Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Participants and Study Protocol
2.2. Cardiovascular Autonomic Function Test
2.3. Statistical Analysis
3. Results
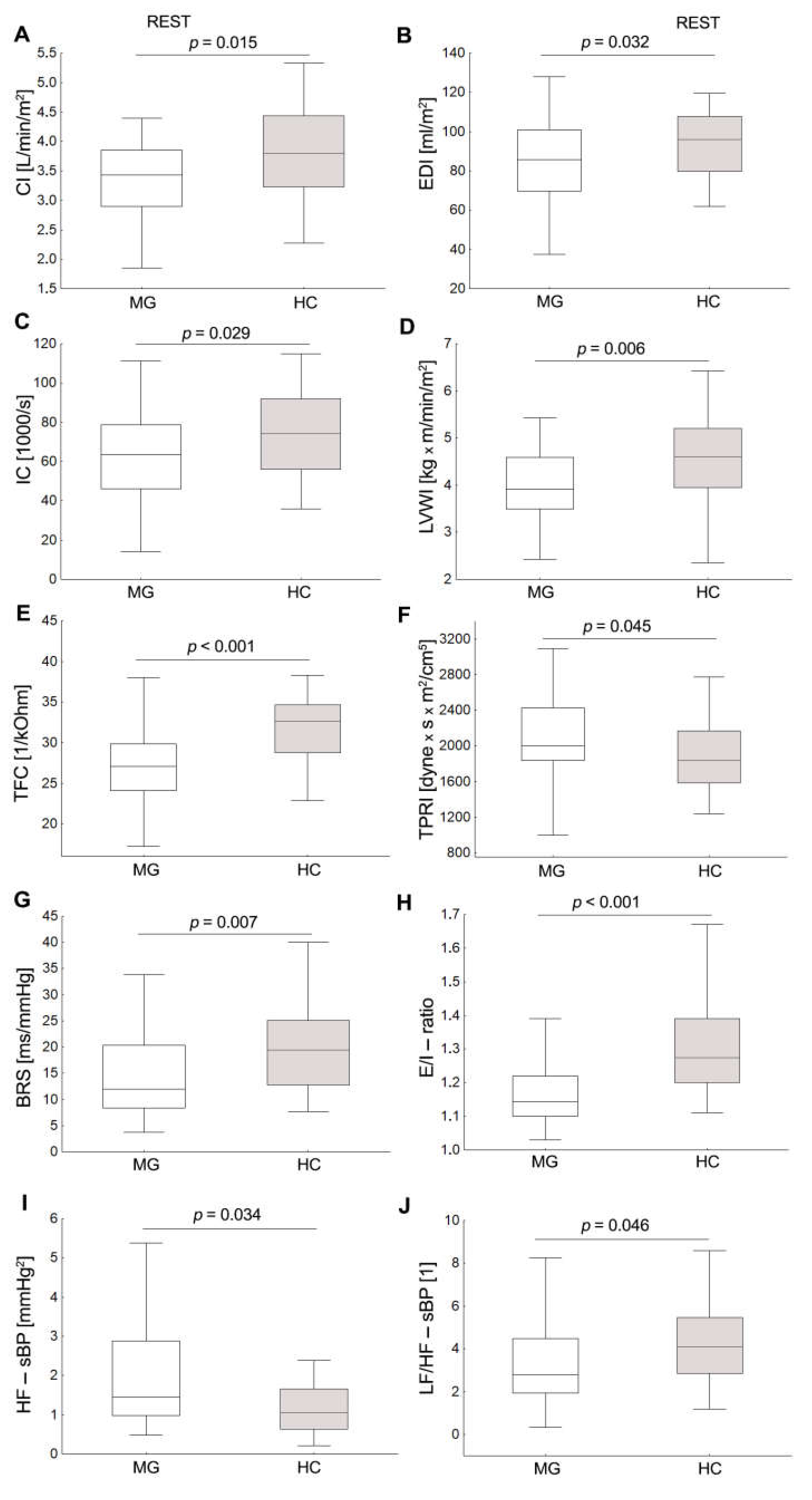
3.1. Baseline Hemodynamic and Autonomic Data
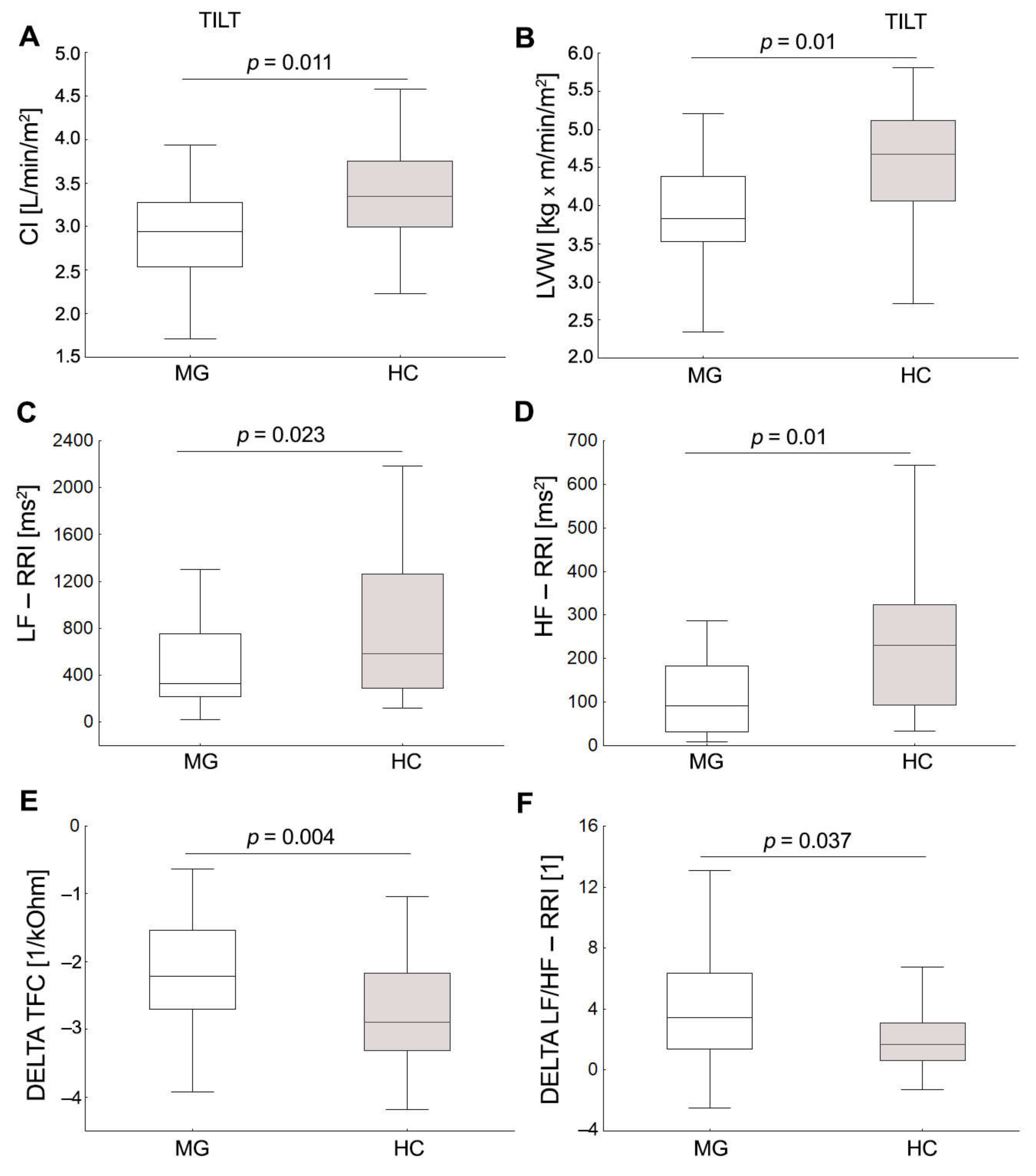
3.2. Cardiovascular Autonomic Function Test
3.3. Relationship between Clinical and Demographic Features of MG and Cardiac Autonomic, Hemodynamic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflamm. 2017, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- De Meel, R.H.; Tannemaat, M.R.; Verschuuren, J.J.G.M. Heterogeneity and shifts in distribution of muscle weakness in myasthenia gravis. Neuromuscul. Disord. 2019, 29, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, L.D.; Homedes, C.; Alberti, M.A.; Santamaria, V.V.; Casasnovas, C. Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales. J. Clin. Med. 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Helgeland, G.; Luckman, S.P.; Romi, F.R.; Jonassen, A.K.; Gilhus, N.E. Myasthenia gravis sera have no effect on cardiomyocytes in vitro. J. Neuroimmunol. 2008, 201, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Shivamurthy, P.; Parker, M.W. Cardiac manifestations of myasthenia gravis: A systematic review. IJC Metab. Endocr. 2014, 5, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.F.; Nacif, S.R.; Pereira, A.N.; Fonseca, N.T.; Urbano, J.J.; Perez, E.A.; Cavalcante, V.; Oliveira, C.S.; Insalaco, G.; Oliveira, A.S.; et al. Sleep disorders in patients with myasthenia gravis: A systematic review. J. Phys. Ther. Sci. 2015, 27, 2013–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaus, B.; Müller, P.; van Wickeren, N.; Dordevic, M.; Schmicker, M.; Zdunczyk, Y.; Brigadski, T.; Leßmann, V.; Vielhaber, S.; Schreiber, S.; et al. Structural and functional brain alterations in patients with myasthenia gravis. Brain Commun. 2022, 4, fcac018. [Google Scholar] [CrossRef]
- Hamed, S.A. Comorbid nervous system manifestations and disorders with myasthenia gravis: Evidences and possible mechanisms. J. Neurol. 2012, 3, 3. [Google Scholar]
- Nikolić, A.; Perić, S.; Nišić, T.; Popović, S.; Ilić, M.; Stojanović, V.R.; Lavrnić, D. The presence of dysautonomia in different subgroups of myasthenia gravis patients. J. Neurol. 2014, 261, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Hopkins, S.; Wang, Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton. Neurosci. 2009, 146, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, L.; Barop, H.; Ludin, S.B.; Schaible, H.-G. Regulation of acute reflectory hyperinflammation in viral and other diseases by means of stellate ganglion block. A conceptual view with a focus on COVID-19. Auton. Neurosci. 2022, 237, 102903. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.T.; Wei, J.C.; Hu, K.C.; Hung, Y.M.; Chou, M.C.; Chang, R. Risk of subsequent atrial fibrillation in patients with myasthenia gravis: A population-based cohort study. Medicine 2021, 100, e26008. [Google Scholar] [CrossRef]
- Peric, S.; Rakocevic-Stojanovic, V.; Nisic, T.; Pavlovic, S.; Basta, I.; Popovic, S.; Damjanovic, S.; Lavrnic, D. Cardiac autonomic control in patients with myasthenia gravis and thymoma. J. Neurol. Sci. 2011, 307, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, Z.U.; Kizilay, F.; Basarici, I.; Uysal, H. Evaluation of cardiac autonomic functions in myasthenia gravis. Neurol. Res. 2018, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Rzepiński, Ł.; Zawadka-Kunikowska, M.; Newton, J.L.; Zalewski, P. Cardiac Autonomic Dysfunction in Myasthenia Gravis and Relapsing-Remitting Multiple Sclerosis-A Pilot Study. J. Clin. Med. 2021, 10, 2173. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Ochoa, J.E.; Bilo, G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr. Hypertens. Rep. 2012, 14, 421–431. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Sever, P.S.; Poulter, N.R. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010, 375, 895–905. [Google Scholar] [CrossRef]
- Shukla, G.; Gupta, S.; Goyal, V.; Singh, S.; Srivastava, A.; Behari, M. Abnormal sympathetic hyper-reactivity in patients with myasthenia gravis: A prospective study. Clin. Neurol. Neurosurg. 2013, 115, 179–186. [Google Scholar] [CrossRef]
- Benjamin, R.N.; Aaron, S.; Sivadasan, A.; Devasahayam, S.; Sebastin, A.; Alexander, M. The Spectrum of Autonomic Dysfunction in Myasthenic Crisis. Ann. Indian Acad. Neurol. 2018, 21, 42–48. [Google Scholar]
- Stoica, E.; Enulescu, O. Deficiency of sympathetic nervous system function in myasthenia. J. Auton. Nerv. Syst. 1992, 38, 69–76. [Google Scholar] [CrossRef]
- Lechin, F.; Van Der Dijs, B.; Pardey-Maldonado, B.; John, E.; Jimenez, V.; Orozco, B.; Baez, S.; Lechin, M.E. Enhancement of noradrenergic neural transmission: An effective therapy of myasthenia gravis: A report on 52 consecutive patients. J. Med. 2000, 31, 333–361. [Google Scholar] [PubMed]
- Owe, F.J.; Davidson, E.S.; Eide, G.E. Left ventricular long axis function in myasthenia gravis. J. Neurol. 2008, 255, 1777–1784. [Google Scholar]
- Johannessen, K.A.; Mygland, A.; Gilhus, N.E.; Aarli, J.; Vik-Mo, H. Left ventricular function in myasthenia gravis. Am. J. Cardiol. 1992, 69, 129–132. [Google Scholar] [CrossRef]
- Kato, T.; Hirose, S.; Kumagai, S.; Ozaki, A.; Matsumoto, S.; Inoko, M. Electrocardiography as the First Step for the Further Examination of Cardiac Involvement in Myasthenia Gravis. BioMed Res. Int. 2016, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Puneeth, C.S.; Chandra, S.R.; Yadav, R.; Sathyaprabha, T.N.; Chandran, S. Heart rate and blood pressure variability in patients with myasthenia gravis. Ann. Indian Acad. Neurol. 2013, 16, 329–332. [Google Scholar]
- Parrott, C.W.; Burnham, K.M.; Quale, C.; Lewis, D.L. Comparison of changes in ejection fraction to changes in impedance cardiography cardiac index and systolic time ratio. Congest. Heart Fail. 2004, 10, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.; Herbelin, L.; Dimachkie, M.M.; Barohn, R.J. Measuring Clinical Treatment Response in Myasthenia Gravis. Neurol. Clin. 2018, 36, 339–353. [Google Scholar] [CrossRef]
- Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology 2000, 55, 16–23. [Google Scholar]
- Fortin, J.; Klinger, T.; Wagner, C.; Sterner, H.; Madritsch, C.; Grüllenberger, R.; Hacker, A.; Habenbacher, W.; Skrabal, F. The task force monitor—A non-invasive beat-to beat monitor for hemodynamic and autonomic function of the human body. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 1 November 1998; Volume 66, pp. 63–151. [Google Scholar]
- Bianchi, A.M.; Mainardi, L.; Meloni, C.; Chierchiu, S.; Cerutti, S. Continuous monitoring of the sympatho-vagal balance through spectral analysis. IEEE Eng. Med. Biol. Mag. 1997, 16, 64–73. [Google Scholar] [CrossRef]
- Parati, G.; Di Rienzo, M.; Mancia, G. How to measure baroreflex sensitivity: From the cardiovascular laboratory to daily life. J. Hypertens. 2000, 18, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilz, M.L.; Dütsch, M. Quantitative studies of autonomic function. Muscle Nerve 2006, 33, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Taskforce of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement. physiological interpretation. And clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment clinical autonomic testing report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology 1996, 46, 873–880. [Google Scholar]
- NIST/SEMATECH e-Handbook of Statistical Methods. Available online: http://www.itl.nist.gov/div898/handbook/.2012/01/20 (accessed on 21 August 2020).
- Kuo, T.B.; Yang, C.C.; Chan, S.H. Transfer function analysis of ventilatory influence on systemic arterial pressure in the rat. Am. J. Physiol. 1996, 271, H2108–H2115. [Google Scholar] [CrossRef]
- Barboza, C.A.; Fukushima, A.R.; Carrozzi, N.; Machi, J.F.; Dourado, P.M.M.; Mostarda, C.T.; Irigoyen, M.C.; Nathanson, L.; Morris, M.; Caperuto, E.C.; et al. Cholinergic Stimulation by Pyridostigmine Bromide Before Myocardial Infarction Prevent Cardiac and Autonomic Dysfunction. Sci. Rep. 2019, 9, 2481. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.H.; Gastaldi, A.C.; Gardim, C.B.; Araujo, J.E.; Simões, M.V.; Oliveira, L.F.; Carvalho, E.E.; Souza, H.C. Chronic cholinergic stimulation promotes changes in cardiovascular autonomic control in spontaneously hypertensive rats. Auton. Neurosci. 2015, 193, 97–103. [Google Scholar] [CrossRef]
- Gardim, C.B.; Veiga, A.C.; Aguilar, B.A.; Philbois, S.V.; Souza, H.C.D. Effects of chronic cholinergic stimulation associated with aerobic physical training on cardiac morphofunctional and autonomic parameters in spontaneously hypertensive rats. Sci. Rep. 2021, 11, 17141. [Google Scholar] [CrossRef]
- Adamec, I.; Crnošija, L.; Junaković, A.; Skorić, M.K.; Habek, M. Progressive multiple sclerosis patients have a higher burden of autonomic dysfunction compared to relapsing remitting phenotype. Clin. Neurophysiol. 2018, 129, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 2449. [Google Scholar] [CrossRef]
- Alston, E.N.; Parrish, D.C.; Hasan, W.; Tharp, K.; Pahlmeyer, L.; Habecker, B.A. Cardiac ischemia-reperfusion regulates sympathetic neuropeptide expression through gp130-dependent and independent mechanisms. Neuropeptides 2011, 45, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Vega Costa, K.P.; Gómez Perez, M.A.; Roqueta, C.; Fischer, L. Effects on hemodynamic variables and echocardiographic parameters after a stellate ganglion block in 15 healthy volunteers. Auton. Neurosci. 2016, 197, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Rice, M.W.; Cai, D. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E32–E41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Ma, N.; Yu, B.; Zhang, W.; Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflammation 2020, 17, 97. [Google Scholar] [CrossRef] [Green Version]
- Zehravi, M.; Kabir, J.; Akter, R.; Malik, S.; Ashraf, G.M.; Tagde, P.; Ramproshad, S.; Mondal, B.; Rahman, M.H.; Mohan, A.G.; et al. A Prospective Viewpoint on Neurological Diseases and Their Biomarkers. Molecules 2022, 27, 3516. [Google Scholar] [CrossRef]
- Tooba, R.; Mayuga, K.A.; Wilson, R.; Tonelli, A.R. Dyspnea in Chronic Low Ventricular Preload States. Ann. Am. Thorac. Soc. 2021, 18, 573–581. [Google Scholar] [CrossRef]
- Oldham, W.M.; Lewis, G.D.; Opotowsky, A.R.; Waxman, A.B.; Systrom, D.M. Unexplained exertional dyspnea caused by low ventricular filling pressures: Results from clinical invasive cardiopulmonary exercise testing. Pulm. Circ. 2016, 6, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Censi, F.; Calcagnini, G.; Lino, S.; Seydnejad, S.R.; Kitney, R.I.; Cerutti, S. Transient phase locking patterns among respiration, heart rate and blood pressure during cardiorespiratory synchronisation in humans. Med. Biol. Eng. Comput. 2000, 38, 416–426. [Google Scholar] [CrossRef]
- Censi, F.; Calcagnini, G.; Strano, S.; Bartolini, P.; Barbaro, V. Nonlinear Coupling Among Heart Rate, Blood Pressure, and Respiration in Patients Susceptible to Neuromediated Syncope. Ann. Biomed. Eng. 2003, 31, 1097–1105. [Google Scholar] [CrossRef]
- Ishbulatov, Y.M.; Karavaev, A.S.; Kiselev, A.R.; Simonyan, M.A.; Prokhorov, M.D.; Ponomarenko, V.I.; Mironov, S.A.; Gridnev, V.I.; Bezruchko, B.P.; Shvartz, V.A. Mathematical modeling of the cardiovascular autonomic control in healthy subjects during a passive head-up tilt test. Sci. Rep. 2020, 10, 16525. [Google Scholar] [CrossRef] [PubMed]
- Skazkina, V.V.; Krasikova, N.S.; Borovkova, E.I.; Ishbulatov, Y.M.; Gorshkov, A.Y.; Korolev, A.I.; Dadaeva, V.A.; Fedorovich, A.A.; Kuligin, A.V.; Drapkina, O.M.; et al. Synchronization of autonomic control loops of blood circulation in patients with COVID-19. Russ. Open Med. J. 2021, 10, 307. [Google Scholar] [CrossRef]
| MG Patients | HC | p-Value | |
|---|---|---|---|
| Number of subjects | 38 | 30 | |
| Sex (male/female), | 5/33 | 7/23 | 0.274 |
| Age, years, median | 40.5 (19–69) | 35.5 (26–59) | 0.054 |
| Age at first manifestation, median, (years) | 32.0 (12–68) | ||
| Early-onset MG (<50 years) | 35 (92.1%) | ||
| Disease duration (years), median (range) | 5.5 (0.5–24) | ||
| First symptom, n (%) | |||
| Seropositivity to AchR antibodies, n (%) | 23 (60.5%) | ||
| Seropositivity to MuSK antibodies | 0 (0%) | ||
| Type of MG, n (%) | |||
| Ocular | 7 (18.4%) | ||
| Generalized | 31 (81.6%) | ||
| Thymectomy, n (%) | 14 (36.8%) | ||
| Severity of disease at the moment of testing (MGFA, %) | |||
| Class 0 | 0 | ||
| Class I (ocular) | 8 (21.1%) | ||
| Class IIa | 19 (50.0%) | ||
| Class IIIa | 11 (28.9%) | ||
| Histology changes, n (%) | |||
| Thymic pathology | 22 (57.9%) | ||
| Thymoma | 1 (2.7%) | ||
| Type of treatment | |||
| Use of an anticholinesterase | 38 (100%) | ||
| Use of corticosteroids | 23 (60.5%) | ||
| Immunosupressive | 13 (34.2%) |
| Group | MG | HC | MG | HC | MG | HC |
|---|---|---|---|---|---|---|
| Rest | 70° Tilt | Change after 70° Tilt (Delta) | ||||
| Hemodynamic measures | ||||||
| HR [1/min] | 62.5 (59.8,69.7) | 68.8 (61.5,71.1) | 75.6 (69.9,83.6) | 82.2 (75.8,86.9) | 10.4 (6.7,16.9) | 14.3 (10.4,18.1) |
| sBP [mmHg] | 113.0 (106.7,120.2) | 110.0 (103.0,121.2) | 120.2 (116.7,126.6) | 122.8 (117.5,132.9) | 6.0 (1.6,19.4) | 14.3 (6.7,20.0) |
| dBP [mmHg] | 72.6 (67.5,75.9) | 73.7 (66.9,78.0) | 85.7 (78.5,88.9) | 89.9 (83.3,92.9) | 12.4 (7.8–17.0) | 15.8 (8.5,21.2) |
| mBP[mmHg] | 89.7 (85.0,93.9) | 90.9 (81.3,97.7) | 99.1 (95.5,104.8) | 103.8 (97.2,110.3) | 10.6 (5.6,16.0) | 14.4 (8.8,21.1) |
| SI [ml/m2] | 55.3 (44.1,61.1) | 59.2 (51.1,66.0) | 39.0 (34.5,42.5) | 41.2 (36.7,45.6) | −16.2 (−20.3,−9.1) | −17.7 (−22.9,−13.6) |
| CI [l/min/m2] | 3.4 (2.9,3.9) | 3.8 (3.2,4.4) * | 2.9 (2.5,3.3) | 3.3 (3.0,3.8) * | −0.5 (−0.8,−0.2) | −0.6 (−0.8,−0.5) |
| TPRI [dyn × s m2/cm5] | 20004.7 (1838.9–2428.6) | 1838.0(1582.6,2167.8) * | 2627.9 (2365.2,3250.5) | 2458.6 (2136.3,2921.7) | 613.0 (249.6,928.3) | 670.0 (392.8,1004.8) |
| LVWI [kg m/min/m2] | 3.9 (3.5,4.6) | 4.6(4.0,5.2) * | 3.8 (3.5,4.4) | 4.7 (4.1,5.1) * | −0.2 (−0.5,0.2) | 0.0 (−0.5,0.4) |
| IC [1000/s] | 63.7 (46.2,78.6) | 74.3 (56.2,92.0) * | 46.8 (35.9,53.9) | 52.9 (44.0,62.2) | −18.7 (−28.0,−6.9) | −23.9 (−31.1,−17.4) |
| EDI [ml/m2] | 85.8 (69.8,100.7) | 96.1 (79.7,107.6) | 69.8 (61.3,76.5) | 75.6 (67.3,84.2) | −18.6 (−25.7,−8.7) | −21.1 (−28.1,−12.9) |
| TFC [1/kOhm] | 27.1 (24.1,29.9) | 32.6 (28.7,34.6) * | 24.6 (22.6,27.5) | 29.4 (26.6,32.1) | −2.2 (−2.7,−1.5) | −2.9 (−3.3,−2.2) * |
| Heart rate variability (HRV) | ||||||
| LFnu-RRI [ms2] | 61.1 (46.8,73.8) | 58.5 (49.2,65.4) | 82.9 (67.4,89.5) | 76.9 (65.0,81.7) | 17.6 (11.1,29.4) | 12.9 (6.3,22.5) |
| HFnu-RRI [ms2] | 38.9 (26.2,53.2) | 41.5 (34.6,50.8) | 17.1 (10.5,32.6) | 23.1 (18.3,35.0) | −17.6 (−29.4,−11.1) | −12.9 (−22.5,−6.3) |
| LF-RRI [ms2] | 570.3 (310.3,1240.6) | 614.8 (392.4,1117.0) | 331.6 (217.9,755.1) | 585.7 (291.1,1262.7) * | −85.1 (−543.7,30.0) | −78.1 (−220.6,232.2) |
| HF-RRI [ms2] | 289.3 (102.6,1012.5) | 364.0 (198.9,882.1) | 90.9 (32.5,183.7) | 230.8 (92.9,324.4) * | −172.4 (−797.3,−67.1) | −161.5 (−640.6,−49.4) |
| PSD-RRI [ms2] | 1246.8 (709.4,2843.7) | 1420.4 (855.8,2458.8) | 762.9 (403.7,1362.7) | 1080.1 (615.1,2096.2) | −447.7 (−1754.4,−145.9) | −443.5 (−1088.6,−57.8) |
| LF/HF-RRI [1] | 1.7 (0.9,3.0) | 1.5 (1.0,2.0) | 5.2 (2.2,9.4) | 3.4 (1.9,5.0) | 3.4 (1.4,6.3) | 1.7 (0.6,3.1) * |
| LF/HF [1] | 1.1 (0.7,1.8) | 1.1 (0.9,1.5) | 3.0 (1.3,5.5) | 2.5 (1.3,3.6) | 1.8 (0.4,4.2) | 1.2 (0.4,2.5) |
| Systolic Blood pressure variability (SBPV) | ||||||
| LFnu-sBP [%] | 41.2 (31.9,48.1) | 40.2 (35.7,45.8) | 51.1 (41.3,62.2) | 46.5 (38.1,54.7) | 10.1 (4.8,17.1) | 11.0 (1.0,17.6) |
| HFnu-sBP [%] | 12.5 (9.0,19.6) | 10.7 (6.9,15.4) | 15.0 (10.7,20.4) | 13.6 (8.3,18.4) | 1.6 (−0.8,3.7) | 2.1 (−0.0,3.2) |
| LF-sBP [mmHg2] | 4.5 (3.4,8.0) | 3.6 (2.9,7.2) | 4.2 (2.7,6.6) | 3.4 (2.1,6.3) | −0.3 (−1.6,0.4) | −0.4 (−1.6,0.1) |
| HF-sBP [mmHg2] | 1.4 (1.0,2.9) | 1.1 (0.6,1.7)* | 1.1 (0.9,2.3) | 1.0 (0.5,1.6) | −0.2 (−0.5,0.0) | −0.1 (−0.5,0.0) |
| PSD-sBP [mmHg2] | 12.1 (7.8,17.3) | 10.4 (7.7,15.7) | 8.4 (6.3,11.9) | 7.8 (5.7,12.4) | −2.6 (−6.7,−1.1) | −2.6 (−5.2,−1.2) |
| LF/HF-sBP [1] | 2.8 (1.9,4.5) | 4.1 (2.9,5.5) * | 3.3 (2.1,5.0) | 4.0 (2.4,5.2) | 0.0 (−0.4,0.7) | 0.1 (−0.7,0.6) |
| BRS [ms/mmHg] | 12.0 (8.4,20.3) | 19.4 (12.8,25.2) * | ||||
| Dependent Variables | Model Variables | BETA (β) | SE | p-Value | R2 |
|---|---|---|---|---|---|
| E/I-ratio | MGFA | −0.36 | 0.16 | 0.034 | 0.15 |
| HF-sBP | Sex | −0.35 | 0.17 | 0.042 | 0.14 |
| BRS | MGFA | −0.44 | 0.15 | 0.005 | 0.33 |
| Age | −0.35 | 0.14 | 0.020 | ||
| CI | Sex | 0.33 | 0.16 | 0.047 | 0.17 |
| TPRI | Age | 0.47 | 0.14 | 0.002 | 0.35 |
| Sex | −0.40 | 0.14 | 0.002 | ||
| EDI | Age | −0.47 | 0.15 | 0.003 | 0.26 |
| IC | Age | −0.41 | 0.15 | 0.011 | 0.21 |
| Tilt CI | Age | −0.68 | 0.12 | <0.001 | 0.52 |
| Sex | 0.30 | 0.12 | 0.024 | ||
| Tilt LVWI | Age | −0.61 | 0.13 | <0.001 | 0.45 |
| Tilt LF-RRI | Age | −0.50 | 0.15 | 0.002 | 0.25 |
| Tilt PSD-RRI | Age | −0.37 | 0.16 | 0.028 | 0.16 |
| Tilt TFC | Sex | −0.32 | 0.15 | 0.043 | 0.30 |
| DELTA LF/HF-RRI | MGFA | 0.34 | 0.16 | 0.047 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadka-Kunikowska, M.; Rzepiński, Ł.; Tafil-Klawe, M.; Klawe, J.J.; Zalewski, P.; Słomko, J. Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability. J. Clin. Med. 2022, 11, 3697. https://doi.org/10.3390/jcm11133697
Zawadka-Kunikowska M, Rzepiński Ł, Tafil-Klawe M, Klawe JJ, Zalewski P, Słomko J. Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability. Journal of Clinical Medicine. 2022; 11(13):3697. https://doi.org/10.3390/jcm11133697
Chicago/Turabian StyleZawadka-Kunikowska, Monika, Łukasz Rzepiński, Małgorzata Tafil-Klawe, Jacek J. Klawe, Paweł Zalewski, and Joanna Słomko. 2022. "Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability" Journal of Clinical Medicine 11, no. 13: 3697. https://doi.org/10.3390/jcm11133697
APA StyleZawadka-Kunikowska, M., Rzepiński, Ł., Tafil-Klawe, M., Klawe, J. J., Zalewski, P., & Słomko, J. (2022). Association of Cardiac Autonomic Responses with Clinical Outcomes of Myasthenia Gravis: Short-Term Analysis of the Heart-Rate and Blood Pressure Variability. Journal of Clinical Medicine, 11(13), 3697. https://doi.org/10.3390/jcm11133697







