Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads
Abstract
1. Introduction
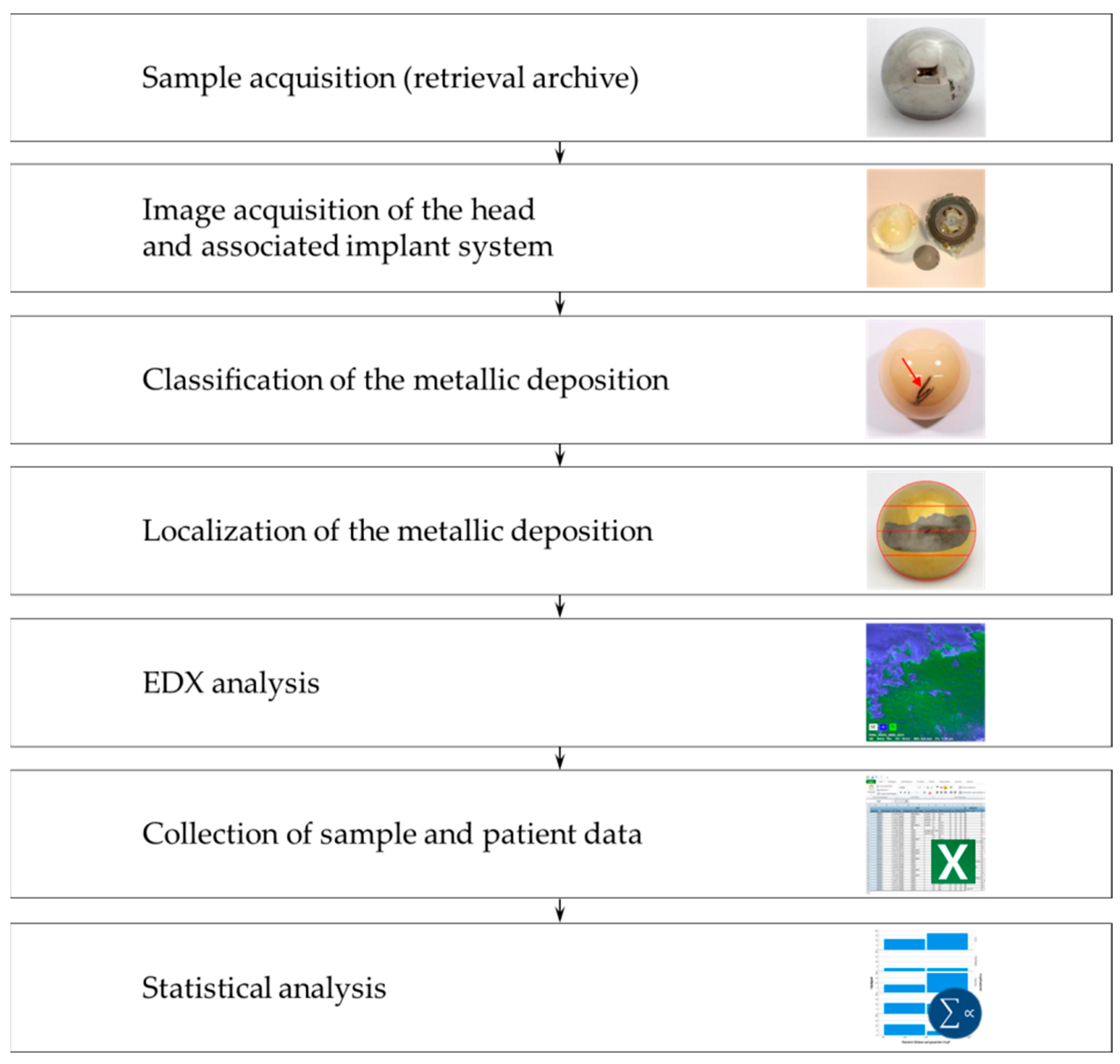
2. Materials and Methods
2.1. Specimens
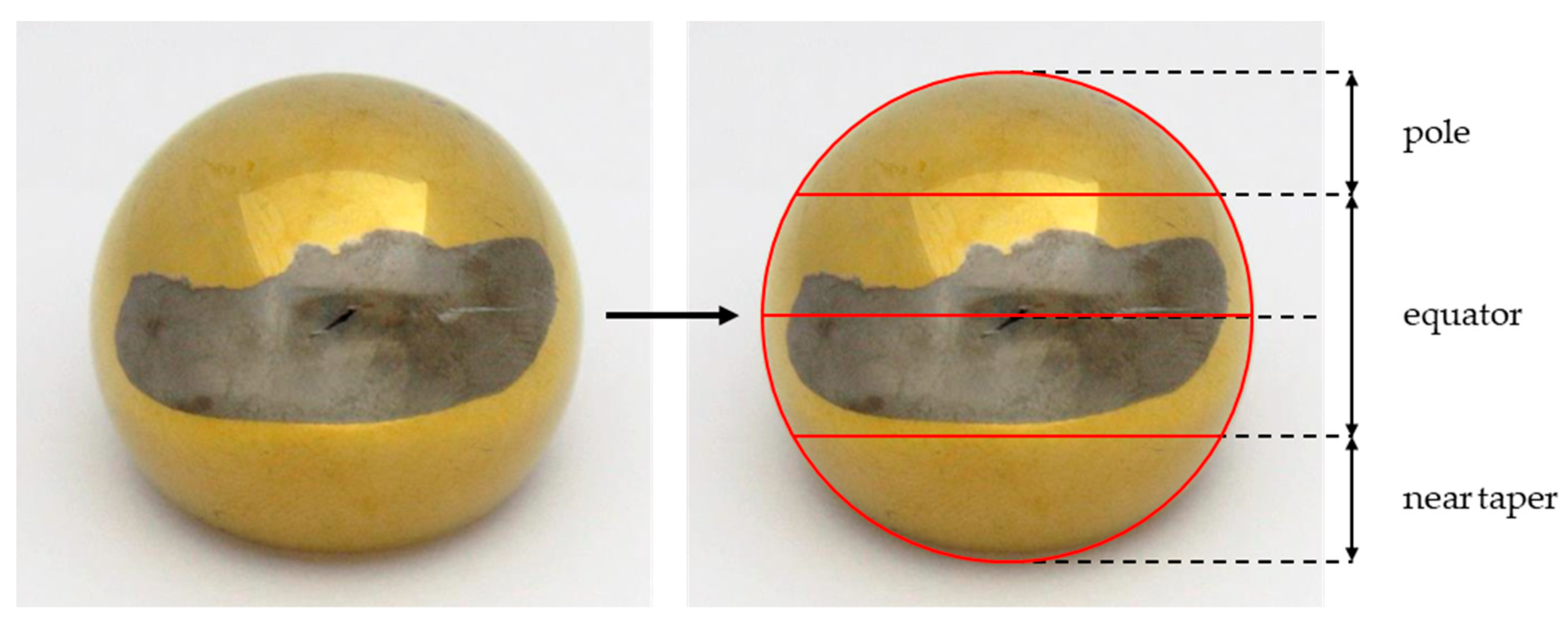
2.2. Classification and Localization of the Metallic Deposition
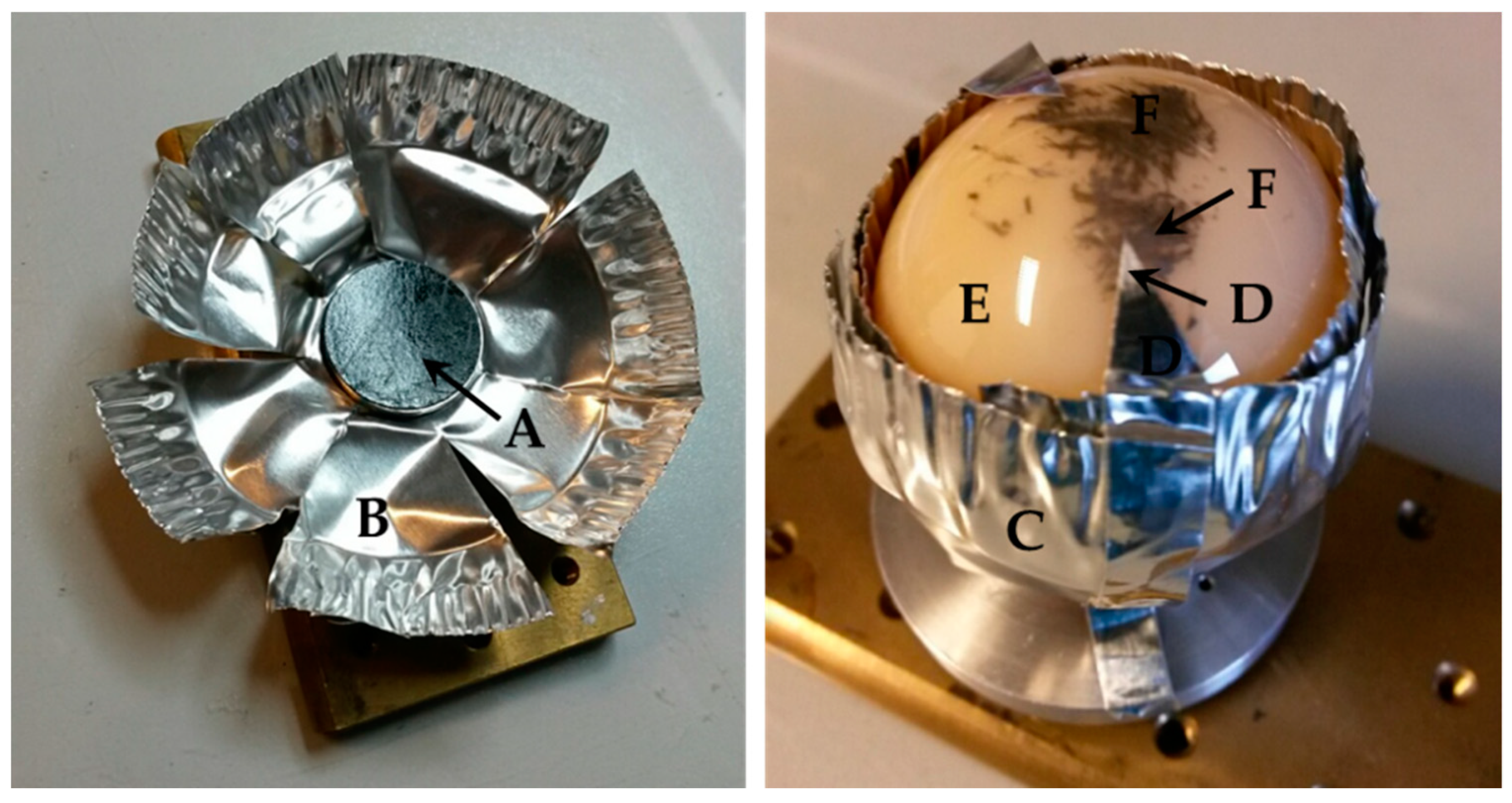
2.3. EDX Analysis of Femoral Heads
2.4. Statistical Analysis
3. Results
3.1. Cause of the Revision of the Examined Femoral Heads
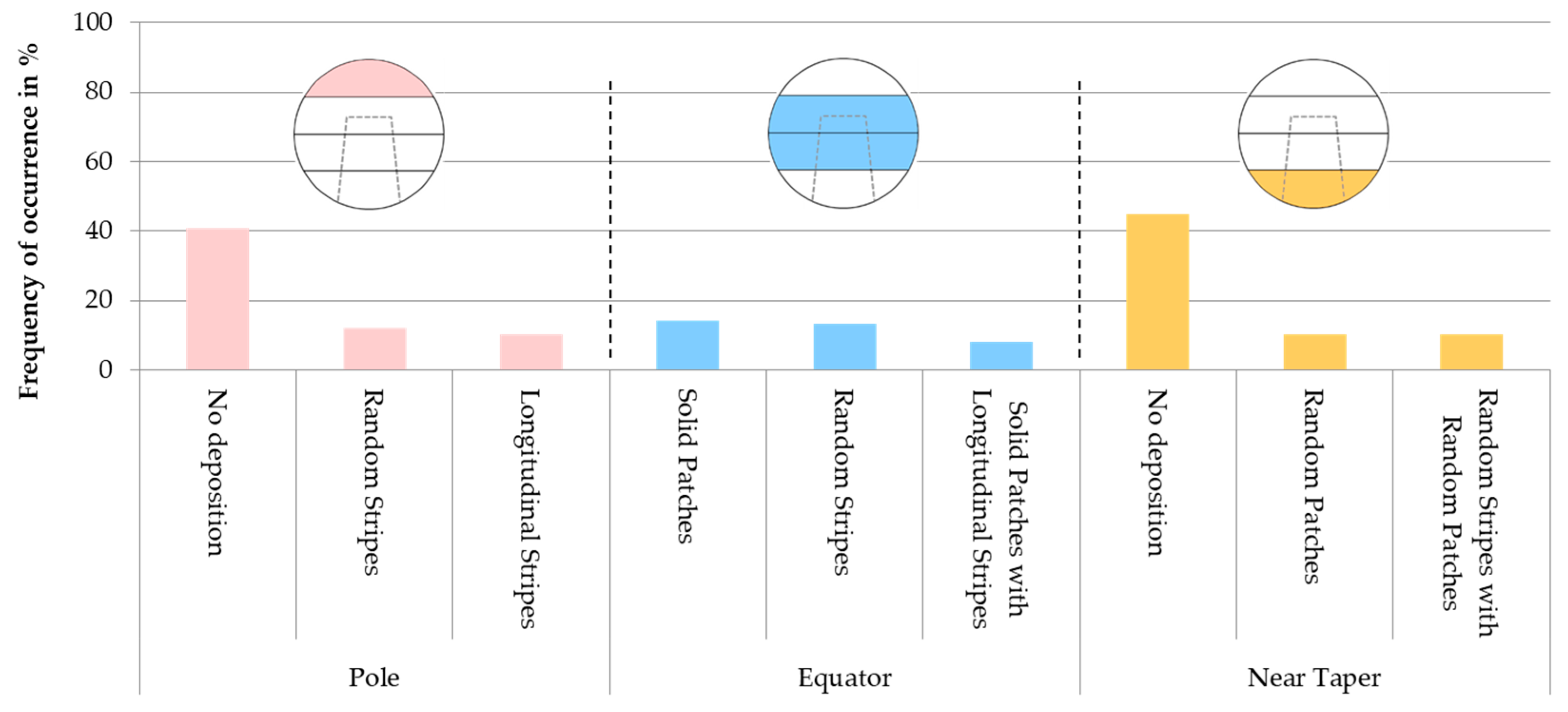
3.2. Classification and Localization of the Metallic Deposition
3.3. Material Analysis of Femoral Heads and EDX Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kärrholm, J.; Rogmark, C.; Nauclér, E.; Nåtman, J.; Vinblad, J.; Mohaddes, M.; Rolfon, O. Swedish Hip Arthroplasty Register—Annual Report 2019; Swedish Hip Arthroplasty Register: Göteborg, Sweden, 2019; p. 195. [Google Scholar]
- Kim, Y.-H.; Choi, Y.; Kim, J.-S. Cementless Total Hip Arthroplasty with Ceramic-on-Ceramic Bearing in Patients Younger than 45 Years with Femoral-Head Osteonecrosis. Int. Orthop. 2010, 34, 1123–1127. [Google Scholar] [CrossRef]
- Müller, F.A.; Hagymási, M.; Greil, P.; Zeiler, G.; Schuh, A. Transfer of Metallic Debris after Dislocation of Ceramic Femoral Heads in Hip Prostheses. Arch. Orthop. Trauma Surg. 2006, 126, 174–180. [Google Scholar] [CrossRef]
- Luchetti, W.T.; Copley, L.A.; Vresilovic, E.J.; Black, J.; Steinberg, M.E. Drain Entrapment and Titanium to Ceramic Head Deposition: Two Unique Complications Following Closed Reduction of a Dislocated Total Hip Arthroplasty. J. Arthroplast. 1998, 13, 713–717. [Google Scholar] [CrossRef]
- Isaac, G.H.; Brockett, C.; Breckon, A.; van der Jagt, D.; Williams, S.; Hardaker, C.; Fisher, J.; Schepers, A. Ceramic-on-Metal Bearings in Total Hip Replacement. J. Bone Jt. Surg. Br. 2009, 91, 1134–1141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eberhardt, A.W.; McKee, R.T.; Cuckler, J.M.; Peterson, D.W.; Beck, P.R.; Lemons, J.E. Surface Roughness of CoCr and ZrO2 Femoral Heads with Metal Transfer: A Retrieval and Wear Simulator Study. Int. J. Biomater. 2009, 2009, e185456. [Google Scholar] [CrossRef] [PubMed]
- Bal, B.S.; Rahaman, M.N.; Aleto, T.; Miller, F.S.; Traina, F.; Toni, A. The Significance of Metal Staining on Alumina Femoral Heads in Total Hip Arthroplasty. J. Arthroplast. 2007, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Firkins, P.J.; Tipper, J.L.; Ingham, E.; Stone, M.H.; Farrar, R.; Fisher, J. A Novel Low Wearing Differential Hardness, Ceramic-on-Metal Hip Joint Prosthesis. J. Biomech. 2001, 34, 1291–1298. [Google Scholar] [CrossRef]
- Affatato, S.; Ruggiero, A.; Merola, M.; Logozzo, S. Does Metal Transfer Differ on Retrieved Biolox® Delta Composites Femoral Heads? Surface Investigation on Three Biolox® Generations from a Biotribological Point of View. Compos. Part B Eng. 2017, 113, 164–173. [Google Scholar] [CrossRef]
- Chen, D.; Lin, S.; Cutrera, N.; Padgett, D.; Wright, T. Ceramic Bearings in Total Hip Replacement: A Retrieval Analysis. Minerva Ortop. E Traumatol. 2010, 61, 43–49. [Google Scholar]
- Fredette, E.K.; MacDonald, D.W.; Underwood, R.J.; Chen, A.F.; Mont, M.A.; Lee, G.-C.; Klein, G.R.; Rimnac, C.M.; Kurtz, S.M. Does Metal Transfer Differ on Retrieved Ceramic and CoCr Femoral Heads? BioMed Res. Int. 2015, 2015, e283038. [Google Scholar] [CrossRef]
- Walter, W.L.; Insley, G.M.; Walter, W.K.; Tuke, M.A. Edge Loading in Third Generation Alumina Ceramic-on-Ceramic Bearings: Stripe Wear. J. Arthroplast. 2004, 19, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Ruggiero, A. Biotribology in Arthroplasty: Worn Surfaces Investigation on Ceramic Hip Femoral Heads Considering Wettability. Appl. Sci. 2020, 10, 8919. [Google Scholar] [CrossRef]
- Hembus, J.; Rößler, L.; Jackszis, M.; Klinder, A.; Bader, R.; Zietz, C. Influence of Metallic Deposition on Ceramic Femoral Heads on the Wear Behavior of Artificial Hip Joints: A Simulator Study. Materials 2020, 13, 3569. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H. Surface Roughness of Ceramic Femoral Heads after In-Vivo Transfer of Metal Correlation to Polyethylene Wear. In Bioceramics and Alternative Bearings in Joint Arthroplasty; Springer: Seoul, Korea, 2007; pp. 49–57. ISBN 978-3-7985-1782-0. [Google Scholar]
- Hembus, J.; Lux, L.; Jackszis, M.; Bader, R.; Zietz, C. Wear Analysis of Cross-Linked Polyethylene Inserts Articulating with Alumina and Ion-Treated Cobalt-Chromium Femoral Heads under Third-Body Conditions. Wear 2018, 402–403, 216–223. [Google Scholar] [CrossRef]
- Chevillotte, C.M.; Trousdale, R.T.M.; Chen, Q.P.; Guyen, O.M.; An, K.-N. The 2009 Frank Stinchfield Award: “Hip Squeaking: A Biomechanical Study of Ceramic-on-ceramic Bearing Surfaces”: Clinical Orthopaedics and Related Research®. Clin. Orthop. 2010, 468, 345–350. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Brandt, J.-M.; Gascoyne, T.C.; Guenther, L.E.; Allen, A.; Hedden, D.R.; Turgeon, T.R.; Bohm, E.R. Clinical Failure Analysis of Contemporary Ceramic-on-Ceramic Total Hip Replacements. Proc. Inst. Mech. Eng. H 2013, 227, 833–846. [Google Scholar] [CrossRef]
- Tomek, I.M.; Currier, J.H.; Mayor, M.B.; Van Citters, D.W. Metal Transfer on a Ceramic Head With a Single Rim Contact. J. Arthroplast. 2012, 27, 324.e1–324.e4. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef]
- Boutin, P. Total Arthroplasty of the Hip by Fritted Alumina Prosthesis. Experimental Study and 1st Clinical Applications. Orthop. Traumatol. Surg. Res. 2014, 100, 15–21. [Google Scholar] [CrossRef]
- Dorlot, J.-M.; Christel, P.; Meunier, A. Wear Analysis of Retrieved Alumina Heads and Sockets of Hip Prostheses. J. Biomed. Mater. Res. 1989, 23, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Bonar, F.; Kirsh, G.; Walter, W.K.; Walter, W.L. Osteolysis in Third-Generation Alumina Ceramic-on-Ceramic Hip Bearings With Severe Impingement and Titanium Metallosis. J. Arthroplast. 2008, 23, 1240.e13–1240.e19. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Modena, E.; Toni, A.; Taddei, P. Retrieval Analysis of Three Generations of Biolox® Femoral Heads: Spectroscopic and SEM Characterisation. J. Mech. Behav. Biomed. Mater. 2012, 13, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Ruggiero, A.; De Mattia, J.S.; Affatato, S. On the Tribological Behavior of Retrieved Hip Femoral Heads Affected by Metallic Debris. A Comparative Investigation by Stylus and Optical Profilometer for a New Roughness Measurement Protocol. Measurement 2016, 90, 365–371. [Google Scholar] [CrossRef]
- Santos, E.M.; Vohra, S.; Catledge, S.A.; McClenny, M.D.; Lemons, J.; Moore, K.D. Examination of Surface and Material Properties of Explanted Zirconia Femoral Heads. J. Arthroplast. 2004, 19, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cadel, E.S.; Topoleski, L.D.T.; Vesnovsky, O.; Anderson, C.R.; Hopper, R.H., Jr.; Engh, C.A., Jr.; Di Prima, M.A. A Comparison of Metal/Metal and Ceramic/Metal Taper-Trunnion Modular Connections in Explanted Total Hip Replacements. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 135–143. [Google Scholar] [CrossRef]
- Fernández-Fairen, M.; Miquel, P.; Murcia-Asensio, A.; Ferrero-Manzanal, F.; Sueiro, J.; Gil, J. Microstructure and Surface Damage in Retrieved Metal-on-Metal Hip Arthroplasties. J. Arthroplast. 2017, 32, 3782–3795. [Google Scholar] [CrossRef]
- Park, S.-H.; Lu, Z.; Hastings, R.S.; Campbell, P.A.; Ebramzadeh, E. Five Hundred Fifty-Five Retrieved Metal-on-Metal Hip Replacements of a Single Design Show a Wide Range of Wear, Surface Features, and Histopathologic Reactions. Clin. Orthop. 2018, 476, 261–278. [Google Scholar] [CrossRef]
- Tikekar, N.M.; Heiner, A.D.; Baer, T.E.; Kruger, K.M.; Callaghan, J.J.; Brown, T.D.; Lannutti, J.J. Bearing-Foreign Material Deposition on Retrieved Co-Cr Femoral Heads: Composition and Morphology. BioMed Res. Int. 2015, 2015, e967278. [Google Scholar] [CrossRef]
- Łapaj, Ł.; Wendland, J.; Markuszewski, J.; Mróz, A.; Wiśniewski, T. Retrieval Analysis of Titanium Nitride (TiN) Coated Prosthetic Femoral Heads Articulating with Polyethylene. J. Mech. Behav. Biomed. Mater. 2015, 55, 127–139. [Google Scholar] [CrossRef]
- Patten, E.W.; Atwood, S.A.; Van Citters, D.W.; Jewett, B.A.; Pruitt, L.A.; Ries, M.D. Delamination of a Highly Cross-Linked Polyethylene Liner Associated with Titanium Deposits on the Cobalt-Chromium Modular Femoral Head Following Dislocation. J. Bone Jt. Surg. Br. 2010, 92, 1306–1311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguado-Maestro, I.; de Blas-Sanz, I.; Sanz-Peñas, A.E.; Campesino-Nieto, S.V.; Diez-Rodríguez, J.; Valle-López, S.; Espinel-Riol, A.; Fernández-Díez, D.; García-Alonso, M. Dual Mobility Cups as the Routine Choice in Total Hip Arthroplasty. Medicina 2022, 58, 528. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.C.; Campbell, P.; Kossovsky, N. Debris-Mediated Osteolysis—A Cascade Phenomenon Involving Motion, Wear, Particulates, Macrophage Induction, and Bone Lysis. Part. Debris Med. Implant. Mech. Form. Biol. Conseq. ASTM Int. 1992, 1144, 7–26. [Google Scholar] [CrossRef]
- Mathys Ltd Bettlach. Ceramys—Eine Perle der Keramiken, Bettlach, Switzerland. 2019. Available online: https://mathysmedical.com/Storages/User/Dokumente/Produktinfo/Huefte/Prospekt_ceramys_DE_V02.Pdf (accessed on 24 March 2022).
- Maruyama, M.; Capello, W.N.; D’Antonio, J.A.; Jaffe, W.L.; Bierbaum, B.E. Effect of Low-Friction Ion-Treated Femoral Heads on Polyethylene Wear Rates. Clin. Orthop. 2000, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, H.; Jonitz-Heincke, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A. Comparison of Inflammatory Effects in THP-1 Monocytes and Macrophages after Exposure to Metal Ions. Materials 2020, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Chamaon, K.; Schönfeld, P.; Awiszus, F.; Bertrand, J.; Lohmann, C.H. Ionic Cobalt but Not Metal Particles Induces ROS Generation in Immune Cells in Vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Hameister, R.; Kaur, C.; Dheen, S.T.; Lohmann, C.H.; Singh, G. Reactive Oxygen/Nitrogen Species (ROS/RNS) and Oxidative Stress in Arthroplasty. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Hegde, V.; Zoller, S.D.; Park, H.Y.; Hart, C.M.; Kondo, T.; Hamad, C.D.; Hu, Y.; Loftin, A.H.; Johansen, D.O.; et al. Point-of-Care Antimicrobial Coating Protects Orthopaedic Implants from Bacterial Challenge. Nat. Commun. 2021, 12, 5473. [Google Scholar] [CrossRef]
- Enz, A.; Mueller, S.C.; Warnke, P.; Ellenrieder, M.; Mittelmeier, W.; Klinder, A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. J. Fungi 2021, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Weikum, J.; Ritzmann, N.; Jelden, N.; Klöckner, A.; Herkersdorf, S.; Josten, M.; Sahl, H.-G.; Grein, F. Sulfide Protects Staphylococcus Aureus from Aminoglycoside Antibiotics but Cannot Be Regarded as a General Defense Mechanism against Antibiotics. Antimicrob. Agents Chemother. 2018, 62, e00602-18. [Google Scholar] [CrossRef]
- Brune, D.C. Isolation and Characterization of Sulfur Globule Proteins from Chromatium Vinosum and Thiocapsa Roseopersicina. Arch. Microbiol. 1995, 163, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A Universal Defense against Antibiotics in Bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Moore, D.R. In Vivo Production of Hydroxyl Radical by Enterococcus Faecalis Colonizing the Intestinal Tract Using Aromatic Hydroxylation. Free Radic. Biol. Med. 2002, 33, 818–826. [Google Scholar] [CrossRef]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent Evolution of Defensin Sequence, Structure and Function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]



| Femoral Head Material | Bearing Partners | Elements Included in Original Surface | Example Image |
|---|---|---|---|
| Metal n = 28 | MoM (n = 8) MoPE (n = 19) Unknown (n = 1) | Co, Cr, Mo |  |
| Coated Metal TiN/TiNbN n = 6 | MoM (n = 6) | Ti, N, Nb (Co, Cr, Mo beneath coating) |  |
| Alumina Ceramic n = 28 | CoC (n = 1) CoPE (n = 26) Unknown (n = 1) | Al, O |  |
| ZTA Ceramic (Zirconia-Toughened Alumina) n = 21 | CoC (n = 1) CoPE (n = 20) | Al, O, Zr, Y |  |
| Other oxide ceramics ATZ/ZTA/ZrO (Zirconia-Toughened Alumina) n = 15 | CoC (n = 1) CoPE (n = 13) Unknown (n = 1) | Al, Zr, O, Y |  |
| Cause for Revision | Frequency of Occurrence in % | Absolute Number (Total n = 98) |
|---|---|---|
| Polyethylene wear (includes decentralization, delamination, linear wear) | 43.9 | 43 |
| Impingement | 40.8 | 40 |
| Aseptic loosening (with 25.5% global loosening, 11.2% cup loosening, 1% stem loosening) | 37.7 | 37 |
| Dislocation (includes single and multiple dislocation) | 20.4 | 20 |
| Particle disease (due to PE and metallic wear particles) | 17.3 | 17 |
| Septic loosening | 11.2 | 11 |
| Septic, without loosening (includes DAIR 1) | 8.2 | 9 |
| Implant migration | 5.1 | 5 |
| Gluteal insufficiency | 3.1 | 3 |
| Osteolysis | 3.1 | 3 |
| Bone fracture | 3.1 | 3 |
| Implant failure | 3.1 | 3 |
| Subluxation | 2.0 | 2 |
| Deposition Patterns on Femoral Heads According to Fredette et al. [11] | Frequency of Occurrence in % | Absolute Number (Total n = 98) | Exemplary Deposition Pattern on Heads of This Study |
|---|---|---|---|
| Random Stripes | 44.9 | 44 |  |
| Random Patches | 41.8 | 41 |  |
| Solid Patch | 35.7 | 35 |  |
| Longitudinal Stripe | 27.6 | 27 |  |
| Directional Scratches | 20.4 | 20 |  |
| Patterned Coverage | 11.2 | 11 |  |
| Miscellaneous | 2.0 | 2 |  |
| Element | Frequency of Occurrence on Femoral Heads % | Absolute Number (Total n = 98) |
|---|---|---|
| Titan (Ti) | 76.5 | 75 |
| Carbon (C) | 49.0 | 48 |
| Iron (Fe) | 34.7 | 34 |
| Chromium (Cr) | 31.6 | 31 |
| Niobium (Nb) | 23.5 | 23 |
| Cobalt (Co) | 16.3 | 16 |
| Aluminum (Al) | 12.2 | 12 |
| Vanadium (V) | 9.2 | 9 |
| Nickel (Ni) | 8.2 | 8 |
| Oxygen (O) | 7.1 | 7 |
| Nitrogen (N) | 6.1 | 6 |
| Silicon (Si) | 5.1 | 5 |
| Molybdenum (Mo) | 4.1 | 4 |
| Sulfur (S) | 4.1 | 4 |
| Magnesium (Mg) | 3.1 | 3 |
| Phosphorus (P) | 2.0 | 2 |
| Copper (Cu) | 1.0 | 1 |
| Zircon (Zr) | 1.0 | 1 |
| Calcium (Ca) | 1.0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hembus, J.; Rößler, L.; Springer, A.; Frank, M.; Klinder, A.; Bader, R.; Zietz, C.; Enz, A. Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads. J. Clin. Med. 2022, 11, 3946. https://doi.org/10.3390/jcm11143946
Hembus J, Rößler L, Springer A, Frank M, Klinder A, Bader R, Zietz C, Enz A. Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads. Journal of Clinical Medicine. 2022; 11(14):3946. https://doi.org/10.3390/jcm11143946
Chicago/Turabian StyleHembus, Jessica, Lisa Rößler, Armin Springer, Marcus Frank, Annett Klinder, Rainer Bader, Carmen Zietz, and Andreas Enz. 2022. "Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads" Journal of Clinical Medicine 11, no. 14: 3946. https://doi.org/10.3390/jcm11143946
APA StyleHembus, J., Rößler, L., Springer, A., Frank, M., Klinder, A., Bader, R., Zietz, C., & Enz, A. (2022). Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads. Journal of Clinical Medicine, 11(14), 3946. https://doi.org/10.3390/jcm11143946






