Combination Therapy of 0.1% Fluorometholone and 0.05% Azelastine in Eyes with Severe Allergic Conjunctival Diseases: A Randomized Controlled Trial
Abstract
:1. Introduction
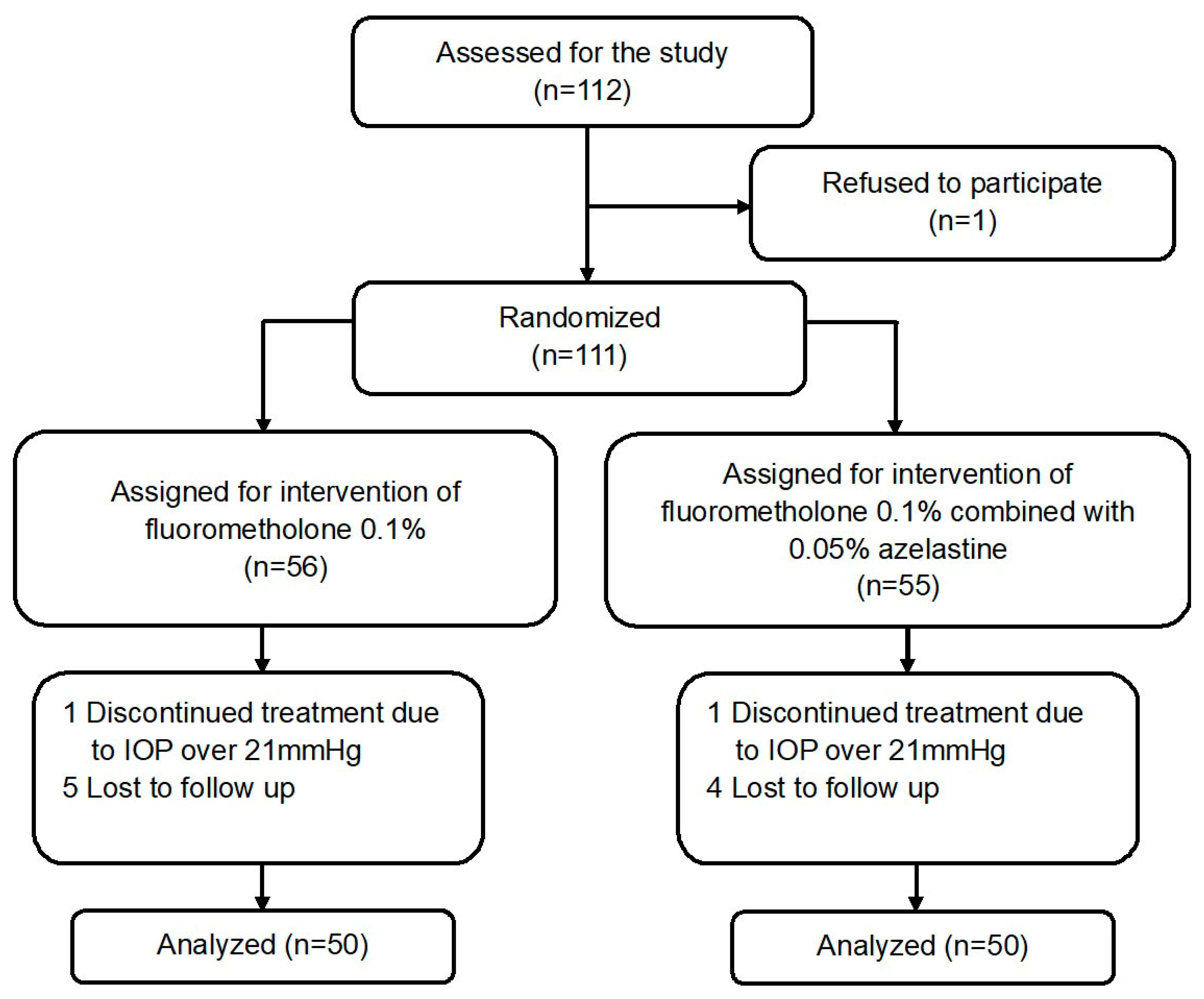
2. Materials and Methods
2.1. Patients
2.2. Outcome Measures
2.3. Safety
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
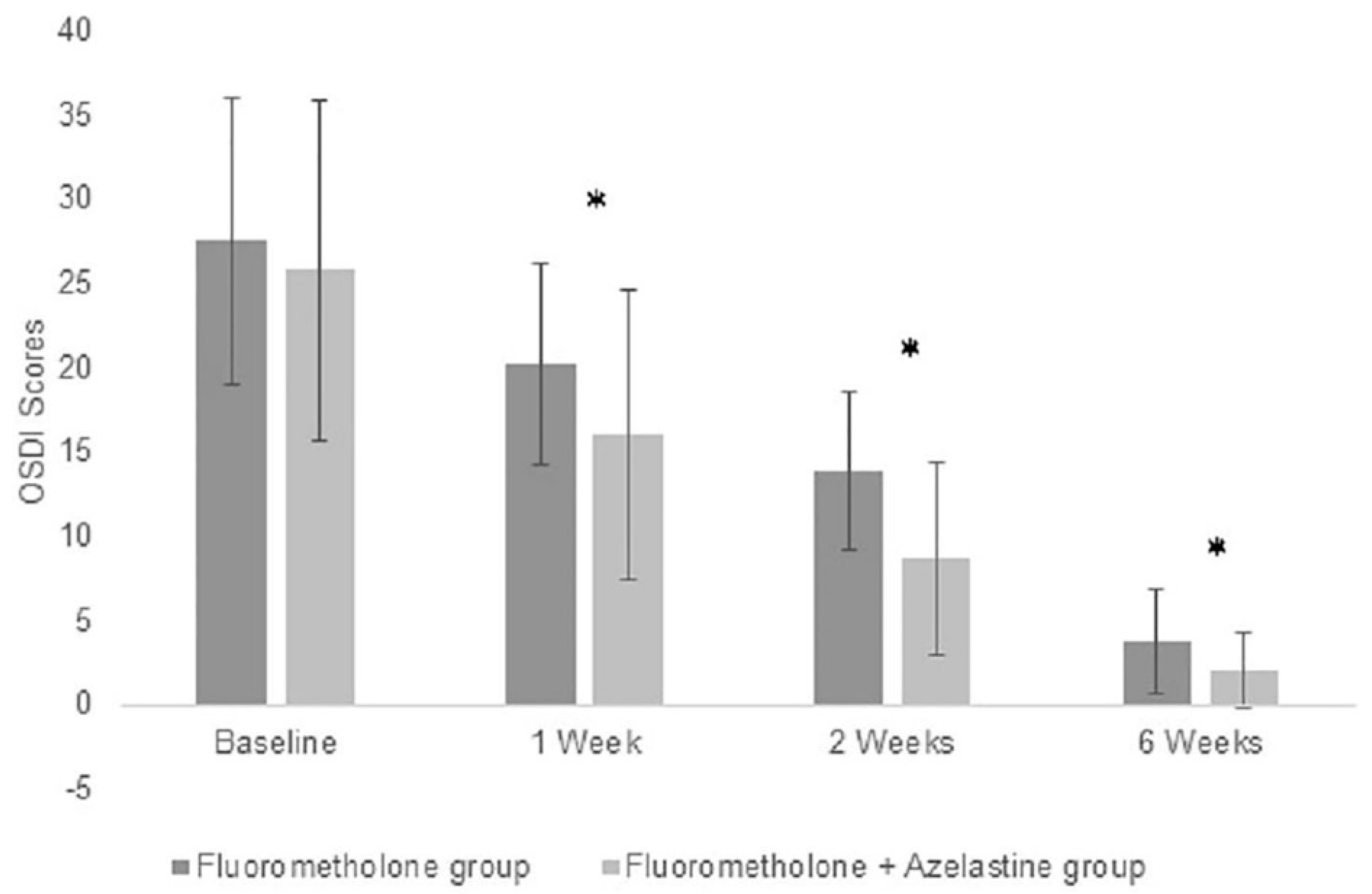
3.2. Improvements of Symptom Scores in Both Groups
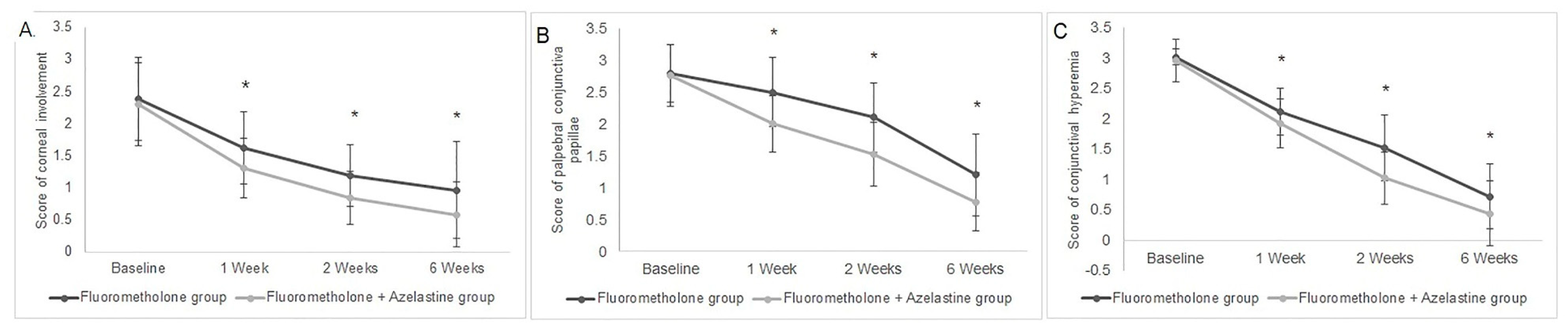
3.3. Improvements of Sign Scores in Both Groups
3.4. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, A.V.; Donthineni, P.R.; Prashanthi, G.S.; Basu, S. Allergic eye disease in children and adolescents seeking eye care in India: Electronic medical records driven big data analytics report II. Ocul. Surf. 2019, 17, 683–689. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Wang, F.; Liu, R.; Chen, L.; Wu, S.; Yang, X.; Chen, M.; Rao, Y.-Q.; Li, J. The prevalence of ocular allergy and comorbidities in Chinese school children in Shanghai. Biomed. Res. Int. 2017, 2017, 7190987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, P.J. Trends in prevalence and treatment of ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ghiglioni, D.G.; Zicari, A.M.; Parisi, G.F.; Marchese, G.; Indolfi, C.; Diaferio, L.; Brindisi, G.; Ciprandi, G.; Marseglia, G.L.; Miraglia Del Giudice, M. Vernal keratoconjunctivitis: An update. Eur. J. Ophthalmol. 2021, 31, 2828–2842. [Google Scholar] [CrossRef]
- Yazu, H.; Fukagawa, K.; Shimizu, E.; Sato, Y.; Fujishima, H. Long-term outcomes of 0.1% tacrolimus eye drops in eyes with severe allergic conjunctival diseases. Allergy Asthma Clin. Immunol. 2021, 17, 11. [Google Scholar] [CrossRef]
- Kenny, S.E.; Tye, C.B.; Johnson, D.A.; Kheirkhah, A. Giant papillary conjunctivitis: A review. Ocul. Surf. 2020, 18, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Sahay, P.; Maharana, P.K.; Raj, N.; Sharma, N.; Titiyal, J.S. Vernal keratoconjunctivitis. Surv. Ophthalmol. 2019, 64, 289–311. [Google Scholar] [CrossRef]
- Chen, M.; Wei, A.; Ke, B.; Zou, J.; Gong, L.; Wang, Y.; Zhang, C.; Xu, J.; Yin, J.; Hong, J. Combination of 0.05% azelastine and 0.1% tacrolimus eye drops in children with vernal keratoconjunctivitis: A prospective study. Front. Med. 2021, 8, 650083. [Google Scholar] [CrossRef]
- Leonardi, A.; Doan, S.; Amrane, M.; Ismail, D.; Montero, J.; Németh, J.; Aragona, P.; Bremond-Gignac, D.; VEKTIS Study Group. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: The VEKTIS Study. Ophthalmology 2019, 126, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, I.T.; Sanghi, P.; Manzouri, B. Pharmacotherapeutic management of atopic keratoconjunctivitis. Expert Opin. Pharmacother. 2020, 21, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Pucci, N.; Caputo, R.; di Grande, L.; de Libero, C.; Mori, F.; Barni, S.; di Simone, L.; Calvani, A.; Rusconi, F.; Novembre, E. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: A randomized, comparative, double-blind, crossover study. Randomized Control. Trial Pediatr. Allergy Immunol. 2015, 26, 256–261. [Google Scholar] [CrossRef]
- Yazu, H.; Shimizu, E.; Aketa, N.; Dogru, M.; Okada, N.; Fukagawa, K.; Fujishima, H. The efficacy of 0.1% tacrolimus ophthalmic suspension in the treatment of severe atopic keratoconjunctivitis. Ann. Allergy Asthma Immunol. 2019, 122, 387–392. [Google Scholar] [CrossRef]
- Jones, R.; Rhee, D.J. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr. Opin. Ophthalmol. 2006, 17, 163–167. [Google Scholar] [PubMed]
- Leonardi, A. Management of vernal keratoconjunctivitis. Ophthalmol. Ther. 2013, 2, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Kumagai, N.; Satake, Y.; Shoji, J.; Nakagawa, Y.; et al. Japanese guidelines for allergic conjunctival diseases 2017. Allergol. Int. 2017, 66, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Kumagai, N.; Satake, Y.; Shoji, J.; et al. Japanese guidelines for allergic conjunctival diseases 2020. Allergol Int. 2020, 69, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Tang, J.; Han, Y.; Wang, D.; Ye, H. Therapeutic effect of 0.1% tacrolimus eye drops in the tarsal form of vernal keratoconjunctivitis. Ophthalmic Res. 2018, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Sharma, N.; Agarwal, T.; Chawla, B.; Velpandian, T.; Tandon, R.; Titiyal, J.S. Comparison of olopatadine and fluorometholone in contact lens-induced papillary conjunctivitis. Eye Contact Lens 2010, 36, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Wajnsztajn, D.; Solomon, A. Vernal keratoconjunctivitis and keratoconus. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Yamamoto, K.; Nishida, T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res. 2006, 25, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Oner, V.; Türkcü, F.M.; Taş, M.; Alakuş, M.F.; Işcan, Y. Topical loteprednol etabonate 0.5% for treatment of vernal keratoconjunctivitis: Efficacy and safety. Jpn. J. Ophthalmol. 2012, 56, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Senthil, S.; Thakur, M.; Rao, H.L.; Mohamed, A.; Jonnadula, G.B.; Sangwan, V.; Garudadri, C.S. Steroid-induced glaucoma and blindness in vernal keratoconjunctivitis. Br. J. Ophthalmol. 2020, 104, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Jain, S.; Mohan, A.; Shah, C.; Sen, A.; Jain, E. Pattern of steroid misuse in vernal keratoconjunctivitis resulting in steroid induced glaucoma and visual disability in Indian rural population: An important public health problem in pediatric age group. Indian J. Ophthalmol. 2019, 67, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Ti, S.E.; Loh, R.; Farzavandi, S.; Zhang, R.; Tan, D.; Chan, C. Steroid-induced ocular hypertension in Asian children with severe vernal keratoconjunctivitis. Clin. Ophthalmol. 2012, 6, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Ang, M.; Ho, C.L.; Tan, D.; Chan, C. Severe vernal keratoconjunctivitis requiring trabeculectomy with mitomycin C for corticosteroid-induced glaucoma. Clin. Exp. Ophthalmol. 2012, 40, e149–e155. [Google Scholar] [CrossRef]
- Cantrill, H.L.; Palmberg, P.F.; Zink, H.A.; Waltman, S.R.; Podos, S.M.; Becker, B. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. Am. J. Ophthalmol. 1975, 79, 1012–1017. [Google Scholar] [CrossRef]
- Gupta, S.; Shah, P.; Grewal, S.; Chaurasia, A.K.; Gupta, V. Steroid-induced glaucoma and childhood blindness. Br. J. Ophthalmol. 2015, 99, 1454–1456. [Google Scholar] [CrossRef]
- Koichi, B.; Noriyasu, H.; Motokazu, T.; Andrew, J.Q.; Kohji, N. The generation of fluorometholone nanocrystal eye drops, their metabolization to dihydrofluorometholone and penetration into rabbit eyes. Int. J. Pharm. 2021, 592, 120067. [Google Scholar]
| Signs | Grading | Definition |
|---|---|---|
| Conjunctiva hyperaemia | 0 | None |
| 1 | Dilatation of several vessels | |
| 2 | Dilatation of many vessels | |
| 3 | Diffuse dilated vessels in all the bulbar conjunctiva | |
| Palpebral conjunctiva papillae | 0 | None |
| 1 | Flat papillae | |
| 2 | Elevated papillae in <1/2 of the upper palpebral conjunctiva | |
| 3 | Elevated papillae in >1/2 or more of the upper palpebral conjunctiva or giant papillae (papillae size ≥1 mm) | |
| Corneal involvement | 0 | None |
| 1 | Superficial punctate keratitis | |
| 2 | Exfoliation superficial punctate keratitis | |
| 3 | Shield ulcer |
| Fluorometholone Group (n = 50 Eyes) | Fluorometholone + Azelastine Group (n = 50 Eyes) | p | |
|---|---|---|---|
| Age (y) | 17.68 ± 11.09 | 19.58 ± 11.74 | 0.4171 |
| Male (%) | 72.0% | 64.0% | 0.391 |
| Duration of course (months) | 47.56 ± 36.24 | 41.4 ± 34.79 | 0.3491 |
| History of contact lens | 25.0% | 30.0% | 0.248 |
| Numbers of VKC/GPC/AKC (eyes) | 31/10/9 | 27/16/7 | 0.385 |
| Corneal Involvement | Palpebral Conjunctiva Papillae | Conjunctival Hyperemia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluorometholone Group | Fluorometholone + Azelastine Group | p | Fluorometholone Group | Fluorometholone + Azelastine Group | p | Fluorometholone Group | Fluorometholone + Azelastine Group | p | |
| Baseline | 2.38 ± 0.64 | 2.30 ± 0.65 | 0.5341 | 2.80 ± 0.45 | 2.76 ± 0.48 | 0.6576 | 3.01 ± 0.14 | 2.96 ± 0.35 | 0.2551 |
| Week 1 | 1.62 ± 0.57 | 1.30 ± 0.46 | 0.0037 | 2.50 ± 0.54 | 2.00 ± 0.45 | <0.0001 | 2.12 ± 0.39 | 1.92 ± 0.40 | 0.0128 |
| Week 2 | 1.18 ± 0.48 | 0.84 ± 0.42 | 0.0004 | 2.1 ± 0.54 | 1.52 ± 0.50 | <0.0001 | 1.52 ± 0.54 | 1.02 ± 0.43 | <0.0001 |
| Week 6 | 0.96 ± 0.75 | 0.58 ± 0.50 | 0.0069 | 1.2 ± 0.64 | 0.78 ± 0.46 | 0.0003 | 0.72 ± 0.54 | 0.44 ± 0.54 | 0.0097 |
| P | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Ke, B.; Zou, J.; Gong, L.; Wang, Y.; Zhang, C.; Xu, J.; Wei, A.; Hong, J. Combination Therapy of 0.1% Fluorometholone and 0.05% Azelastine in Eyes with Severe Allergic Conjunctival Diseases: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 3877. https://doi.org/10.3390/jcm11133877
Chen M, Ke B, Zou J, Gong L, Wang Y, Zhang C, Xu J, Wei A, Hong J. Combination Therapy of 0.1% Fluorometholone and 0.05% Azelastine in Eyes with Severe Allergic Conjunctival Diseases: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(13):3877. https://doi.org/10.3390/jcm11133877
Chicago/Turabian StyleChen, Minjie, Bilian Ke, Jun Zou, Lan Gong, Yan Wang, Chaoran Zhang, Jianjiang Xu, Anji Wei, and Jiaxu Hong. 2022. "Combination Therapy of 0.1% Fluorometholone and 0.05% Azelastine in Eyes with Severe Allergic Conjunctival Diseases: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 13: 3877. https://doi.org/10.3390/jcm11133877
APA StyleChen, M., Ke, B., Zou, J., Gong, L., Wang, Y., Zhang, C., Xu, J., Wei, A., & Hong, J. (2022). Combination Therapy of 0.1% Fluorometholone and 0.05% Azelastine in Eyes with Severe Allergic Conjunctival Diseases: A Randomized Controlled Trial. Journal of Clinical Medicine, 11(13), 3877. https://doi.org/10.3390/jcm11133877






