High First Trimester Levels of TSH as an Independent Risk Factor for Gestational Diabetes Mellitus: A Retrospective Cohort Study
Abstract
:1. Introduction
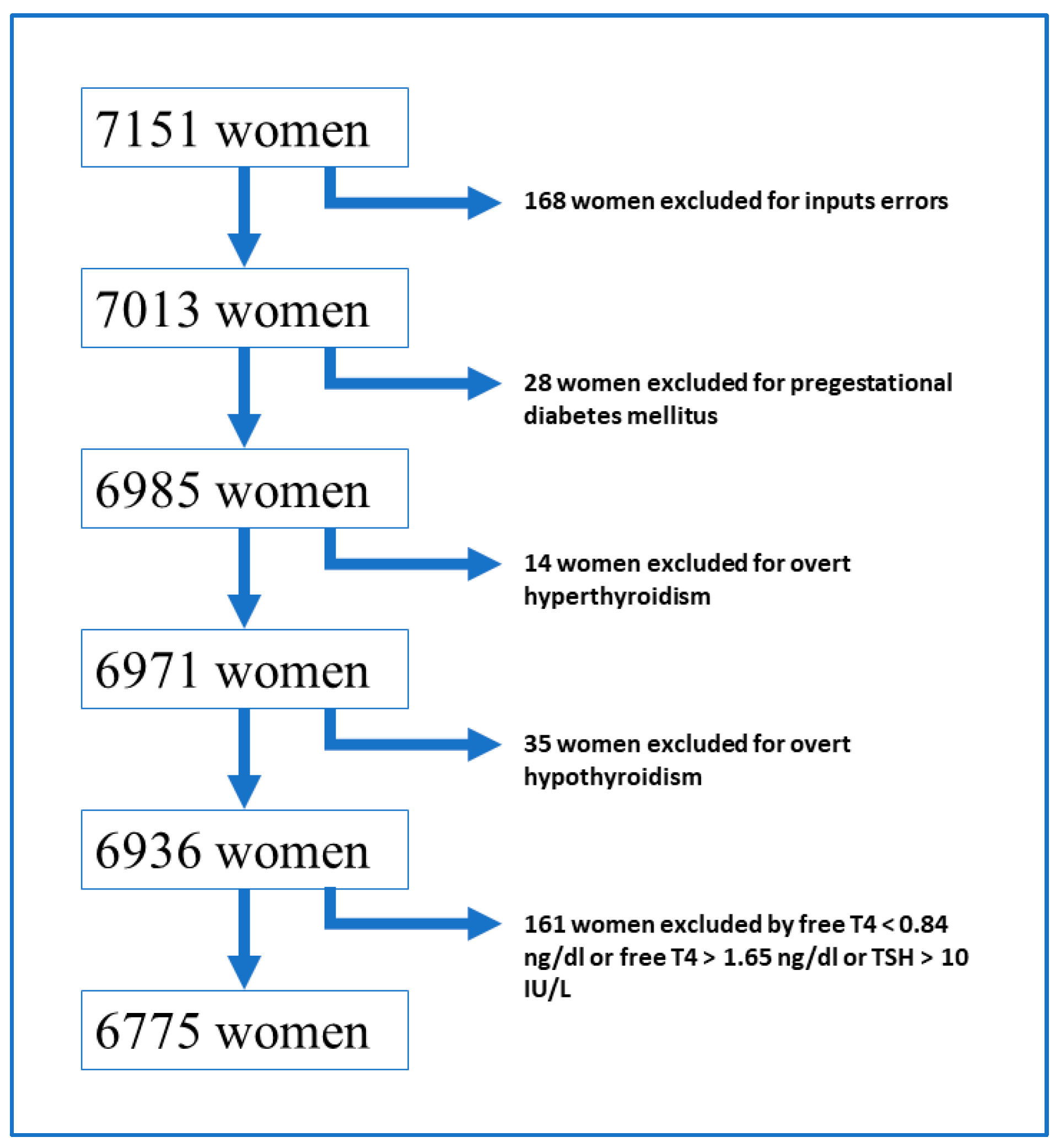
2. Materials and Methods
2.1. Definitions
2.1.1. Thyroid Function
Reference Ranges
- All pregnant women with a TSH value > 10 mIU/L were excluded from the study because we considered that with this TSH level, it was overt hypothyroidism, regardless of the fT4 values.
- Normal fT4 level ranges from 0.84 ng/dL to 1.65 ng/dL. All women with fT4 values outside the above range were excluded from the study.
- Normal TSH level ranges from 0.13 mIU/L to 4.72 mIU/L.
Thyroid Antibodies
2.1.2. Screening and Diagnosis of GDM
2.2. Statistical Analysis
2.2.1. Bivariate Analysis
2.2.2. Multivariate Analysis
- -
- <0.131 mIU/L (subclinical hyperthyroidism)
- -
- 0.131–2.49 mIU/L (Euthyroid 1)
- -
- 2.5–4.71 mIU/L (Euthyroid 2)
- -
- ≥4.72 mIU/L (subclinical hypothyroidism)
3. Results
3.1. Clinical and Demographic Characteristics
3.2. Bivariate Analysis
3.3. Multivariate Analysis
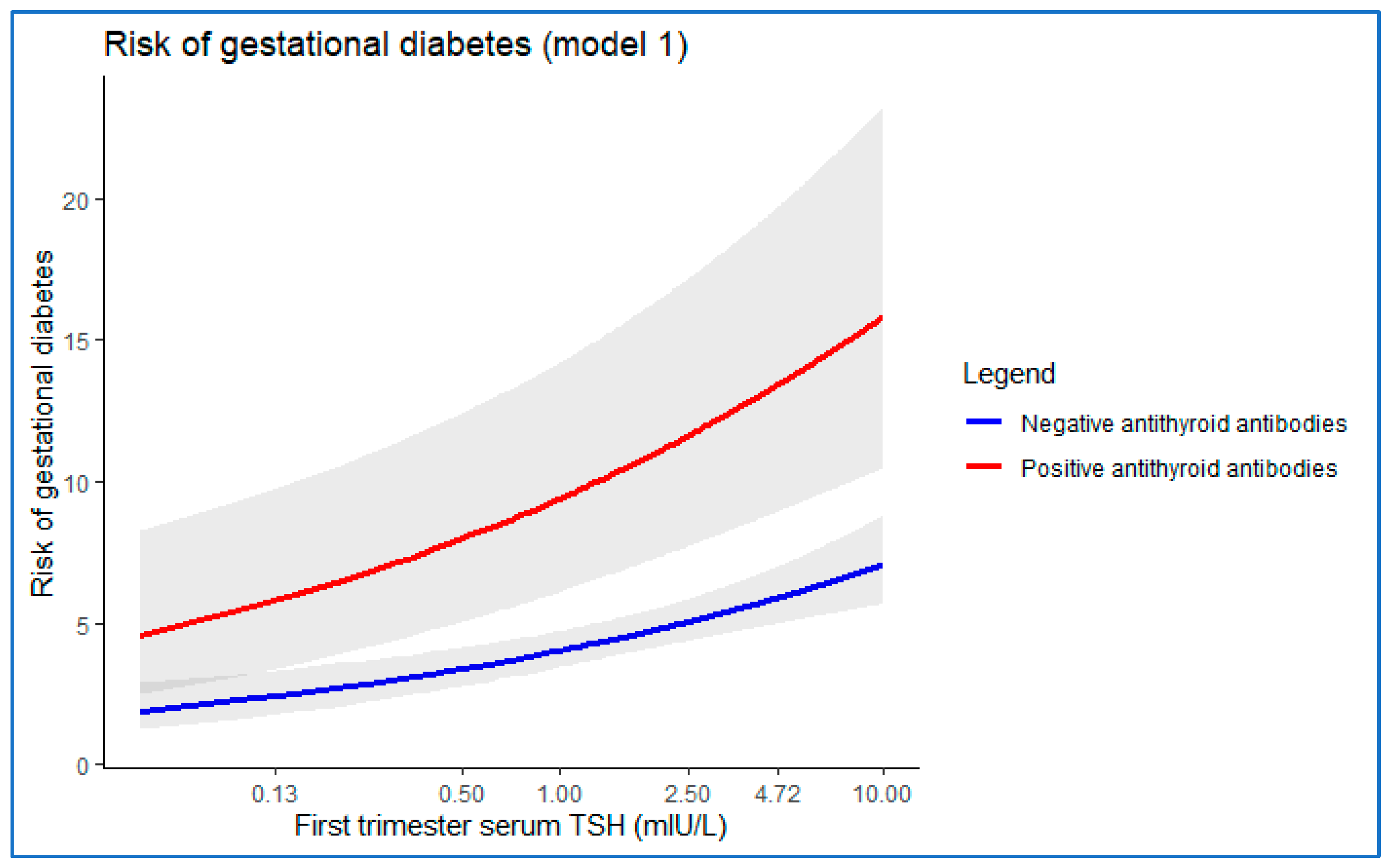
3.3.1. Model 1
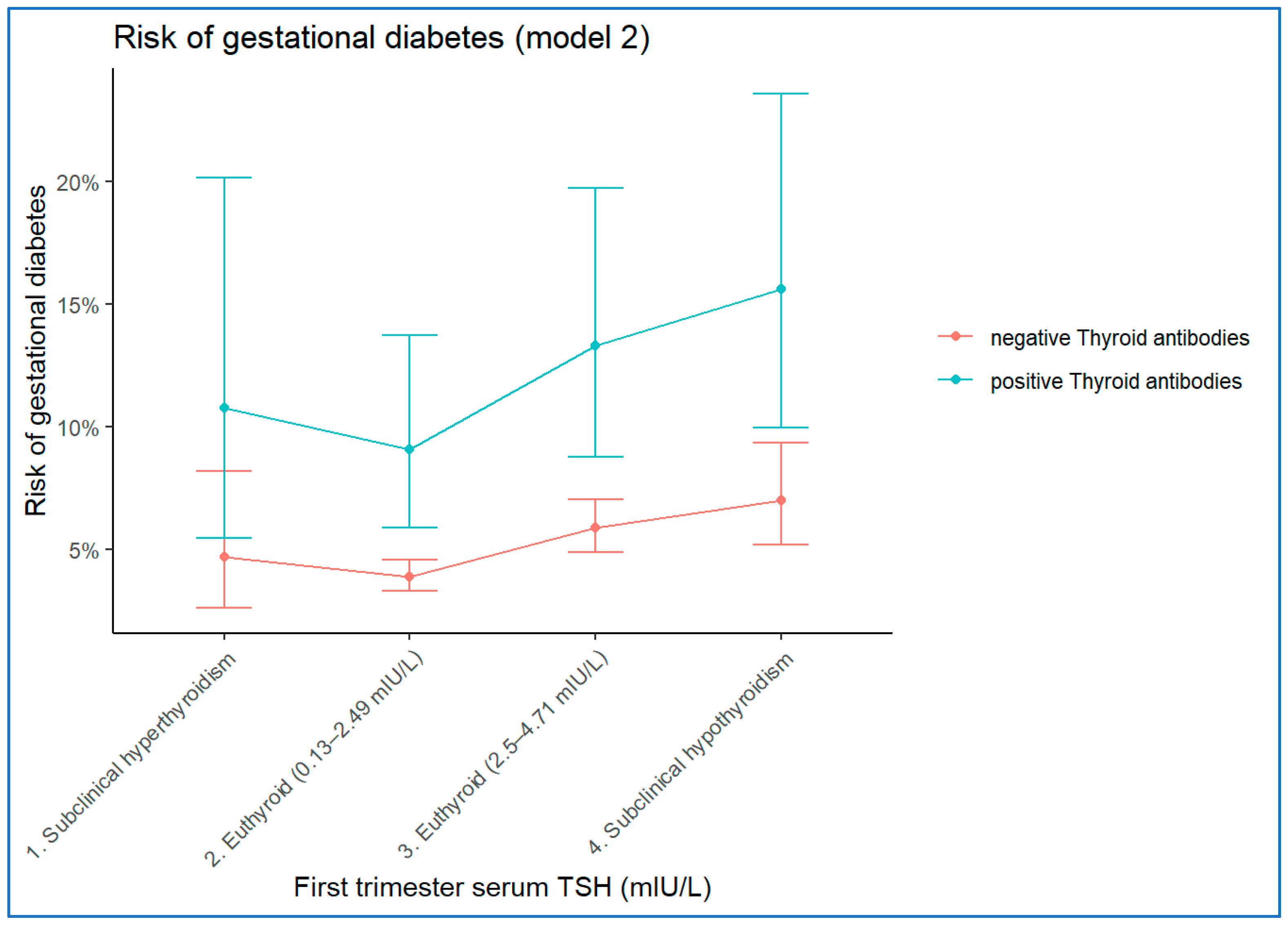
3.3.2. Model 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reece, E.A.; Leguizamon, G.; Wiznitzer, A. Gestational diabetes: The need for a common ground. Lancet 2009, 373, 1789–1797. [Google Scholar] [CrossRef]
- Hillier, T.A.; Pedula, K.L.; Vesco, K.K.; Schmidt, M.M.; Mullen, J.A.; LeBlanc, E.S.; Pettitt, D.J. Excess gestational weight gain. Obstet. Gynecol. 2008, 112, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Coustan, D.R.; Trimble, E.R. Hyperglycemia and adverse pregnancy outcomes. Clin. Chem. 2019, 65, 937–938. [Google Scholar] [CrossRef] [Green Version]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational diabetes risk factors and long-term consequences for both mother and offspring: A literature review. Expert Rev. Endocrinol. Metab. 2018, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Maratou, E.; Hadjidakis, D.J.; Kollias, A.; Tsegka, K.; Peppa, M.; Alevizaki, M.; Mitrou, P.; Lambadiari, V.; Boutati, E.; Nikzas, D.; et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur. J. Endocrinol. 2009, 160, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Association of thyroid disorders with gestational diabetes mellitus: A meta-analysis. Endocrine 2021, 73, 550–560, Epub 13 May 2021. [Google Scholar] [CrossRef]
- Toulis, K.A.; Stagnaro-Green, A.; Negro, R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: A meta-analysis. Endocr. Pr. 2014, 20, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Ospina, N.M.; O’Keeffe, D.T.; Espinosa De Ycaza, A.E.; Gionfriddo, M.R.; Erwin, P.J.; Coddington, C.C., 3rd; Stan, M.N.; Murad, M.H.; Montori, V.M. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 580–590, Epub 3 March 2016. [Google Scholar] [CrossRef] [Green Version]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389, Erratum in 2017, 27, 1212. [Google Scholar] [CrossRef] [Green Version]
- Kent, N.L.; Young, S.L.; Akison, L.K.; Cuffe, J.S.M. Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: A systematic review and meta-analysis. Endocrine 2021, 74, 38–49, Epub 15 May 2021. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Ginecología y Obstetricia. Enfermedad Tiroidea y Gestación. Prog. Obstet. Ginecol. 2013, 58, 101–111. [Google Scholar] [CrossRef]
- Grupo Español de Diabetes y Embarazo. Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada en 2014. Av Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes 2017. Diabetes Care 2017, 40 (Suppl. S1), S1–S135. Available online: http://care.diabetesjournals.org/content/40/Supplement_1 (accessed on 15 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 15 May 2022).
- Ying, H.; Tang, Y.P.; Bao, Y.R.; Su, X.J.; Cai, X.; Li, Y.H.; Wang, D.F. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine 2016, 54, 742–750, Epub 16 July 2016. [Google Scholar] [CrossRef] [PubMed]
- De Garduno-Garcia, J.; Alvirde-Garcia, U.; López-Carrasco, G.; Mendoza, M.E.P.; Mehta, R.; Arellano-Campos, O.; Choza, R.; Sauque, L.; Garay-Sevilla, M.E.; Malacara, J.M.; et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur. J. Endocrinol. 2010, 163, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Handisurya, A.; Pacini, G.; Tura, A.; Gessl, A.; Kautzky-Willer, A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin. Endocrinol. 2008, 69, 963–969. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Boutati, E.; Maratou, E.; Panagiotakos, D.B.; Koukkou, E.; Tzanela, M.; Thalassinos, N.; Raptis, S.A. Insulin action in adipose tissue and muscle in hypothyroidism. J. Clin. Endocrinol. Metab. 2006, 91, 4930–4937. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, W.C.; Alkan, Z.; Lang, K.; King, J.C. Plasma selenium decrease during pregnancy is associated with glucose intolerance. Biol. Trace Elem. Res. 2004, 100, 19–29. [Google Scholar] [CrossRef]
- Al-Khaldi, A.; Sultan, S. The expression of sirtuins, superoxide dismutase, and lipid peroxidation status in peripheral blood from patients with diabetes and hypothyroidism. BMC Endocr. Disord. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, Q.; Wang, Q.; Ma, X. Thyroid antibodies and gestational diabetes mellitus: A meta-analysis. Fertil. Steril. 2015, 104, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaner, P.; Juan, L.; Campos, R.; Gil, L.; Corcoy, R. Is thyroid autoimmunity associated with gestational diabetes mellitus? Metabolism 2008, 57, 522–525. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 6775) | GDM (N = 690, 10.2%) | Non GDM (N = 6085, 89.8%) | p | ||||
|---|---|---|---|---|---|---|---|
| Number (%) | Median (IQR) | Number (%) | Median (IQR) | Number (%) | Median (IQR) | ||
| Age (years) | 32.87 (7.27) | 34.39 (6.96) | 32.68 (7.32) | <0.001 | |||
| Maternal BMI (kg/m2) | 24.33 (6.21) | 26.15 (7.76) | 24.10 (6.05) | <0.001 <0.001 | |||
| Underweight (BMI < 18.5) | 177 (2.6) | 18 (2.6) | 159 (2.6) | ||||
| Normal weight (BMI between 18.5–24.9) | 3620 (53.4) | 255 (37) | 3365 (55.3) | ||||
| Overweight (BMI between 25–29.9) | 1833 (27.1) | 231 (33.5) | 1602 (26.3) | ||||
| Obesity (BMI ≥ 30) | 1145 (16.9) | 186 (27) | 959 (15.8) | ||||
| Family history of type 2 diabetes mellitus | 1146 (16.9) | 231 (33.5) | 915 (15) | <0.001 | |||
| Gravidity | 0.264 | ||||||
| 1 | 2699 (39.8) | 243 (35.2) | 2456 (40.4) | ||||
| >1 | 4076 (60.2) | 447 (64.8) | 3629 (59.6) | ||||
| Parity | 0.752 | ||||||
| Primiparous | 2721 (59.2) | 399 (57.8) | 3691 (60.7) | ||||
| Multiparous | 1878 (40.8) | 291 (42.2) | 2394 (39.3) | ||||
| Recurrent abortion | 409 (6%) | 63 (9.1%) | 346 (5.6%) | <0.001 | |||
| Chronic hypertension | 65(1%) | 24(3.5%) | 41 (0.7%) | <0.001 | |||
| Fetus number | <0.001 | ||||||
| Singleton | 6511 (96.1) | 642 (93) | 5869 (96.5) | ||||
| Multiple | 264 (3.9) | 48 (7) | 216 (3.5) | ||||
| Gestational week at delivery | <0.001 | ||||||
| Preterm birth | 468 (6.9) | 66 (9.6) | 402 (6.6) | ||||
| Full-term birth | 6307 (93.1) | 624 (90.4) | 5683 (93.4) | ||||
| Delivery mode | <0.001 | ||||||
| Vaginal | 5144 (75.9) | 474 (68.7) | 4670 (76.7) | ||||
| Caesarean section | 1631 (24.1) | 216 (31.3) | 1415 (23.3) | ||||
| Newborn weight (g) | 3280 (620) | 3290 (630) | 3280 (610) | 0.330 | |||
| TSH (mIU/L) | 1.89 (1.63) | 2.13 (1.99) | 1.86 (1.61) | <0.001 <0.001 | |||
| Subclinical hyperthyroidism | 109 (1.61) | 12 (1.74) | 97 (1.59) | ||||
| Euthyroid | |||||||
| TSH between 0.13–2.49 | 4501 (66.44) | 399 (57.82) | 4102 (67.41) | ||||
| TSH between 2.5–4.71 | 1782 (26.30) | 219 (31.74) | 1563 (25.69) | ||||
| Subclinical hypothyroidism | 383 (5.65) | 60 (8.7) | 323 (5.31) | ||||
| Thyroid-antibody positive (IU/L) (anti-TG > 115 and/or anti-TPO > 34) | 383 (5.65) | 126 (18.26) | 257 (4.22) | <0.001 | |||
| Unadjusted OR | 95%CI | p Value | |
|---|---|---|---|
| Age (Years) | 1.088 | 1.071–1.105 | <0.001 |
| Maternal BMI (kg/m2) | 1.069 | 1.056–1.083 | <0.001 |
| Underweight (BMI < 18.5) | 1.494 | 0.903–2.472 | 0.121 |
| Overweight (BMI between 25–29.9) | 1.903 | 1.577–2.296 | <0.001 |
| Obesity (BMI ≥ 30) | 2.559 | 2.091–3.133 | <0.001 |
| Family history of type 2 diabetes mellitus | 2.844 | 2.392–3.381 | <0.001 |
| Gravidity (>1) | 1.245 | 1.056–1.467 | <0.05 |
| Parity (multiparous) | 1.124 | 0.959–1.319 | 0.189 |
| Recurrent abortion | 1.668 | 1.260–2.209 | <0.001 |
| Chronic hypertension | 5.317 | 3.193–8.855 | <0.001 |
| Fetus number (Multiple) | 2.031 | 1.470–2.807 | <0.001 |
| TSH level at first trimester of gestation (mIU/L) | 1.150 | 1.093–1.210 | <0.001 |
| Subclinical hyperthyroidism | 1.272 | 0.692–2.337 | 0.536 |
| TSH between 2.5–4.71 | 1.440 | 1.209–1.716 | <0.001 |
| Subclinical hypothyroidism | 1.910 | 1.423–2.563 | <0.001 |
| Anti-TPO antibodies > 34 IU/L | 2.146 | 1.275–3.612 | <0.01 |
| Anti-TG antibodies > 115 IU/L | 3.108 | 1.808–5.343 | <0.001 |
| Thyroid-antibodies positive | 2.568 | 1.662–3.967 | <0.001 |
| (anti-TG >115 IU/L and/or anti-TPO > 34 IU/L) |
| B | SE | Wald | df | Sig. | Adjusted OR | 95% CI by Adjusted OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age (years) | 0.079 | 0.008 | 92.686 | 1 | <0.001 | 1.083 | 1.065 | 1.100 |
| Family history of type 2 DM | 0.930 | 0.091 | 105.125 | 1 | <0.001 | 2.533 | 2.121 | 3.026 |
| Normal BMI (18.5–24.9 kg/m2) | 63.030 | 3 | <0.001 | |||||
| Underweight (BMI < 18.5 kg/m2) | 0.601 | 0.265 | 5.149 | 1 | 0.023 | 1.825 | 1.085 | 3.068 |
| Overweight (BMI 25–29.9 kg/m2) | 0.584 | 0.098 | 35.564 | 1 | <0.001 | 1.793 | 1.480 | 2.172 |
| Obesity (BMI ≥ 30 kg/m2) | 0.785 | 0.109 | 52.103 | 1 | <0.001 | 2.193 | 1.772 | 2.714 |
| Chronic hypertension | 1.220 | 0.276 | 19.562 | 1 | <0.001 | 3.388 | 1.973 | 5.818 |
| Fetus number (multiple) | 0.669 | 0.173 | 14.864 | 1 | <0.001 | 1.952 | 1.389 | 2.742 |
| Log10(TSH mIU/L) at first trimester of gestation | 0.591 | 0.128 | 21.397 | 1 | <0.001 | 1.806 | 1.406 | 2.320 |
| Positive antithyroid-antibodies | 0.905 | 0.232 | 15.233 | 1 | <0.001 | 2.471 | 1.569 | 3.893 |
| Constant | −5.629 | 0.295 | 364.750 | 1 | <0.001 | 0.004 | ||
| B | SE | Wald | df | Sig. | Adjusted OR | 95% CI by Adjusted OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age (years) | 0.079 | 0.008 | 91.606 | 1 | <0.001 | 1.082 | 1.065 | 1.101 |
| Family history of type 2 DM | 0.932 | 0.091 | 105.646 | 1 | <0.001 | 2.541 | 2.127 | 3.035 |
| Normal BMI (18.5–24.9 kg/m2) | 66.087 | 3 | <0.001 | |||||
| Underweight (BMI < 18.5 kg/m2) | 0.627 | 0.265 | 5.578 | 1 | 0.018 | 1.872 | 1.113 | 3.150 |
| Overweight (BMI 25–29.9 kg/m2) | 0.597 | 0.098 | 37.217 | 1 | <0.001 | 1.817 | 1.500 | 2.202 |
| Obesity (BMI ≥ 30 kg/m2) | 0.803 | 0.109 | 54.560 | 1 | <0.001 | 2.233 | 1.804 | 2.763 |
| Chronic hypertension | 1.210 | 0.277 | 19.024 | 1 | <0.001 | 3.354 | 1.947 | 5.779 |
| Fetus number (multiple) | 0.658 | 0.173 | 14.544 | 1 | <0.001 | 1.931 | 1.377 | 2.709 |
| TSH between 0.13–2.49 mIU/L | 30.354 | 3 | <0.001 | |||||
| TSH < 0.13 mIU/L (Subclinical hyperthyroidism) | 0.190 | 0.303 | 0.392 | 1 | 0.531 | 1.209 | 0.667 | 2.191 |
| TSH between 2.5 mIU/L–4.70 mIU/L | 0.429 | 0.093 | 21.422 | 1 | <0.001 | 1.536 | 1.281 | 1.842 |
| TSH > 4.7 mIU/L (Subclinical hypothyroidism) | 0.615 | 0.158 | 15.172 | 1 | <0.001 | 1.849 | 1.357 | 2.519 |
| Thyroid-antibody positive | 0.897 | 0.231 | 15.058 | 1 | <0.001 | 2.453 | 1.559 | 3.859 |
| Constant | −5.638 | 0.294 | 368.382 | 1 | <0.001 | 0.004 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Alba, J.J.; Castillo Lara, M.; Jiménez Heras, J.M.; Moreno Cortés, R.; González Macías, C.; Vilar Sánchez, Á.; San Laureano, F.C.; Moreno Corral, L.J. High First Trimester Levels of TSH as an Independent Risk Factor for Gestational Diabetes Mellitus: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 3776. https://doi.org/10.3390/jcm11133776
Fernández Alba JJ, Castillo Lara M, Jiménez Heras JM, Moreno Cortés R, González Macías C, Vilar Sánchez Á, San Laureano FC, Moreno Corral LJ. High First Trimester Levels of TSH as an Independent Risk Factor for Gestational Diabetes Mellitus: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(13):3776. https://doi.org/10.3390/jcm11133776
Chicago/Turabian StyleFernández Alba, Juan Jesús, María Castillo Lara, José Manuel Jiménez Heras, Rocío Moreno Cortés, Carmen González Macías, Ángel Vilar Sánchez, Florentino Carral San Laureano, and Luis Javier Moreno Corral. 2022. "High First Trimester Levels of TSH as an Independent Risk Factor for Gestational Diabetes Mellitus: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 13: 3776. https://doi.org/10.3390/jcm11133776
APA StyleFernández Alba, J. J., Castillo Lara, M., Jiménez Heras, J. M., Moreno Cortés, R., González Macías, C., Vilar Sánchez, Á., San Laureano, F. C., & Moreno Corral, L. J. (2022). High First Trimester Levels of TSH as an Independent Risk Factor for Gestational Diabetes Mellitus: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(13), 3776. https://doi.org/10.3390/jcm11133776






