Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age–Period–Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analysis
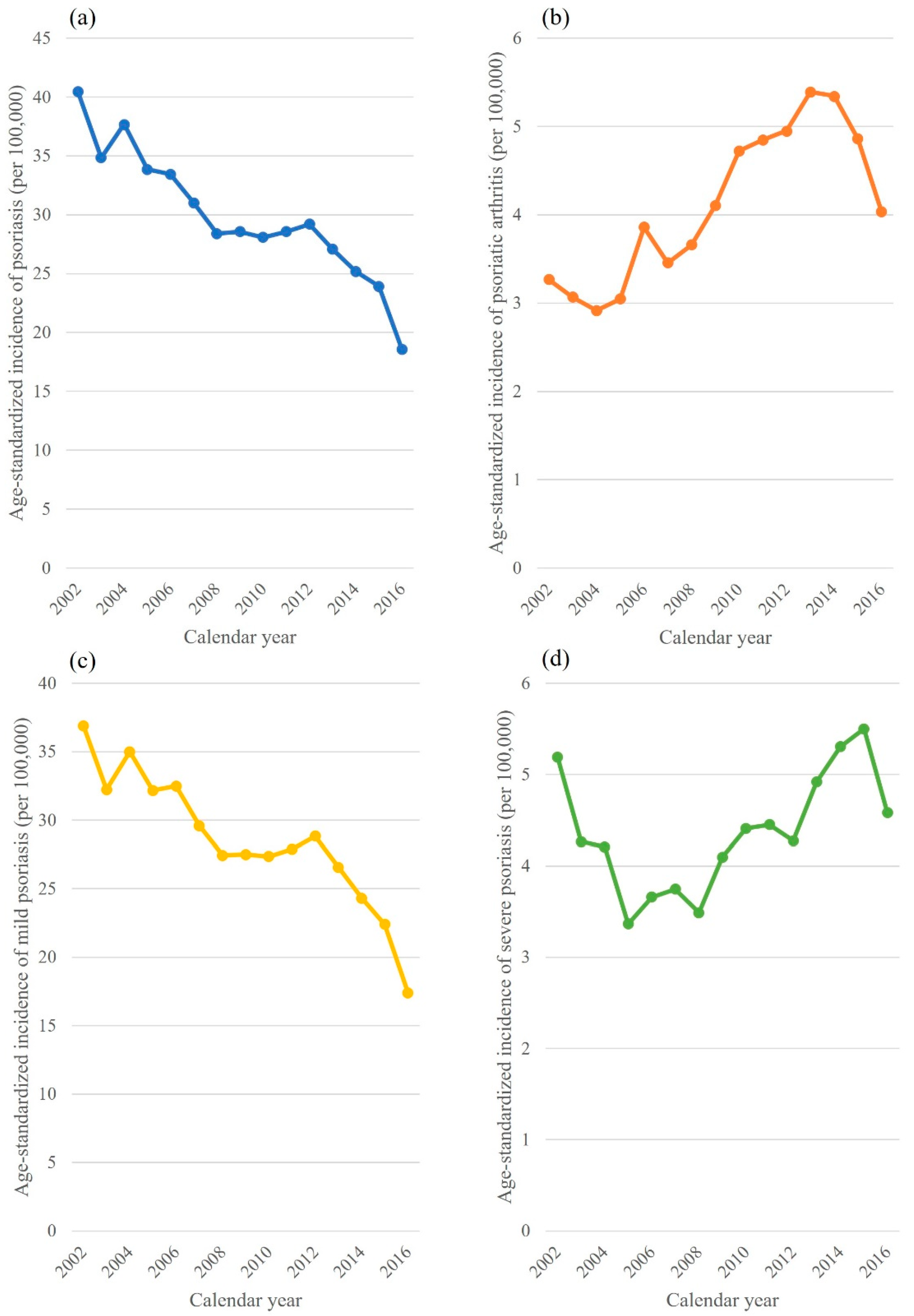
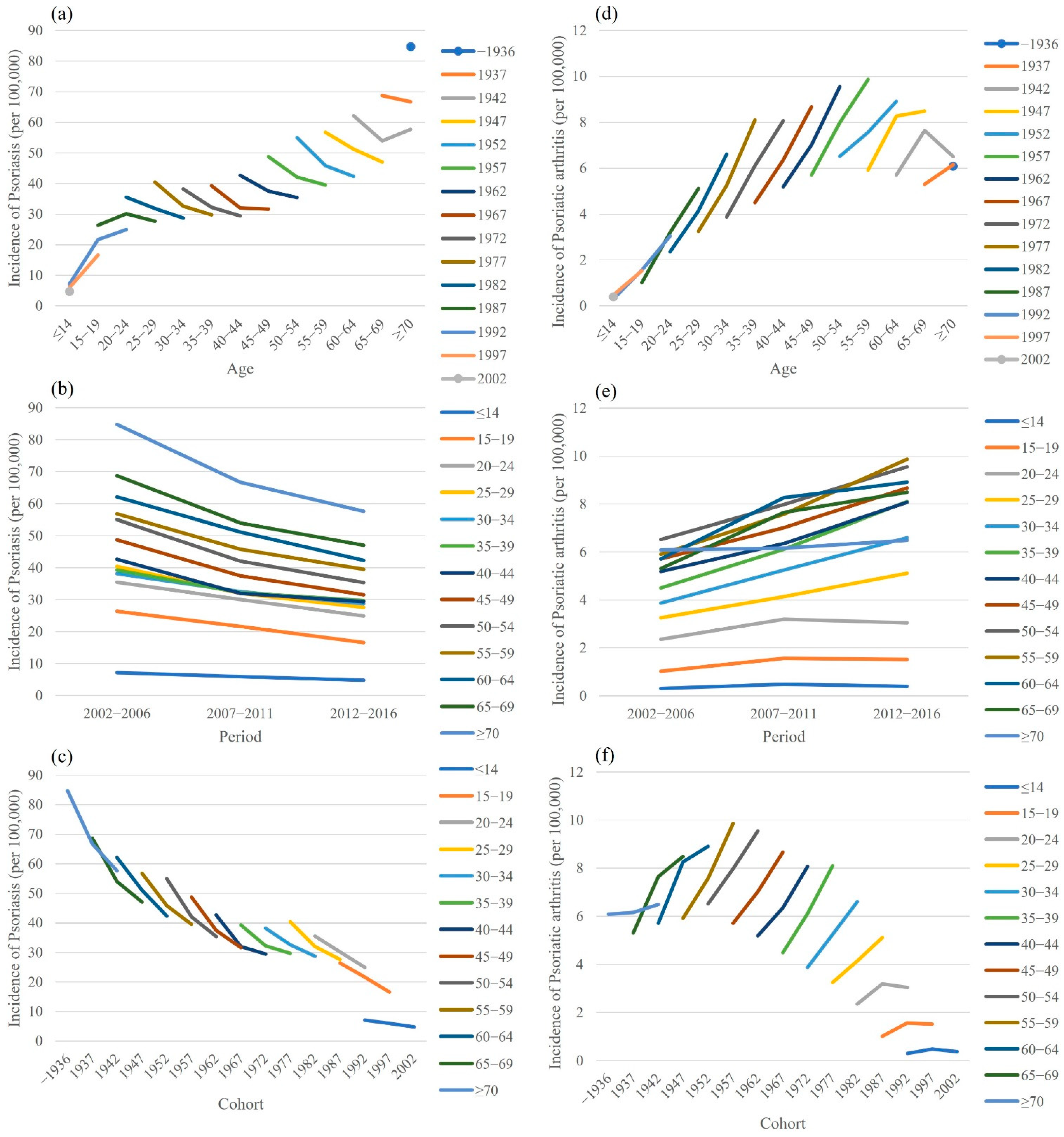
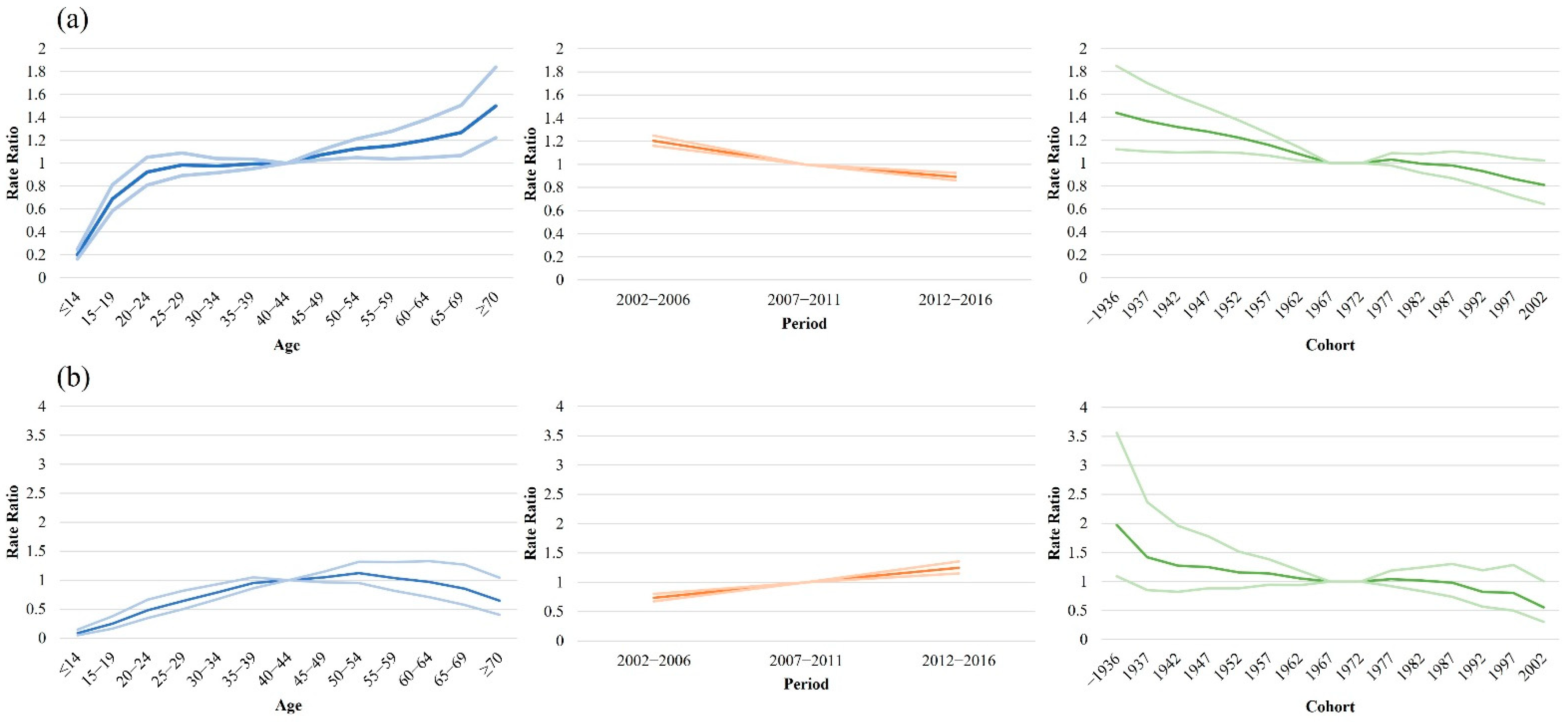
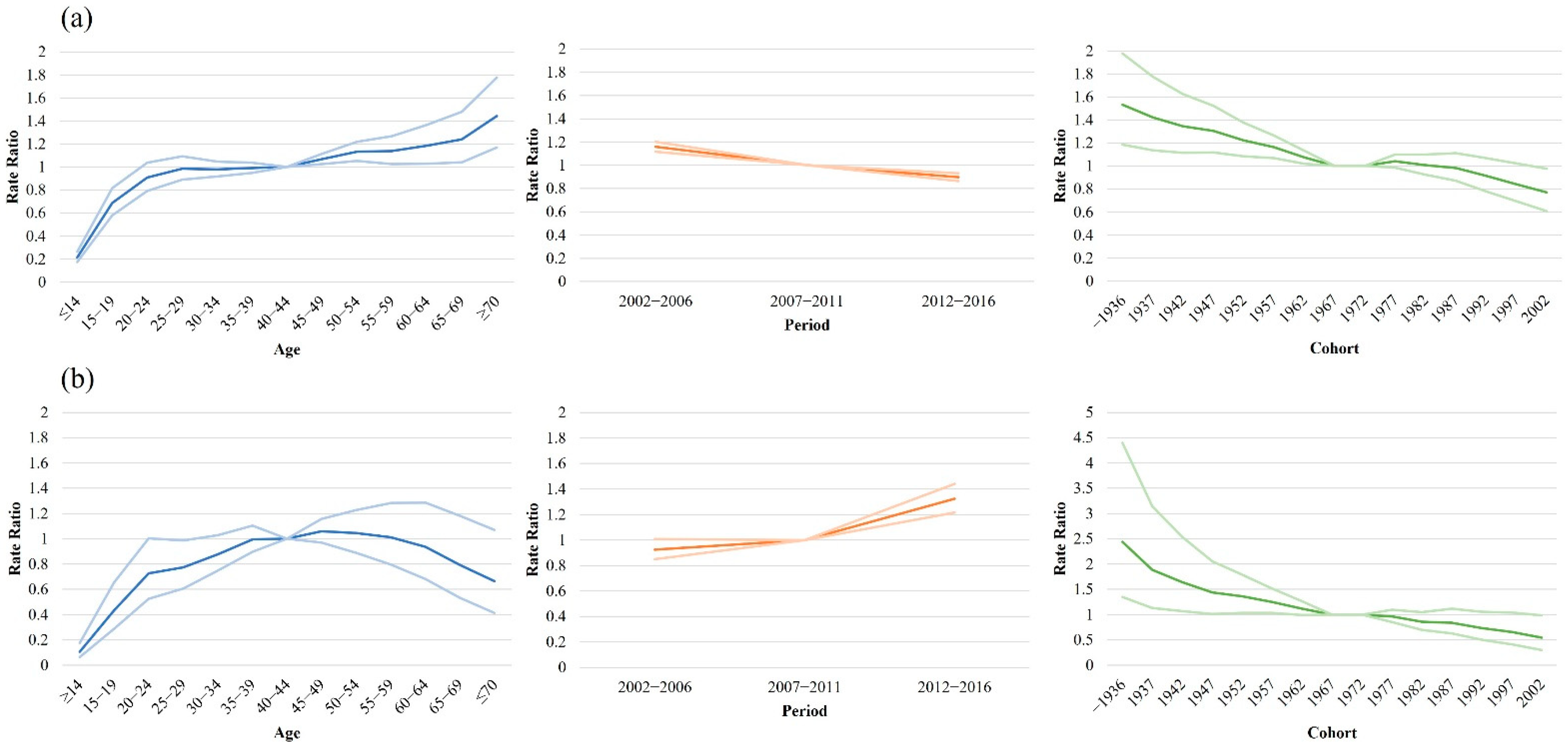
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Sterry, W.; Strober, B.; Menter, A.; On behalf of the International Psoriasis Council. Obesity in psoriasis: The metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br. J. Dermatol. 2007, 157, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Bø, K.; Thoresen, M.; Dalgard, F. Smokers Report More Psoriasis, but Not Atopic Dermatitis or Hand Eczema: Results from a Norwegian Population Survey among Adults. Dermatology 2007, 216, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.P.; Farber, E.M. The prevalence of psoriasis in the world. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 16–17. [Google Scholar] [CrossRef]
- Tsai, T.-F.; Wang, T.-S.; Hung, S.-T.; Tsai, P.I.-C.; Schenkel, B.; Zhang, M.; Tang, C.-H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011, 63, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.-C.; Shi, L.-H.; Huang, J.-Y.; Wu, X.-F.; Wu, R.; Chiou, J.-Y. Epidemiology and Medication Pattern Change of Psoriatic Diseases in Taiwan from 2000 to 2013: A Nationwide, Population-based Cohort Study. J. Rheumatol. 2018, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-Y.; Wang, T.-S.; Chen, P.-H.; Hsu, S.-H.; Tsai, Y.-C.; Tsai, T.-F. Psoriasis in Taiwan: From epidemiology to new treatments. Dermatol. Sin. 2018, 36, 115–123. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N.W.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Chiu, H.; Huang, P.-Y.; Jee, S.-H.; Hu, C.-Y.; Chou, C.-T.; Chang, Y.-T.; Hwang, C.-Y.; Tsai, T.-F. HLA polymorphism among Chinese patients with chronic plaque psoriasis: Subgroup analysis. Br. J. Dermatol. 2011, 166, 288–297. [Google Scholar] [CrossRef]
- Cantarutti, A.; Donà, D.; Visentin, F.; Borgia, E.; Scamarcia, A.; Cantarutti, L.; Peruzzi, E.; Egan, C.G.; Villa, M.; Giaquinto, C.; et al. Epidemiology of Frequently Occurring Skin Diseases in Italian Children from 2006 to 2012: A Retrospective, Population-Based Study. Pediatr. Dermatol. 2015, 32, 668–678. [Google Scholar] [CrossRef]
- Springate, D.; Parisi, R.; Kontopantelis, E.; Reeves, D.; Griffiths, C.; Ashcroft, D. Incidence, prevalence and mortality of patients with psoriasis: A U.K. population-based cohort study. Br. J. Dermatol. 2016, 176, 650–658. [Google Scholar] [CrossRef] [PubMed]
- A Vena, G.; Altomare, G.; Ayala, F.; Berardesca, E.; Calzavara-Pinton, P.; Chimenti, S.; Giannetti, A.; Girolomoni, G.; Lotti, T.; Martini, P.; et al. Incidence of psoriasis and association with comorbidities in Italy: A 5-year observational study from a national primary care database. Eur. J. Dermatol. 2010, 20, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Kim, D.K.; Doo, S.W.; Yang, W.J.; Song, Y.S.; Lee, B.; Kim, J.H. Time Trends for Prostate Cancer Incidence from 2003 to 2013 in South Korea: An Age-Period-Cohort Analysis. Cancer Res. Treat. 2020, 52, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Jee, Y.H. Age-Period-Cohort Analysis of Liver Cancer Mortality in Korea. Asian Pac. J. Cancer Prev. 2015, 16, 8589–8594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reither, E.N.; Hauser, R.M.; Yang, Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc. Sci. Med. 2009, 69, 1439–1448. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.-C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Lee, M.-S.; Yeh, Y.-C.; Chang, Y.-T.; Lai, M.-S. All-Cause and Cause-Specific Mortality in Patients with Psoriasis in Taiwan: A Nationwide Population-Based Study. J. Investig. Dermatol. 2017, 137, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Clayton, D.; Schifflers, E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat. Med. 1987, 6, 449–467. [Google Scholar] [CrossRef]
- Clayton, D.; Schifflers, E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat. Med. 1987, 6, 469–481. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tsai, S.; Lee, D.; Shiao, Y.; Huang, C.; Liu, H.; Wang, W.; Wong, C. A study of candidate genes for psoriasis near HLA-C in Chinese patients with psoriasis. Br. J. Dermatol. 2003, 148, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, I.; Parisi, R.; Griffiths, C.; Ashcroft, D.; The Global Psoriasis Atlas. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br. J. Dermatol. 2020, 184, 243–258. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Carrascosa, J.; Toro, M. Prevalence of Psoriasis in Spain in the Age of Biologics. Actas Dermosifiliogr. 2014, 105, 504–509. [Google Scholar] [CrossRef]
- Schonmann, Y.; Ashcroft, D.M.; Iskandar, I.Y.K.; Parisi, R.; Sde-Or, S.; Comaneshter, D.; Batat, E.; Shani, M.; Vinker, S.; Griffiths, C.E.; et al. Incidence and prevalence of psoriasis in Israel between 2011 and 2017. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Chen, T.J.; Liu, P.C.; Chen, Y.C.; Chen, Y.J.; Huang, Y.L.; Jih, J.S.; Chen, C.C.; Lee, D.D.; Wang, W.J.; et al. Epidemiological Study of Psoriasis in the National Health Insurance Database in Taiwan. Acta Derm. Venereol. 2009, 89, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Han, K.D.; Lee, J.H. Bodyweight variability and the risk of psoriasis: A nationwide population-based cohort study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1019–1025. [Google Scholar] [CrossRef]
- Kim, H.-N.; Han, K.; Song, S.-W.; Lee, J.H. Hypertension and risk of psoriasis incidence: An 11-year nationwide population-based cohort study. PLoS ONE 2018, 13, e0202854. [Google Scholar] [CrossRef]
- Kim, E.S.; Han, K.; Kim, M.K.; Park, Y.-M.; Baek, K.-H.; Moon, S.D.; Han, J.-H.; Song, K.-H.; Kwon, H.-S. Impact of metabolic status on the incidence of psoriasis: A Korean nationwide cohort study. Sci. Rep. 2017, 7, 1989. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.-H.; Lee, J.J.; Yu, E.W.-R.; Hu, H.-Y.; Lin, S.-Y.; Ho, C.-Y. Association between obesity and education level among the elderly in Taipei, Taiwan between 2013 and 2015: A cross-sectional study. Sci. Rep. 2020, 10, 20285. [Google Scholar] [CrossRef] [PubMed]
- Directorate-General of Budget, Accounting and Statistics, Executive Yuan, R.O.C. (Taiwan). Available online: https://www.stat.gov.tw/public/Data/169152483HCL2D3O.pdf (accessed on 9 June 2021).
- Armstrong, A.W.; Robertson, A.D.; Wu, J.; Schupp, C.; Lebwohl, M.G. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: Findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013, 149, 1180–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stem, R.S. Utilization of outpatient care for psoriasis. J. Am. Acad. Dermatol. 1996, 35, 543–545. [Google Scholar] [PubMed]
- Wilson, F.C.; Icen, M.; Crowson, C.S.; McEvoy, M.T.; Gabriel, S.E.; Kremers, H.M. Time Trends in Epidemiology and Characteristics of Psoriatic Arthritis over 3 Decades: A Population-based Study. J. Rheumatol. 2009, 36, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeberg, A.; Kristensen, L.E.; Thyssen, J.P.; Gislason, G.; Gottlieb, A.B.; Coates, L.C.; Jullien, D.; Gisondi, P.; Gladman, D.D.; Skov, L.; et al. Incidence and prevalence of psoriatic arthritis in Denmark: A nationwide register linkage study. Ann. Rheum. Dis. 2017, 76, 1591–1597. [Google Scholar] [CrossRef]
- Wang, T.-S.; Hsieh, C.-F.; Tsai, T.-F. Epidemiology of psoriatic disease and current treatment patterns from 2003 to 2013: A nationwide, population-based observational study in Taiwan. J. Dermatol. Sci. 2016, 84, 340–345. [Google Scholar] [CrossRef]
- Merola, J.F.; Tian, H.; Patil, D.; Richardson, C.; Scott, A.; Chen, Y.-H.; Kim, N.; Hur, P.; Armstrong, A.W. Incidence and prevalence of psoriatic arthritis in patients with psoriasis stratified by psoriasis disease severity: Retrospective analysis of an electronic health records database in the United States. J. Am. Acad. Dermatol. 2021, 86, 748–757. [Google Scholar] [CrossRef]
- Karmacharya, P.; Wright, K.; Achenbach, S.J.; Crowson, C.S.; Ogdie, A.; Bekele, D.; Duarte-García, A.; Ernste, F.C.; Tollefson, M.M.; Iii, J.M.D. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin. Arthritis Rheum. 2021, 52, 151949. [Google Scholar] [CrossRef]
- Ogdie, A. The preclinical phase of PsA: A challenge for the epidemiologist. Ann. Rheum. Dis. 2017, 76, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- Umberson, D.; Crosnoe, R.; Reczek, C. Social Relationships and Health Behavior Across the Life Course. Annu. Rev. Sociol. 2010, 36, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Wethington, E. An Overview of the Life Course Perspective: Implications for Health and Nutrition. J. Nutr. Educ. Behav. 2005, 37, 115–120. [Google Scholar] [CrossRef]
- Yang, Y. Trends in U.S. adult chronic disease mortality, 1960–1999: Age, period, and cohort variations. Demography 2008, 45, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.K.; Syme, S.L. Education: A Missed Opportunity for Public Health Intervention. Am. J. Public Health 2013, 103, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Rosenberg, P.S.; Chen, W.-Q.; Hartman, M.; Lim, W.-Y.; Chia, K.S.; Mang, O.W.-K.; Chiang, C.-J.; Kang, D.; Ngan, R.K.-C.; et al. Female Breast Cancer Incidence Among Asian and Western Populations: More Similar Than Expected. JNCI J. Nat. Cancer Inst. 2015, 107, djv107. [Google Scholar] [CrossRef] [Green Version]
- Chandran, V.; Raychaudhuri, S.P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J. Autoimmun. 2010, 34, J314–J321. [Google Scholar] [CrossRef]
- Lopes, N.; Dias, L.L.S.; Azulay-Abulafia, L.; Oyafuso, L.K.M.; Suarez, M.V.; Fabricio, L.; Kobata, C.M.; Cestari, T.; Gontijo, B.; Sabbag, C.Y.; et al. Humanistic and Economic Impact of Moderate to Severe Plaque Psoriasis in Brazil. Adv. Ther. 2019, 36, 2849–2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häbel, H.; Wettermark, B.; Hägg, D.; Villacorta, R.; Wennerström, E.M.; Linder, M. Societal impact for patients with psoriasis: A nationwide Swedish register study. JAAD Int. 2021, 3, 63–75. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Skov, L.; Dodge, R.; Hedegaard, M.S.; Kjellberg, J. Socioeconomic Costs and Health Inequalities from Psoriasis: A Cohort Study. Dermatology 2019, 235, 372–379. [Google Scholar] [CrossRef]
- Kristensen, L.E.; Jørgensen, T.S.; Christensen, R.; Gudbergsen, H.; Dreyer, L.; Ballegaard, C.; Jacobsson, L.T.H.; Strand, V.; Mease, P.J.; Kjellberg, J. Societal costs and patients’ experience of health inequities before and after diagnosis of psoriatic arthritis: A Danish cohort study. Ann. Rheum. Dis. 2017, 76, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Raciborski, F.; Śliwczyński, A.; Kłak, A.; Kwiatkowska, B.; Brzozowska, M.; Tłustochowicz, M. Prevalence of psoriatic arthritis and costs generated by treatment of psoriatic arthritis patients in the public health system–the case of Poland. Rheumatology 2016, 54, 278–284. [Google Scholar] [CrossRef]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2014, 74, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Petrie, M.; O’Rourke, K.S.; Feldman, S.R. Rheumatologists’ recommendations on what to do in the dermatology office to evaluate and manage psoriasis patients’ joint symptoms. J. Dermatolog. Treat. 2009, 20, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Kanekura, T.; Kawabata, H.; Maruyama, I.; Kanzaki, T. Treatment of psoriatic arthritis with granulocyte and monocyte adsorption apheresis. J. Am. Acad. Dermatol. 2004, 50, 242–246. [Google Scholar] [CrossRef]
- Marchetti, G.; Vittori, A.; Mascilini, I.; Francia, E.; Picardo, S.G. Acupuncture for pain management in pediatric psoriatic arthritis: A case report. Acupunct. Med. 2020, 38, 440–442. [Google Scholar] [CrossRef] [PubMed]
| Psoriasis | Psoriatic Arthritis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Population | Incident Case | Incidence Rate * | Prevalent Case | Prevalence Rate * | Incident Case | Incidence Rate * | Prevalent Case | Prevalence Rate * |
| 2002 | 21,598,394 | 9358 | 43.33 | 29,049 | 134.50 | 771 | 3.57 | 1824 | 8.45 |
| 2003 | 21,452,868 | 8147 | 37.98 | 30,457 | 141.97 | 735 | 3.43 | 2064 | 9.62 |
| 2004 | 21,545,933 | 8880 | 41.21 | 33,702 | 156.42 | 713 | 3.31 | 2278 | 10.57 |
| 2005 | 21,369,558 | 7990 | 37.39 | 35,132 | 164.40 | 747 | 3.50 | 2479 | 11.60 |
| 2006 | 21,637,563 | 8080 | 37.34 | 36,759 | 169.89 | 956 | 4.42 | 2908 | 13.44 |
| 2007 | 21,816,951 | 7596 | 34.82 | 38,283 | 175.47 | 874 | 4.01 | 3109 | 14.25 |
| 2008 | 21,993,473 | 7070 | 32.15 | 38,786 | 176.35 | 940 | 4.27 | 3455 | 15.71 |
| 2009 | 22,116,920 | 7232 | 32.70 | 40,329 | 182.34 | 1067 | 4.82 | 3957 | 17.89 |
| 2010 | 22,176,505 | 7245 | 32.67 | 41,603 | 187.60 | 1235 | 5.57 | 4534 | 20.45 |
| 2011 | 22,263,419 | 7497 | 33.67 | 43,870 | 197.05 | 1318 | 5.92 | 5141 | 23.09 |
| 2012 | 22,362,328 | 7697 | 34.42 | 46,345 | 207.25 | 1350 | 6.04 | 5740 | 25.67 |
| 2013 | 22,477,022 | 7240 | 32.21 | 48,087 | 213.94 | 1503 | 6.69 | 6514 | 28.98 |
| 2014 | 22,564,915 | 6896 | 30.56 | 48,692 | 215.79 | 1525 | 6.76 | 7224 | 32.01 |
| 2015 | 22,633,061 | 6687 | 29.55 | 49,797 | 220.02 | 1383 | 6.11 | 7702 | 34.03 |
| 2016 | 22,692,606 | 5250 | 23.14 | 50,248 | 221.43 | 1184 | 5.22 | 7625 | 33.60 |
| Average rate | 34.21 | 184.29 | 4.91 | 19.96 | |||||
| APC | −2.80 | 3.40 | 6.80 | 11.00 | |||||
| 95% CI | −3.7 to −2 | 3 to 3.8 | 5.4 to 8.2 | 10.2 to 11.8 | |||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Models | df | Deviance | Likelihood-Ratio Statistic (df *) | p-Value |
|---|---|---|---|---|
| Psoriasis | ||||
| Age | 26 | 2769.396 | 2749.1268 (15) | <0.0001 |
| Period | 36 | 32,449.15 | 32,428.8771 (25) | <0.0001 |
| Cohort | 24 | 3005.044 | 2984.7749 (13) | <0.0001 |
| Age–Period | 24 | 72.3037 | 52.0346 (13) | <0.0001 |
| Age–Cohort | 12 | 51.2918 | 31.0227 (1) | <0.0001 |
| Period–Cohort | 22 | 2824.326 | 2804.0565 (11) | <0.0001 |
| Age–Period–Cohort | 11 | 20.2691 | reference | reference |
| Psoriatic arthritis | ||||
| Age | 26 | 511.265 | 492.4695 (15) | <0.0001 |
| Period | 36 | 6474.931 | 6456.1351 (25) | <0.0001 |
| Cohort | 24 | 1553.344 | 1534.5484 (13) | <0.0001 |
| Age–Period | 24 | 68.0293 | 49.2338 (13) | 0.0002 |
| Age–Cohort | 12 | 25.0532 | 6.2577 (1) | <0.0001 |
| Period–Cohort | 22 | 444.6162 | 425.8207 (11) | <0.0001 |
| Age–Period–Cohort | 11 | 18.7955 | reference | reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Wu, C.-Y.; Li, Y.-L.; Chen, L.-Y.; Chiou, H.-Y. Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age–Period–Cohort Analysis. J. Clin. Med. 2022, 11, 3744. https://doi.org/10.3390/jcm11133744
Chen Y-T, Wu C-Y, Li Y-L, Chen L-Y, Chiou H-Y. Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age–Period–Cohort Analysis. Journal of Clinical Medicine. 2022; 11(13):3744. https://doi.org/10.3390/jcm11133744
Chicago/Turabian StyleChen, Yu-Tsung, Chih-Yi Wu, Yu-Ling Li, Li-Ying Chen, and Hung-Yi Chiou. 2022. "Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age–Period–Cohort Analysis" Journal of Clinical Medicine 11, no. 13: 3744. https://doi.org/10.3390/jcm11133744
APA StyleChen, Y.-T., Wu, C.-Y., Li, Y.-L., Chen, L.-Y., & Chiou, H.-Y. (2022). Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age–Period–Cohort Analysis. Journal of Clinical Medicine, 11(13), 3744. https://doi.org/10.3390/jcm11133744






