Safety and Effectiveness of Intravenous Iron Therapy in Patients Supported by Durable Left Ventricular Assist Devices
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Effectiveness and Safety Endpoints
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary and Secondary Endpoints
3.3. Adverse Events and Safety Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LVAD | left ventricular assist device |
| NYHA | New York Heart Association |
| MCV | mean corpuscular volume |
| ACE | angiotensin-converting enzyme inhibitors |
| ARB | angiotensin II receptor blocker |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
References
- Milano, C.A.; Rogers, J.G.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Mokadam, N.A.; Mahr, C.; Miller, J.S.; Markham, D.W.; et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018, 6, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Sajgalik, P.; Grupper, A.; Edwards, B.S.; Kushwaha, S.S.; Stulak, J.M.; Joyce, D.L.; Joyce, L.D.; Daly, R.C.; Kara, T.; Schirger, J.A. Current Status of Left Ventricular Assist Device Therapy. Mayo Clin. Proc. 2016, 91, 927–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.G.; Aaronson, K.D.; Boyle, A.J.; Russell, S.D.; Milano, C.A.; Pagani, F.D.; Edwards, B.S.; Park, S.; John, R.; Conte, J.V.; et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J. Am. Coll. Cardiol. 2010, 55, 1826–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahfood Haddad, T.; Saurav, A.; Smer, A.; Azzouz, M.S.; Akinapelli, A.; Williams, M.A.; Alla, V.M. Cardiac Rehabilitation in Patients with Left Ventricular Assist Device: A systematic review and meta-analysis. J. Cardiopulm. Rehabil. Prev. 2017, 37, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Burkhoff, D.; Rich, J.D.; Drakos, S.G.; Teuteberg, J.J.; Imamura, T.; Rodgers, D.; Raikhelkar, J.; Vorovich, E.E.; Selzman, C.H.; et al. Impact of Hemodynamic Ramp Test-Guided HVAD Speed and Medication Adjustments on Clinical Outcomes. Circ. Heart Fail. 2019, 12, e006067. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; McAlister, F.A.; Armstrong, P.W. Anemia is common in heart failure and is associated with poor outcomes: Insights from a cohort of 12 065 patients with new-onset heart failure. Circulation 2003, 107, 223–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Nye, R.; Levy, W.C. Anemia predicts mortality in severe heart failure: The prospective randomized amlodipine survival evaluation (PRAISE). J. Am. Coll. Cardiol. 2003, 41, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.D.; Katz, S.D. Anemia in chronic heart failure: Prevalence, etiology, clinical correlates, and treatment options. Circulation 2006, 113, 2454–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Amione-Guerra, J.; Cruz-Solbes, A.S.; Bhimaraj, A.; Trachtenberg, B.H.; Pingali, S.R.; Estep, J.D.; Park, M.H.; Guha, A. Anemia after continuous-flow left ventricular assist device implantation: Characteristics and implications. Int. J. Artif. Organs 2017, 40, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, F.J.; Slaughter, J. Biostatistics for Biomedical Research 2021. Available online: http://hbiostat.org/doc/bbr.pdf (accessed on 24 May 2020).
- von Haehling, S.; Gremmler, U.; Krumm, M.; Mibach, F.; Schön, N.; Taggeselle, J.; Dahm, J.B.; Angermann, C.E. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: The PrEP Registry. Clin. Res. Cardiol. 2017, 106, 436–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L.E.; Wesner, S.; Katz, J.N.; Chien, C.V.; Hollis, I. Intravenous Versus Oral Iron Replacement in Patients with a Continuous-Flow Left Ventricular Assist Device. ASAIO J. 2019, 65, e90–e91. [Google Scholar] [CrossRef] [PubMed]
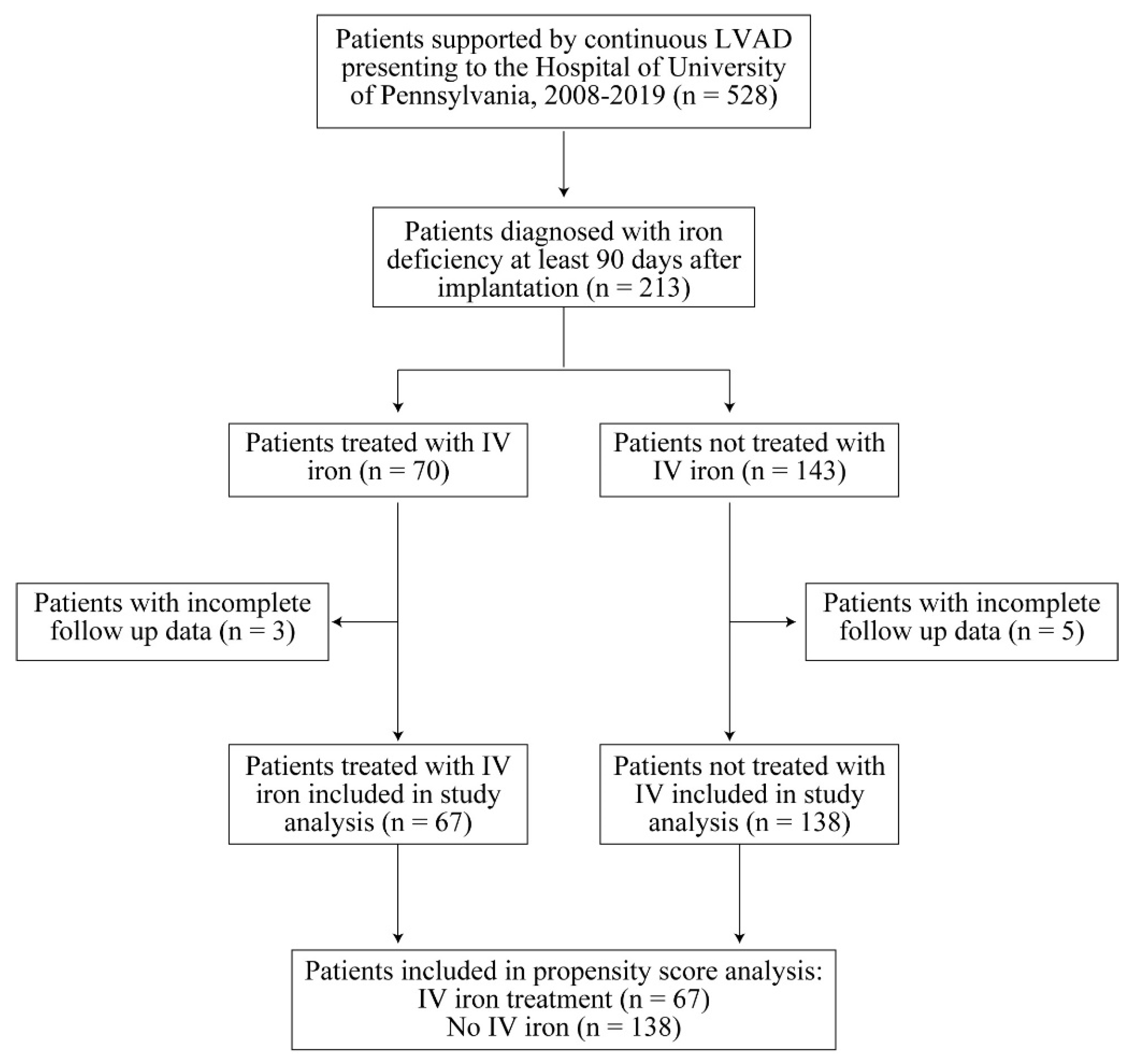

| Characteristic | Iron Treatment (n = 67) | Control (n = 138) | p-Value |
|---|---|---|---|
| Age—yr | 62.0 (54.0, 69.0) | 59.0 (50.0, 68.0) | 0.15 |
| Male sex—No. (%) | 56 (83.6%) | 119 (86.2%) | 0.61 |
| Race—No. (%) | 0.10 | ||
| White | 34 (50.7%) | 89 (64.5%) | |
| Black | 30 (44.8%) | 41 (29.7%) | |
| Other | 3 (4.5%) | 8 (5.8%) | |
| BMI—kg/m2 ‡ | 31.7 (26.3, 36.4) | 29.8 (25.6, 34.3) | 0.23 |
| NYHA Class—No. (%) | 0.006 | ||
| I | 8 (11.9%) | 33 (23.9%) | |
| II | 26 (38.8%) | 69 (50.0%) | |
| III | 23 (34.3%) | 28 (20.3%) | |
| IV | 10 (14.9%) | 8 (5.8%) | |
| Inpatient status—No. (%) | 46 (68.7%) | 43 (31.2%) | <0.001 |
| Ischemic etiology—No. (%) | 27 (40.3%) | 56 (40.6%) | 0.97 |
| LVAD type—No. (%) | 0.77 | ||
| Heartmate II | 16 (23.9%) | 38 (27.5%) | |
| Heartmate III | 37 (55.2%) | 69 (50.0%) | |
| Heartware HVAD | 14 (20.9%) | 31 (22.5%) | |
| Goals of therapy—No. (%) | 0.77 | ||
| Bridge to transplant | 18 (26.9%) | 40 (29.0%) | |
| Destination | 48 (71.6%) | 94 (68.1%) | |
| Bridge to recovery | 1 (1.5%) | 4 (2.9%) | |
| Medical history—No. (%) | |||
| Gastrointestinal bleed | 44 (65.7%) | 52 (37.7%) | <0.001 |
| Diabetes mellitus | 26 (38.8%) | 42 (30.4%) | 0.23 |
| COPD | 7 (10.4%) | 13 (9.4%) | 0.82 |
| Laboratory measurements | |||
| Hemoglobin—g/dL | 9.2 (8.5, 10.6) | 11.0 (9.6, 12.8) | <0.001 |
| Mean corpuscular volume—μm3 | 79.0 (73.0, 86.0) | 86.0 (81.0, 90.0) | <0.001 |
| Serum ferritin—μg/L | 46.0 (30.0, 117.0) | 71.0 (45.0, 104.0) | 0.049 |
| Aspartate aminotransferase—U/L | 19.0 (15.0, 29.0) | 23.0 (20.0, 29.0) | 0.005 |
| Alanine aminotransferase—U/L | 15.0 (11.0, 24.0) | 19.0 (15.0, 26.0) | 0.008 |
| Creatinine—mg/dL | 1.3 (1.0, 1.8) | 1.2 (1.0, 1.5) | 0.044 |
| Concomitant treatment—No. (%) | |||
| ACE inhibitor or ARB | 33 (49.3%) | 103 (74.6%) | <0.001 |
| Beta-blocker | 50 (74.6%) | 99 (71.7%) | 0.66 |
| Digoxin | 18 (26.9%) | 45 (32.6%) | 0.40 |
| Antiplatelet therapy | 46 (68.7%) | 110 (79.7%) | 0.082 |
| Anticoagulant therapy | 60 (89.6%) | 132 (95.7%) | 0.093 |
| Proton pump inhibitor | 48 (71.6%) | 80 (58.0%) | 0.058 |
| H2 blocker | 13 (19.4%) | 28 (20.3%) | 0.88 |
| Oral iron | 23 (34.3%) | 54 (39.1%) | 0.51 |
| Time between LVAD implant and hemoglobin—days | 575.0 (232.0, 904.0) | 377.5 (203.0, 655.0) | 0.026 |
| Variable | Iron Treatment Mean (95% CI) | Control Mean (95% CI) | Mean Difference or Odds Ratio Adjusted for Baseline | p-Value † |
|---|---|---|---|---|
| Hemoglobin—g/dL | 11.0 (10.5–11.4) | 11.7 (11.3–12.0) | 0.6 (0.1–1.1) * | 0.01 |
| MCV—μm3 | 84.5 (82.4–86.5) | 85.8 (84.6–87.1) | 0.7 (−1.3–2.7) * | 0.50 |
| NYHA Class—% | 2.84 (1.42–5.68) º | 0.003 | ||
| I | 17.9 | 21.0 | ||
| II | 56.7 | 48.3 | ||
| III | 20.9 | 26.1 | ||
| IV | 3.0 | 2.2 |
| Iron Treatment | Control | |||
|---|---|---|---|---|
| Safety end Point | No. of Patients with End Point or Event (Percent) | No. of Patients with End Point or Event (Percent) | Time to First Event Hazard Ratio (95% CI) * | p-value |
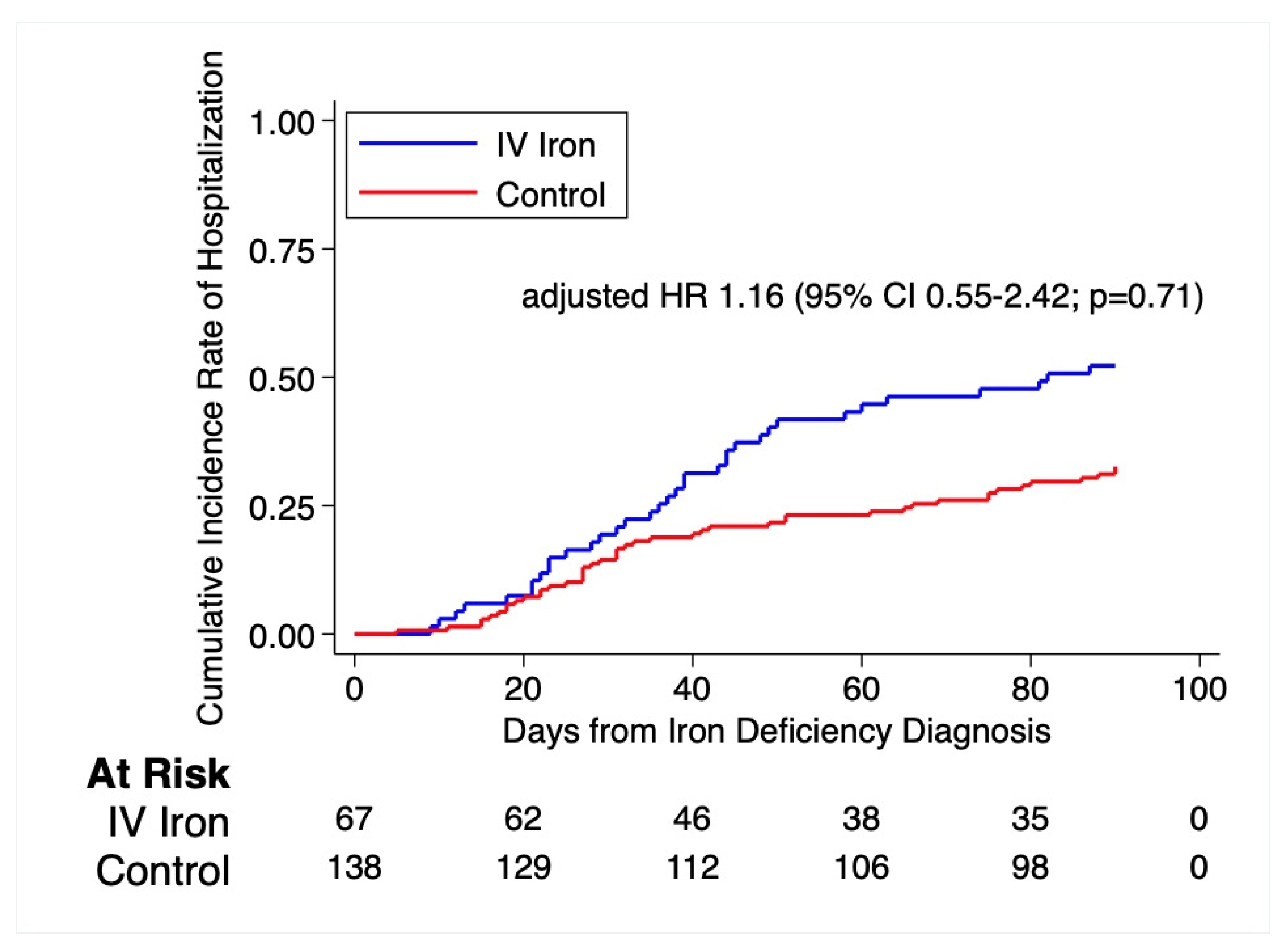
| Hospitalization within 90 days | 35 (52.2) | 45 (32.6) | 1.15 (0.55–2.42) | 0.71 |
| Hospitalization for any cardiovascular cause within 90 days | 8 (17.9) | 11 (8.0) | 1.67 (0.52–5.37) | 0.39 |
| Adverse event | ||||
| Gastrointestinal bleed | 7 (10.4) | 7 (5.1) | 1.86 (0.56–6.22) | 0.31 |
| Infection | 8 (11.9) | 13 (9.4) | 0.50 (0.12–2.05) | 0.34 |
| Arrhythmia | 8 (11.9) | 6 (4.3) | 1.94 (0.59–6.40) | 0.28 |
| Pump thrombosis | 0 (0.0) | 0 (0.0) | 1.00 | 1.00 |
| Anaphylaxis | 0 (0.0) | 0 (0.0) | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, C.J.; Hanff, T.C.; Genuardi, M.V.; Zhang, R.; Domenico, C.; Atluri, P.; Mazurek, J.A.; Urgo, K.; Wald, J.; Tanna, M.S.; et al. Safety and Effectiveness of Intravenous Iron Therapy in Patients Supported by Durable Left Ventricular Assist Devices. J. Clin. Med. 2022, 11, 3900. https://doi.org/10.3390/jcm11133900
Peters CJ, Hanff TC, Genuardi MV, Zhang R, Domenico C, Atluri P, Mazurek JA, Urgo K, Wald J, Tanna MS, et al. Safety and Effectiveness of Intravenous Iron Therapy in Patients Supported by Durable Left Ventricular Assist Devices. Journal of Clinical Medicine. 2022; 11(13):3900. https://doi.org/10.3390/jcm11133900
Chicago/Turabian StylePeters, Carli J., Thomas C. Hanff, Michael V. Genuardi, Robert Zhang, Christopher Domenico, Pavan Atluri, Jeremy A. Mazurek, Kim Urgo, Joyce Wald, Monique S. Tanna, and et al. 2022. "Safety and Effectiveness of Intravenous Iron Therapy in Patients Supported by Durable Left Ventricular Assist Devices" Journal of Clinical Medicine 11, no. 13: 3900. https://doi.org/10.3390/jcm11133900
APA StylePeters, C. J., Hanff, T. C., Genuardi, M. V., Zhang, R., Domenico, C., Atluri, P., Mazurek, J. A., Urgo, K., Wald, J., Tanna, M. S., Shore, S., Acker, M. A., Goldberg, L. R., Margulies, K. B., & Birati, E. Y. (2022). Safety and Effectiveness of Intravenous Iron Therapy in Patients Supported by Durable Left Ventricular Assist Devices. Journal of Clinical Medicine, 11(13), 3900. https://doi.org/10.3390/jcm11133900






