Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis
Abstract
:1. Introduction
2. Methods
Study Population
3. Study Design
4. Measurement of Serum Fibrinogen, Adiponectin, and Angiopoietn-2 Levels
5. Statistical Analysis
6. Results
6.1. Baseline Demographic and Clinical Characteristics
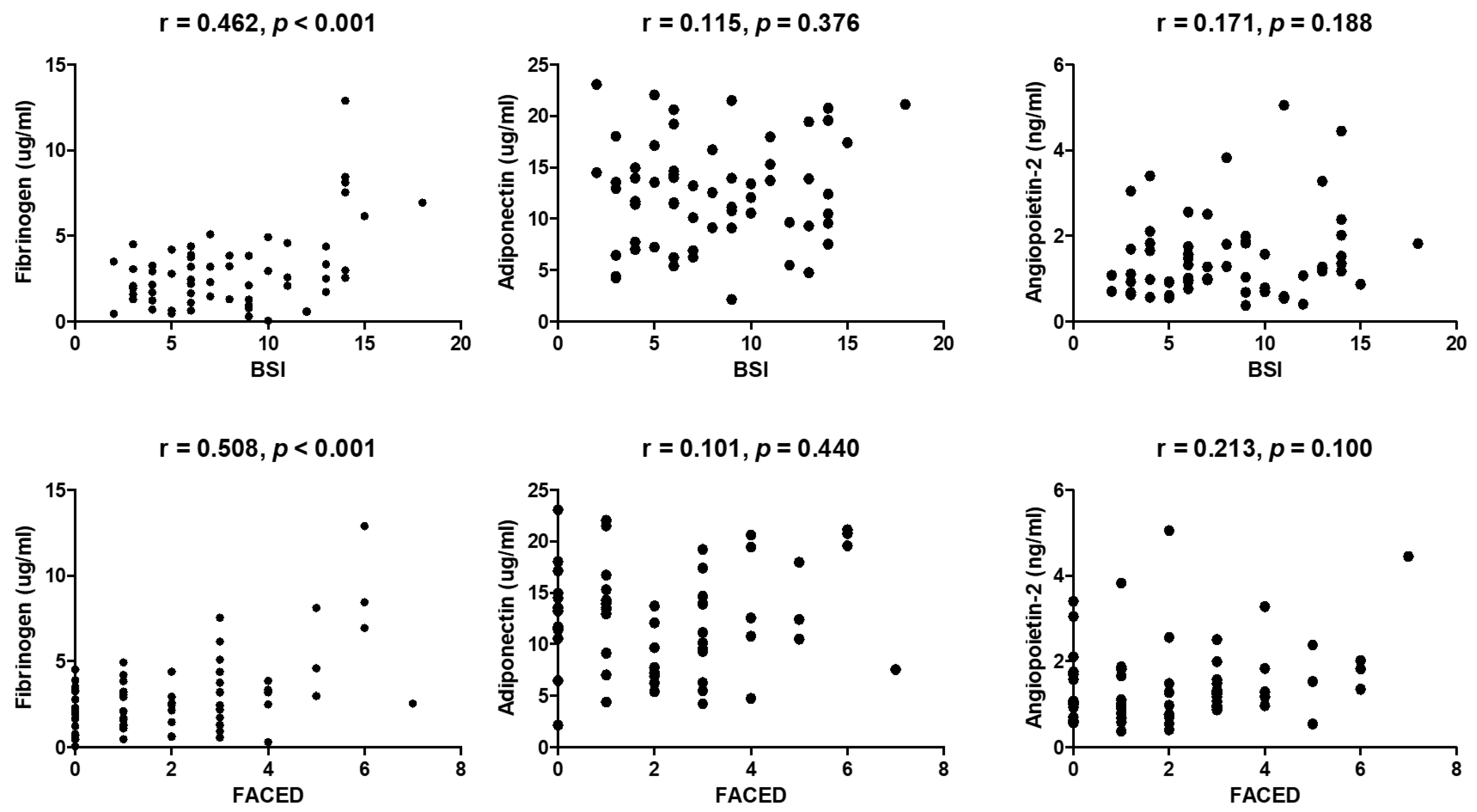
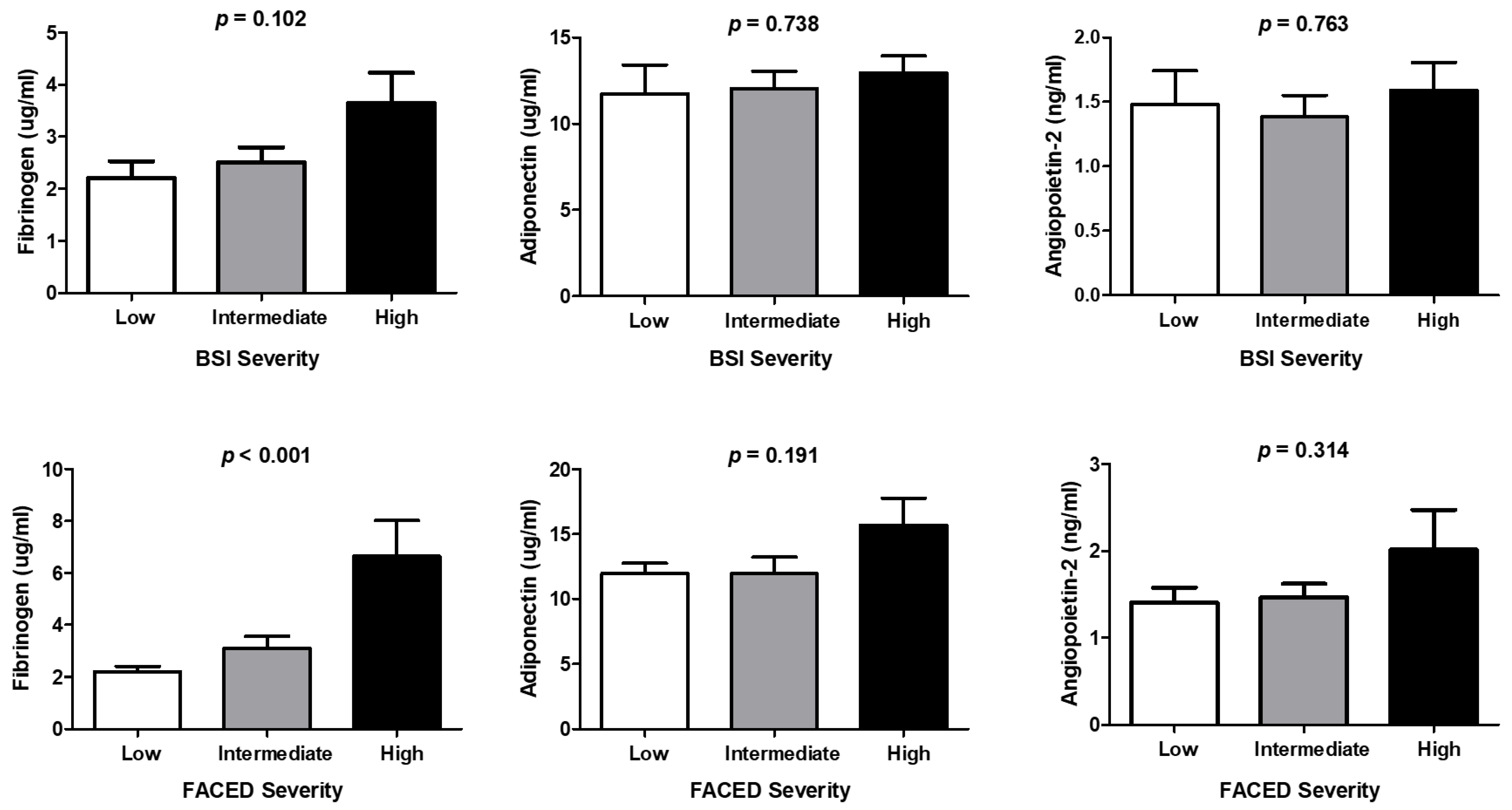
6.2. Comparison of Biomarkers between Patients with Bronchiectasis and Control Subjects, Correlation between Biomarkers and Severity Scores, and Differences in Biomarker Levels Associated with Severity
6.3. Independent Factors Associated with Severity Scoring Systems
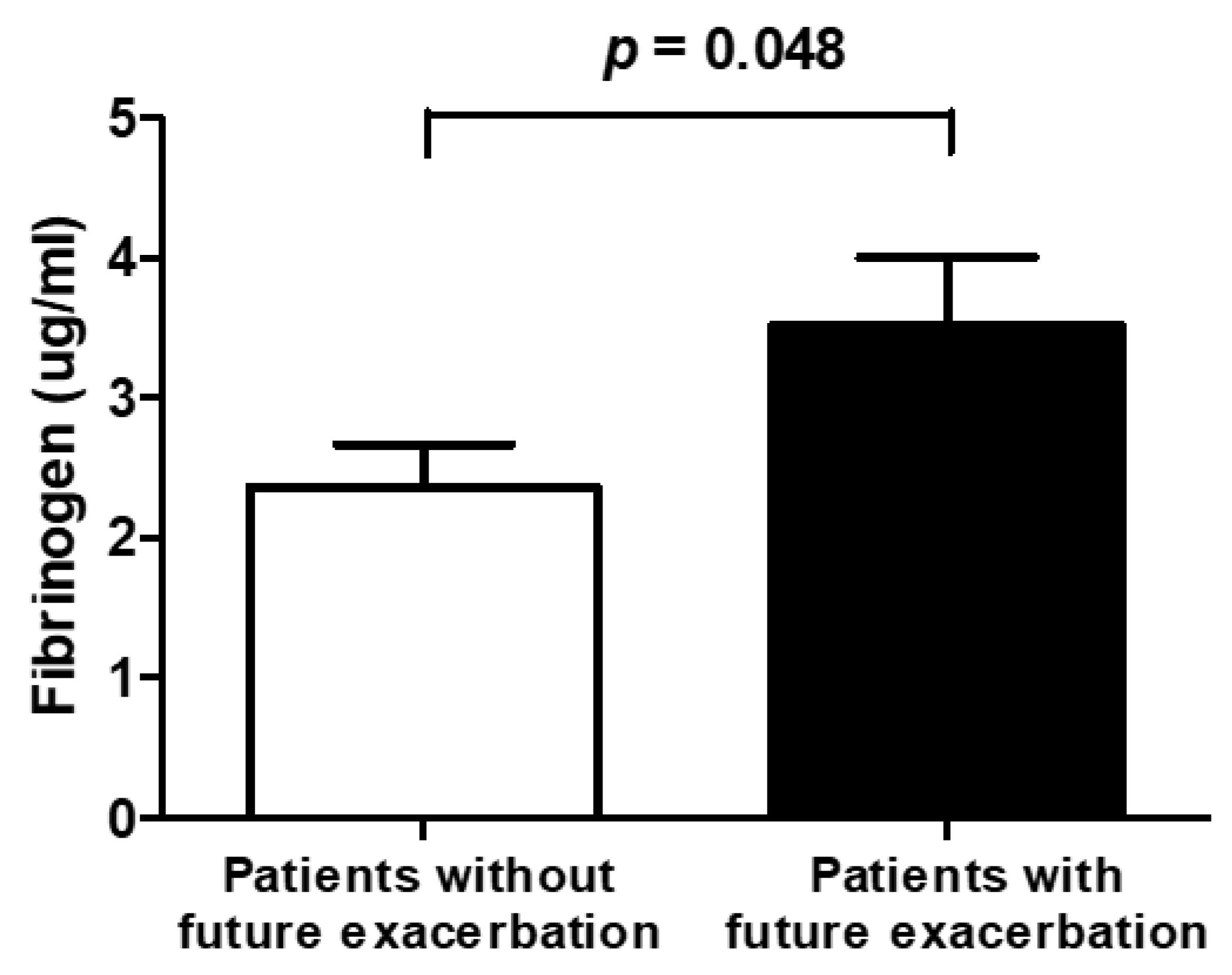
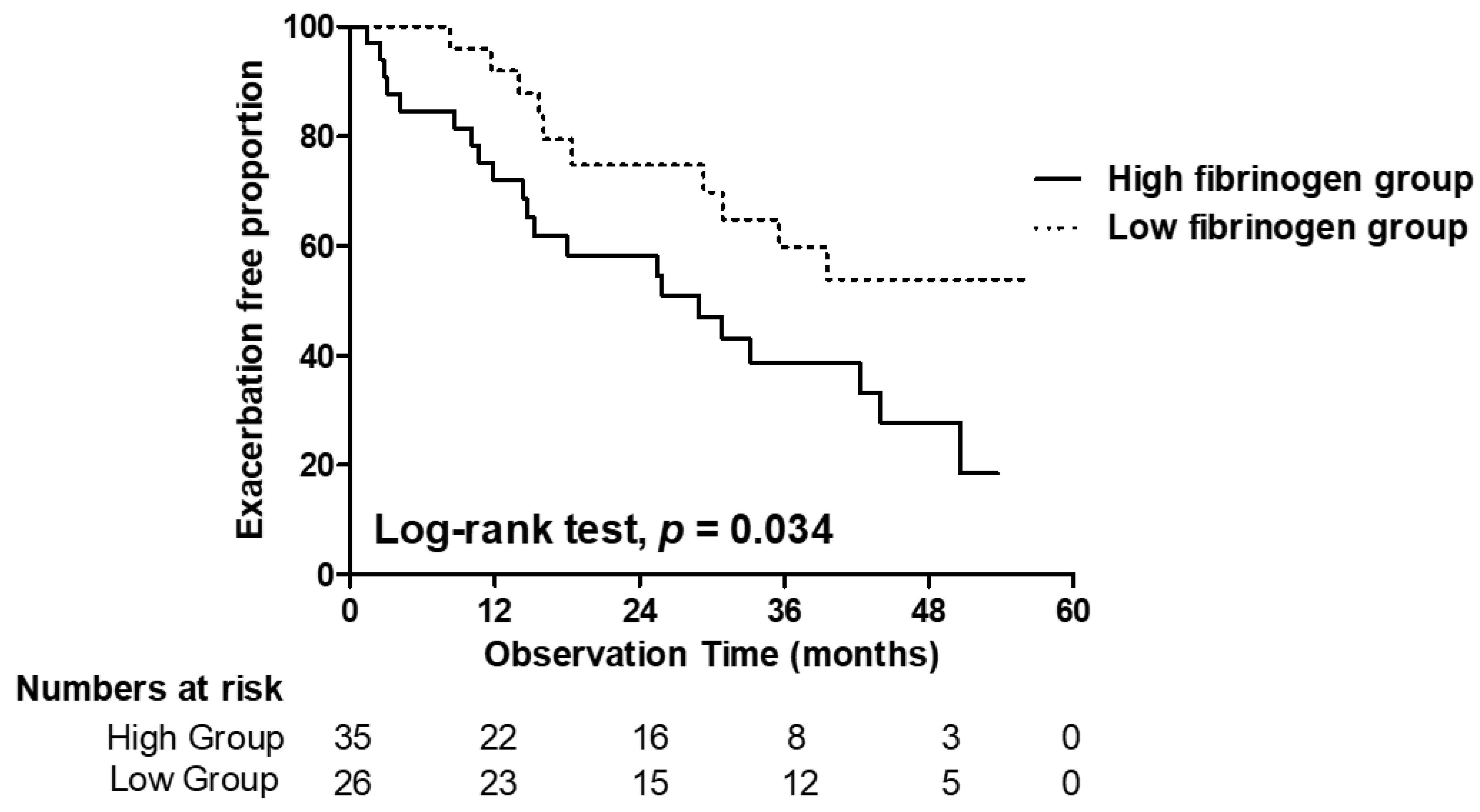
6.4. Exacerbation
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Garcia, M.A.; de Gracia, J.; Vendrell Relat, M.; Giron, R.M.; Maiz Carro, L.; de la Rosa Carrillo, D.; Olveira, C. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2014, 43, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.D.; Chalmers, J.D.; De Soyza, A.; Fardon, T.C.; Koustas, S.O.; Scott, J.; Simpson, A.J.; Brown, J.S.; Hurst, J.R. The heterogeneity of systemic inflammation in bronchiectasis. Respir. Med. 2017, 127, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.T.; Kuzmanova, E.; Dicker, A.J.; Keir, H.R.; Finch, S.; Aliberti, S.; Fardon, T.C.; Chalmers, J.D. Serum Desmosine Is Associated with Long-Term All-Cause and Cardiovascular Mortality in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 202, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.J.; Kim, J.Y.; Ju, S.; Lim, S.; Yoo, J.W.; Nam, S.J.; Lee, G.D.; Cho, H.S.; Kim, R.B.; et al. Serum Albumin and Disease Severity of Non-Cystic Fibrosis Bronchiectasis. Respir. Care 2017, 62, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Jeong, J.H.; Heo, M.; Heo, I.R.; Kim, T.H.; Kim, H.C.; Yoo, J.W.; Cho, Y.J.; Jeong, Y.Y.; Lee, J.D.; et al. Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis. Chronic Respir. Dis. 2021, 18, 14799731211017548. [Google Scholar] [CrossRef]
- Jeong, J.H.; Heo, M.; Kim, E.J.; Hah, Y.S.; Heo, I.R.; Kim, T.H.; Kim, H.C.; Ju, S.; Yoo, J.W.; Jeong, Y.Y.; et al. Serum hepatocyte growth factor as a predictor of disease severity and future exacerbations in patients with non-cystic fibrosis bronchiectasis. Respir. Med. 2021, 185, 106505. [Google Scholar] [CrossRef]
- Lowe, G.D.; Rumley, A.; Mackie, I.J. Plasma fibrinogen. Ann. Clin. Biochem. 2004, 41 Pt 6, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Duvoix, A.; Dickens, J.; Haq, I.; Mannino, D.; Miller, B.; Tal-Singer, R.; Lomas, D.A. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013, 68, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Mazzarella, G.; Turchiarelli, V.; Nigro, E.; Corbi, G.; Scudiero, O.; Sofia, M.; Daniele, A. Adiponectin: An attractive marker for metabolic disorders in Chronic Obstructive Pulmonary Disease (COPD). Nutrients 2013, 5, 4115–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelkel, N.F.; Douglas, I.S.; Nicolls, M. Angiogenesis in chronic lung disease. Chest 2007, 131, 874–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiff, D.B.; Wells, A.U.; Carr, D.H.; Cole, P.J.; Hansell, D.M. CT findings in bronchiectasis: Limited value in distinguishing between idiopathic and specific types. Am. J. Roentgenol. 1995, 165, 261–267. [Google Scholar] [CrossRef]
- Hill, A.T.; Haworth, C.S.; Aliberti, S.; Barker, A.; Blasi, F.; Boersma, W.; Chalmers, J.D.; De Soyza, A.; Dimakou, K.; Elborn, J.S.; et al. Pulmonary exacerbation in adults with bronchiectasis: A consensus definition for clinical research. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2017, 49, 1700051. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Jiang, H.; Lin, Y.; Wu, Y.; Cai, W.; Shi, B.; Luo, Y.; Jian, Z.; Zhou, X. Prognostic value of plasma fibrinogen in hepatocellular carcinoma: A meta-analysis. Cancer Manag. Res. 2018, 10, 5027–5041. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Qian, Y.; Fang, S.; Wang, Y.; Tang, Y.; Gu, W. Prognostic Value of Plasma Fibrinogen in Lung Cancer Patients: A Meta-Analysis. J. Cancer 2018, 9, 3904–3911. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.E.; Tal-Singer, R.; Rennard, S.I.; Furtwaengler, A.; Leidy, N.; Lowings, M.; Martin, U.J.; Martin, T.R.; Merrill, D.D.; Snyder, J.; et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am. J. Respir. Crit. Care Med. 2016, 193, 607–613. [Google Scholar] [CrossRef]
- Fermont, J.M.; Masconi, K.L.; Jensen, M.T.; Ferrari, R.; Di Lorenzo, V.A.P.; Marott, J.M.; Schuetz, P.; Watz, H.; Waschki, B.; Müllerova, H.; et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax 2019, 74, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Coban, H.; Gungen, A.C. Is There a Correlation between New Scoring Systems and Systemic Inflammation in Stable Bronchiectasis? Can. Respir. J. 2017, 2017, 9874068. [Google Scholar] [CrossRef]
- Aliberti, S.; Sotgiu, G.; Gramegna, A.; McDonnell, M.J.; Polverino, E.; Torres, A.; Goeminne, P.C.; Dimakou, K.; Shteinberg, M.; Sibila, O.; et al. Thrombocytosis during Stable State Predicts Mortality in Bronchiectasis. Ann. Am. Thorac. Soc. 2021, 18, 1316–1325. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramegna, A.; Aliberti, S.; Sibila, O.; Di Francesco, C.; Sotgiu, G.; Perea, L.; Terranova, L.; Oriano, M.; Pilocane, T.; Saderi, L.; et al. Sputum neutrophil elastase in bronchiectasis: A Southern European cohort study. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2020, 56, 2001702. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Haworth, C.S.; Metersky, M.L.; Loebinger, M.R.; Blasi, F.; Sibila, O.; O’Donnell, A.E.; Sullivan, E.J.; Mange, K.C.; Fernandez, C.; et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N. Engl. J. Med. 2020, 383, 2127–2137. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total Patients (n = 61) |
|---|---|
| Age, years | 65.3 ± 9.12 |
| Male | 19 (31.1) |
| Body mass index, kg/m2 | 22.6 ± 3.44 |
| FEV1/FVC, % | 65.3 ± 12.9 |
| FEV1, L | 1.54 ± 0.62 |
| FEV1, % | 61.8 ± 20.2 |
| Smoking status Current smoker Former smoker Never smoker | 3 (4.9) 9 (14.8) 49 (80.3) |
| History of respiratory hospitalization Yes No | 23 (37.7) 38 (62.3) |
| History of acute exacerbation Yes No | 36 (59.0) 25 (41.0) |
| Number of exacerbations in previous year | 0.97 ± 1.21 |
| Chronic colonization with Pseudomonas Yes No | 12 (19.7) 49 (80.3) |
| Number of affected lobes | 2.90 ± 1.34 |
| Pattern of bronchiectasis Cylindrical Varicose Cystic | 24 (39.3) 4 (6.6) 33 (54.1) |
| FACED score | 2.10 ± 1.83 |
| BSI | 8.00 ± 3.96 |
| Comorbid diseases COPD Bronchial asthma Hypertension Previous history of tuberculosis Diabetes Cardiovascular disease | 32 (52.5) 15 (24.6) 14 (23.0) 9 (14.8) 5 (8.2) 3 (4.9) |
| Dependent Variables | Explanatory Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| β Coefficient | 95% CI | p-Value | β Coefficient | 95% CI | p-Value | ||
| BSI | Fibrinogen, µg/mL | 0.794 | 0.397; 1.191 | <0.001 | 0.646 | 0.244; 1.149 | 0.002 |
| Albumin, g/dL | −2.909 | −5.537; −0.280 | 0.031 | −1.512 | −3.965; 0.940 | 0.222 | |
| CRP, mg/L | 0.189 | 0.057; 0.321 | 0.006 | 0.118 | −0.010; 0.246 | 0.071 | |
| FACED score | Fibrinogen, µg/mL | 0.404 | 0.226; 0.583 | <0.001 | 0.348 | 0.171; 0.525 | <0.001 |
| WBC, 109/L | 0.295 | 0.074; 0.516 | 0.01 | 0.261 | 0.066; 0.456 | 0.01 | |
| PLR | 0.014 | 0.002; 0.025 | 0.022 | 0.003 | −0.008; 0.015 | 0.573 | |
| Uric acid, mg/dL | 0.424 | 0.089; 0.759 | 0.014 | 0.230 | −0.063; 0.523 | 0.121 | |
| CRP, mg/L | 0.080 | 0.018; 0.142 | 0.012 | 0.026 | −0.034; 0.087 | 0.385 | |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age, years | 1.015 | 0.978–1.053 | 0.424 | |||
| Sex | ||||||
| Male | Ref | 1 | ||||
| Female | 1.086 | 0.508–2.323 | 0.831 | |||
| Body mass index, kg/m2 | 0.970 | 0.873–1.077 | 0.564 | |||
| FEV1% predicted | 0.991 | 0.971–1.012 | 0.406 | |||
| Hospital admission before study | ||||||
| No | Ref | 1 | ||||
| Yes | 1.656 | 0.814–3.372 | 0.164 | |||
| Exacerbations before the study | ||||||
| 0–2 | Ref | 1 | ||||
| 3 or more | 1.197 | 0.490–2.925 | 0.694 | |||
| mMRC dyspnea score | ||||||
| 0–1 | Ref | 1 | ||||
| ≥2 | 0.871 | 0.332–2.183 | 0.779 | |||
| Pseudomonas colonization | ||||||
| No | Ref | 1 | 1 | |||
| Yes | 2.420 | 1.103–5.308 | 0.027 | 2.555 | 1.116–5.620 | 0.02 |
| Number of affected lobes | ||||||
| 0–2 lobes | Ref | 1 | ||||
| ≥3 lobes | 1.245 | 0.555–2.790 | 0.595 | |||
| Fibrinogen | ||||||
| Low (<2.182 µg/mL) | Ref | 1 | 1 | |||
| High (≥2.182 µg/mL) | 2.215 | 1.041–4.713 | 0.039 | 2.308 | 1.083–4.919 | 0.03 |
| Adiponectin | ||||||
| Low (<11.447 µg/mL) | Ref | 1 | ||||
| High (≥11.447 µg/mL) | 0.986 | 0.476–2.039 | 0.969 | |||
| Angiopoietin-2 | ||||||
| Low (<1.177 ng/mL) | Ref | 1 | ||||
| High (≥1.177 ng/mL) | 1.117 | 0.550–2.269 | 0.759 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Jeong, J.H.; Heo, M.; Ju, S.; Yoo, J.-W.; Jeong, Y.Y.; Lee, J.D. Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis. J. Clin. Med. 2022, 11, 3948. https://doi.org/10.3390/jcm11143948
Lee SJ, Jeong JH, Heo M, Ju S, Yoo J-W, Jeong YY, Lee JD. Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis. Journal of Clinical Medicine. 2022; 11(14):3948. https://doi.org/10.3390/jcm11143948
Chicago/Turabian StyleLee, Seung Jun, Jong Hwan Jeong, Manbong Heo, Sunmi Ju, Jung-Wan Yoo, Yi Yeong Jeong, and Jong Deog Lee. 2022. "Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis" Journal of Clinical Medicine 11, no. 14: 3948. https://doi.org/10.3390/jcm11143948
APA StyleLee, S. J., Jeong, J. H., Heo, M., Ju, S., Yoo, J.-W., Jeong, Y. Y., & Lee, J. D. (2022). Serum Fibrinogen as a Biomarker for Disease Severity and Exacerbation in Patients with Non-Cystic Fibrosis Bronchiectasis. Journal of Clinical Medicine, 11(14), 3948. https://doi.org/10.3390/jcm11143948






