistical Analysis
3. Results
3.1. Population
3.2. Retinal Layers Volume and Thickness
3.3. OCT Angiography Parameters
3.4. Correlations of Glycemic Indices with OCT and OCTA Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L.; et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA J. Am. Med. Assoc. 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Hautala, N.; Hannula, V.; Palosaari, T.; Ebeling, T.; Falck, A. Prevalence of diabetic retinopathy in young adults with type 1 diabetes since childhood: The oulu cohort study of diabetic retinopathy. Acta Ophthalmol. 2014, 92, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.B.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.J.; van Velthoven, M.E.J.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pritchard, N.; Sampson, G.P.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Retinal tissue thickness in type 1 and type 2 diabetes. Clin. Exp. Optom. 2016, 99, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, F.; Picconi, F.; Virgili, G.; Giorno, P.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Single retinal layer evaluation in patients with type 1 diabetes with no or early signs of diabetic retinopathy: The first hint of neurovascular crosstalk damage between neurons and capillaries? Ophthalmologica 2017, 237, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Zozulińska-Ziółkiewicz, D.; Meller, M.; Bernardczyk-Meller, J.; Piłaciński, S.; Rogowicz-Frontczak, A.; Naskrȩt, D.; Wierusz-Wysocka, B. Neurodegeneration of the retina in type 1 diabetic patients. Pol. Arch. Med. Wewn. 2012, 122, 464–470. [Google Scholar] [CrossRef]
- Mameli, C.; Invernizzi, A.; Bolchini, A.; Bedogni, G.; Giani, E.; MacEdoni, M.; Zuccotti, G.; Preziosa, C.; Pellegrini, M. Analysis of retinal perfusion in children, adolescents, and young adults with type 1 diabetes using optical coherence tomography angiography. J. Diabetes Res. 2019, 2019. [Google Scholar] [CrossRef]
- Li, T.; Jia, Y.; Wang, S.; Wang, A.; Gao, L.; Yang, C.; Zou, H. Retinal microvascular abnormalities in children with type 1 diabetes mellitus without visual impairment or diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 990–998. [Google Scholar] [CrossRef]
- Niestrata-Ortiz, M.; Fichna, P.; Stankiewicz, W.; Stopa, M. Enlargement of the foveal avascular zone detected by optical coherence tomography angiography in diabetic children without diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 689–697. [Google Scholar] [CrossRef]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.E.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G.; Rangasamy, S. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, E.E.B.; Schouten, J.S.A.G.; Berendschot, T.T.J.M.; Kessels, A.G.H.; Nuijts, R.M.M.A.; Beckers, H.J.M.; Schram, M.T.; Stehouwer, C.D.A.; Webers, C.A.B. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015, 3, 653–663. [Google Scholar] [CrossRef]
- Sohn, E.H.; Van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; Van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- De Clerck, E.E.B.; Schouten, J.S.A.G.; Berendschot, T.T.J.M.; Goezinne, F.; Dagnelie, P.C.; Schaper, N.C.; Schram, M.T.; Stehouwer, C.D.A.; Webers, C.A.B. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: The maastricht study. Acta Ophthalmol. 2018, 96, 174–182. [Google Scholar] [CrossRef]
- Lynch, S.K.; Abràmoff, M.D. Diabetic retinopathy is a neurodegenerative disorder. Vis. Res. 2017, 139, 101–107. [Google Scholar] [CrossRef]
- Vujosevic, S.; Micera, A.; Bini, S.; Berton, M.; Esposito, G.; Midena, E. Proteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humour of diabetic patients. Acta Ophthalmol. 2016, 94, 56–64. [Google Scholar] [CrossRef]
- Vujosevic, S.; Micera, A.; Bini, S.; Berton, M.; Esposito, G.; Midena, E. Aqueous humor biomarkers of müller cell activation in diabetic eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3913–3918. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Frizziero, L.; Midena, G.; Longhin, E.; Berton, M.; Torresin, T.; Parrozzani, R.; Pilotto, E. Early retinal changes by OCT angiography and multifocal electroretinography in diabetes. J. Clin. Med. 2020, 9, 3514. [Google Scholar] [CrossRef]
- Pilotto, E.; Nacci, E.B.; Ferrara, A.M.; De Mojà, G.; Zovato, S.; Midena, E. Macular perfusion impairment in von Hippel-Lindau disease suggests a generalized retinal vessel alteration. J. Clin. Med. 2020, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, E.; Nacci, E.B.; De Mojà, G.; Ferrara, A.M.; Parrozzani, R.; Londei, D.; Zovato, S.; Midena, E. Structural and microvascular changes of the peripapillary retinal nerve fiber layer in von Hippel–Lindau disease: An OCT and OCT angiography study. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Midena, E.; Torresin, T.; Longhin, E.; Midena, G.; Pilotto, E.; Frizziero, L. Early microvascular and oscillatory potentials changes in human diabetic retina: Amacrine cells and the intraretinal neurovascular crosstalk. J. Clin. Med. 2021, 10, 4035. [Google Scholar] [CrossRef]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Odrobina, D.; Baszyńska-Wilk, M.; Groszek, A.; Szalecki, M.; Hautz, W. Optical coherence tomography angiography vessel density in children with type 1 diabetes. PLoS ONE 2017, 12, e0186479. [Google Scholar] [CrossRef]
- Ong, J.X.; Fawzi, A.A. Perspectives on diabetic retinopathy from advanced retinal vascular imaging. Eye 2022, 36, 319–327. [Google Scholar] [CrossRef]
- Rosen, R.B.; Andrade Romo, J.S.; Krawitz, B.D.; Mo, S.; Fawzi, A.A.; Linderman, R.E.; Carroll, J.; Pinhas, A.; Chui, T.Y.P. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am. J. Ophthalmol. 2019, 203, 103–115. [Google Scholar] [CrossRef]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal nerve fiber layer and ganglion cell complex thicknesses are reduced in children with type 1 diabetes with no evidence of vascular retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5355–5360. [Google Scholar] [CrossRef]
- Gołȩbiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Baszyńska-Wilk, M.; Groszek, A.; Czeszyk-Piotrowicz, A.; Szalecki, M.; Hautz, W. Choroidal thickness and ganglion cell complex in pubescent children with type 1 diabetes without diabetic retinopathy analyzed by spectral domain optical coherence tomography. J. Diabetes Res. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Prada, D.; Harris, A.; Guidoboni, G.; Siesky, B.; Huang, A.M.; Arciero, J. Autoregulation and neurovascular coupling in the optic nerve head. Surv. Ophthalmol. 2016, 61, 164–186. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of topically administered neuroprotective drugs in early stages of diabetic retinopathy: Results of the EUROCONDOR clinical trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Chen, H.; Zheng, S. Alterations of retinal pigment epithelium-photoreceptor complex in patients with type 2 diabetes mellitus without diabetic retinopathy: A cross-sectional study. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Énzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, Á.; Németh, J.; Lukáts, Á. Pathologic alterations of the outer retina in streptozotocin-induced diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Frydkjaer-Olsen, U.; Grauslund, J. Vascular changes and neurodegeneration in the early stages of diabetic retinopathy: Which comes first? Ophthalmic Res. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Hegazy, A.I.; Zedan, R.H.; Macky, T.A.; Esmat, S.M. Retinal ganglion cell complex changes using spectral domain optical coherence tomography in diabetic patients without retinopathy. Int. J. Ophthalmol. 2017, 10, 427–433. [Google Scholar] [CrossRef]
- Kern, T.S.; Barber, A.J. Retinal ganglion cells in diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Dehghani, C.; Srinivasan, S.; Edwards, K.; Pritchard, N.; Russell, A.W.; Malik, R.A.; Efron, N. Presence of peripheral neuropathy is associated with progressive thinning of retinal nerve fiber layer in type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO234–BIO239. [Google Scholar] [CrossRef][Green Version]
- Kern, T.S.; Berkowitz, B.A. Photoreceptors in diabetic retinopathy. J. Diabetes Investig. 2015, 6, 371–380. [Google Scholar] [CrossRef]
- Wysocka-Mincewicz, M.; Baszyńska-Wilk, M.; Gołębiewska, J.; Olechowski, A.; Byczyńska, A.; Hautz, W.; Szalecki, M. Influence of metabolic parameters and treatment method on OCT angiography results in children with type 1 diabetes. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Casaluci, M.; Borrelli, E.; Mulinacci, G.; Lamanna, F.; Gelormini, F.; Carnevali, A.; Querques, L.; Zerbini, G.; Bandello, F.; et al. Multimodal imaging assessment of vascular and neurodegenerative retinal alterations in type 1 diabetic patients without fundoscopic signs of diabetic retinopathy. J. Clin. Med. 2019, 8, 1409. [Google Scholar] [CrossRef] [PubMed]
| HC Group | noDR Group | DR Group | p-Value noDR vs. DR | |
|---|---|---|---|---|
| Eyes | 40 | 68 | 10 | |
| Mean age ± SD (years) | 17.3 ± 3.1 | 17.2 ± 2.0 | 17.0 ± 1.6 | 0.8602 a |
| Mean duration T1D ± SD (years) | n.a. | 12.5 ± 2.1 | 12.8 ± 2.0 | 0.8202 a |
| Mean glucose ± SD (mg/dL) | n.a. | 178.2 ± 30.9 | 179.6 ± 14.0 | 0.8640 a |
| Glycemic variability (SD) ± SD (mg/dL) | n.a. | 80.0 ± 14.7 | 84.4 ± 7.0 | 0.3392 a |
| TIR ± SD (%) | n.a. | 46.6 ± 11.2 | 48.3 ± 3.9 | 0.5495 a |
| TBR ± SD (%) | n.a. | 9.7 ± 6.9 | 9.3 ± 4.7 | 0.8806 a |
| HbA1c ± SD (%) | n.a. | 7.6 ± 1.0 | 7.7 ± 0.9 | 0.7737 a |
| AGE ± SD (AU) | n.a. | 1.3 ± 0.2 | 1.5 ± 0.4 | 0.2478 a |
| HC Group Mean ± SD (mm3) | noDR Group Mean ± SD (mm3) | DR Group Mean ± SD (mm3) | p-Value noDR vs. HC | p-Value DR vs. HC | p-Value DR vs. noDR | |
|---|---|---|---|---|---|---|
| Full retina | 8.79 ± 0.33 | 8.98 ± 0.35 | 8.77 ± 0.29 | 0.0632 | 0.8716 | 0.8892 |
| RNFL | 0.90 ± 0.06 | 0.94 ± 0.09 | 0.88 ± 0.06 | 0.1460 | 0.2266 | 0.4465 |
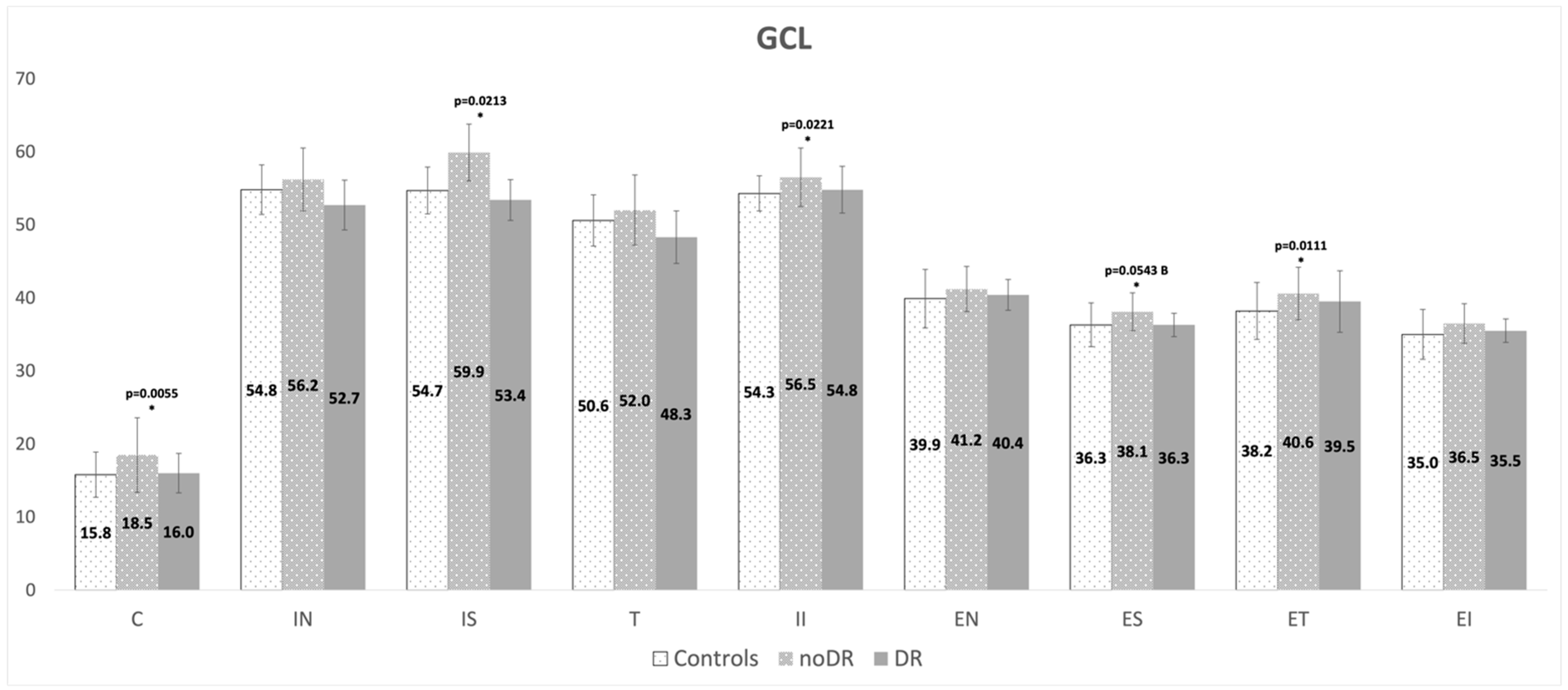
| GCL | 1.14 ± 0.08 | 1.19 ± 0.08 | 1.15 ± 0.05 | 0.0433 | 0.9625 | 0.7731 |
| IPL | 0.94 ± 0.07 | 0.97 ± 0.06 | 0.94 ± 0.04 | 0.1409 | 0.7403 | 0.8478 |
| INL | 0.99 ± 0.07 | 1.00 ± 0.06 | 0.97 ± 0.04 | 0.6313 | 0.5663 | 0.5393 |
| OPL | 0.80 ± 0.07 | 0.79 ± 0.05 | 0.80 ± 0.05 | 0.7010 | 0.9923 | 0.7368 |
| ONL | 1.77 ± 0.17 | 1.80 ± 0.17 | 1.73 ± 0.18 | 0.5484 | 0.7663 | 0.9041 |
| ORL | 2.26 ± 0.06 | 2.29 ± 0.07 | 2.32 ± 0.06 | 0.0346 | 0.0340 | 0.2872 |
| Sector | HC Group Mean ± SD (mm) | noDR Group mean ± SD (mm) | DR Group Mean ± SD (mm) | p-Value noDR vs. HC | p-Value DR vs. HC | p-Value DR vs. noDR |
|---|---|---|---|---|---|---|
| Global | 101.0 ± 9.6 | 106.3 ± 9.6 | 103.2 ± 9.5 | 0.2025 | 0.6723 | 0.8380 |
| Nasal | 75.2 ± 16.9 | 78.3 ± 13.5 | 74.1 ± 10.4 | 0.4709 | 0.9533 | 0.9935 |
| Nasal Superior | 115.1 ± 22.8 | 116.7±19.2 | 106.8 ± 17.1 | 0.7175 | 0.2667 | 0.2943 |
| Temporal Superior | 147.3 ± 15.0 | 148.6 ± 18.3 | 149.6 ± 17.1 | 0.7778 | 0.6617 | 0.3378 |
| Temporal | 72.7 ± 16.3 | 77.2 ± 16.8 | 73.1 ± 8.4 | 0.2860 | 0.8755 | 0.9826 |
| Temporal Inferior | 147.4 ± 13.8 | 155.7 ± 17.0 | 156.1 ± 14.0 | 0.0447 | 0.1715 | 0.3952 |
| Nasal Inferior | 106.9 ± 26.7 | 121.5 ± 23.3 | 118.1 ± 26.4 | 0.0004 | 0.0853 | 0.8826 |
| Sector | HC Group Mean ± SD (mm) | noDR Group Mean ± SD (mm) | DR Group Mean ± SD (mm) | p-Value noDR vs. HC | p-Value DR vs. HC | p-Value DR vs. noDR |
|---|---|---|---|---|---|---|
| Central | 93.2 ± 4.5 | 94.3 ± 3.5 | 93.6 ± 4.7 | 0.1515 | 0.9135 | 0.2725 |
| Internal Nasal | 84.1 ± 2.9 | 85.3 ± 2.7 | 85.3 ± 2.4 | 0.0957 | 0.4109 | 0.8446 |
| Internal Superior | 81.4 ± 2.6 | 83.1 ± 2.6 | 83.4 ± 2.4 | 0.0209 | 0.1472 | 0.8472 |
| Internal Temporal | 82.4 ± 3.5 | 83.9 ± 2.9 | 84.1 ± 2.8 | 0.0378 | 0.2225 | 0.9621 |
| Internal Inferior | 80.8 ± 2.9 | 82.2 ± 2.7 | 82.8 ± 2.8 | 0.0556 B | 0.1472 | 0.5700 |
| External Nasal | 79.9 ± 2.0 | 81.1 ± 2.6 | 82.6 ± 2.4 | 0.0944 | 0.0363 | 0.0760 |
| External Superior | 79.1 ± 2.4 | 80.8 ± 2.7 | 82.0 ± 2.5 | 0.0253 | 0.0281 | 0.1516 |
| External Temporal | 77.8 ± 2.8 | 78.9 ± 2.8 | 81.1 ± 2.9 | 0.1038 | 0.0097 | 0.0123 |
| External Inferior | 77.2 ± 2.3 | 79.0 ± 2.8 | 80.6 ± 2.9 | 0.0129 | 0.0091 | 0.0676 |
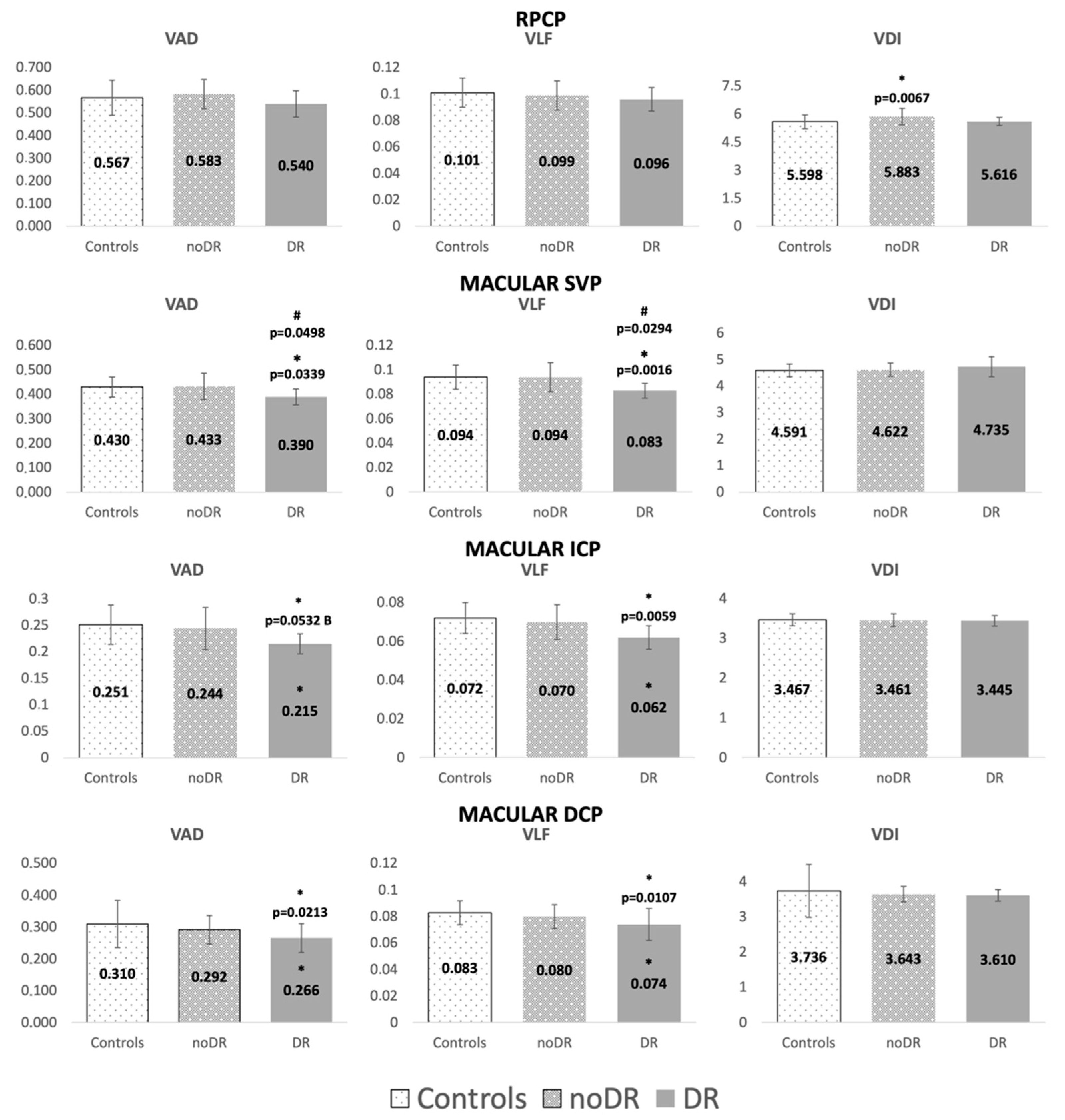
| OCT (Volume) | Correlation: (+ or −) p = Value | OCTA (Vascular Parameter) | Correlation: (+ or −) p = Value | |
|---|---|---|---|---|
| Mean glucose | none | ICP (VAD, VLF, VDI) | (−) p = 0.0035, p = 0.0168, p = 0.0226 | |
| Mean glucose variability | ORL | (−) p = 0.0402 | none | |
| Time in range mean | none | ICP (VAD, VLF, VDI) | (+) p = 0.0095, p = 0.0470, p = 0.009 | |
| Time below range mean | none | RPCP (VAD, VLF) | (+) p = 0.0539, p = 0.0395 | |
| HbA1c mean | none | ICP (VAD, VLF, VDI) | (−) p = 0.0039; p = 0.0211; p = 0.0184 | |
| HbA1c variability | Total Retina | (−) p = 0.0312 | SVP (VAD, VLF) | (−) p = 0.0010, p = 0.0047 |
| GCL | (−) p = 0.0311 | DCP (VLF) | (+) p = 0.0320 | |
| IPL | (−) p = 0.0241 | |||
| ONL | (−) p = 0.0515 | |||
| Glycemic variability (SD) mean | none | ICP (VAD, VFL, VDI) | (−) p = 0.0030, p = 0.0354, p = 0.0045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilotto, E.; Torresin, T.; Leonardi, F.; Gutierrez De Rubalcava Doblas, J.; Midena, G.; Moretti, C.; Midena, E. Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy. J. Clin. Med. 2022, 11, 3982. https://doi.org/10.3390/jcm11143982
Pilotto E, Torresin T, Leonardi F, Gutierrez De Rubalcava Doblas J, Midena G, Moretti C, Midena E. Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy. Journal of Clinical Medicine. 2022; 11(14):3982. https://doi.org/10.3390/jcm11143982
Chicago/Turabian StylePilotto, Elisabetta, Tommaso Torresin, Francesca Leonardi, Joaquin Gutierrez De Rubalcava Doblas, Giulia Midena, Carlo Moretti, and Edoardo Midena. 2022. "Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy" Journal of Clinical Medicine 11, no. 14: 3982. https://doi.org/10.3390/jcm11143982
APA StylePilotto, E., Torresin, T., Leonardi, F., Gutierrez De Rubalcava Doblas, J., Midena, G., Moretti, C., & Midena, E. (2022). Retinal Microvascular and Neuronal Changes Are Also Present, Even If Differently, in Adolescents with Type 1 Diabetes without Clinical Diabetic Retinopathy. Journal of Clinical Medicine, 11(14), 3982. https://doi.org/10.3390/jcm11143982









