Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors?
Abstract
:1. Introduction
2. Materials and Methods
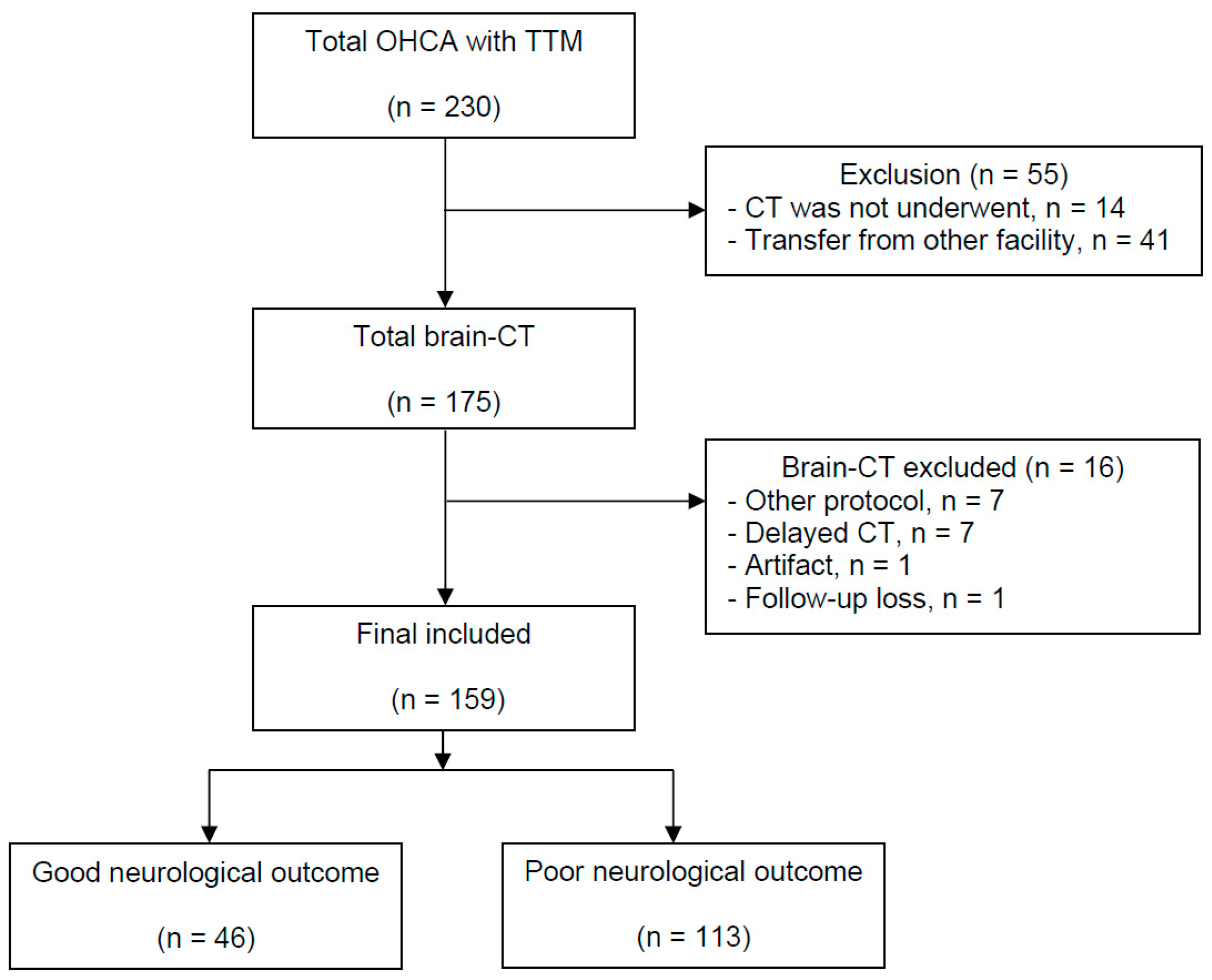
2.1. Study Design and Subjects
2.2. Brain CT Acquisitions and Post-Resuscitation Care
2.3. Interpretation of the Brain CTs
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. GWR Variables between the Outcome Groups
3.3. ONSD Variables between the Outcome Groups
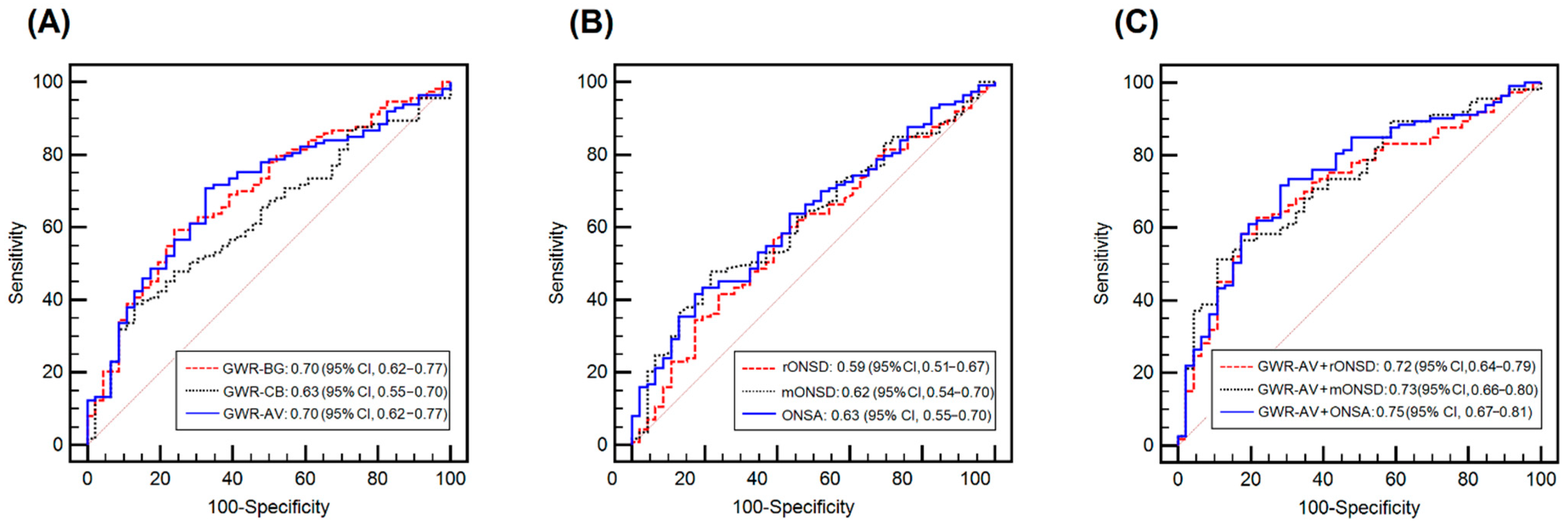
3.4. Prognostic Performances of the Single GWR or ONSD Variables Alone
3.5. Association between the GWR and ONSD Variables and the Combined Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamorro, C.; Borrallo, J.M.; Romera, M.A.; Silva, J.A.; Balandín, B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: A systematic review. Anesth. Analg. 2010, 110, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl. S2), S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.M.; Wu, O. Neuroimaging in Cardiac Arrest Prognostication. Semin. Neurol. 2017, 37, 66–74. [Google Scholar]
- Friberg, H.; Cronberg, T.; Dünser, M.W.; Duranteau, J.; Horn, J.; Oddo, M. Survey on current practices for neurological prognostication after cardiac arrest. Resuscitation 2015, 90, 158–162. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, D.H.; Oh, J.H.; Lee, S.H.; Choi, Y.H.; Kim, S.H.; Min, J.H.; Kim, S.J.; Park, Y.S.; Korean Hypothermia Network Investigators; et al. Grey-white matter ratio measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation 2019, 140, 161–169. [Google Scholar] [CrossRef]
- Na, M.K.; Kim, W.; Lim, T.H.; Jang, B.; Cho, Y.; Choi, K.S.; Shin, H.G.; Ahn, C.; Lee, J.; Kim, J.G. Gray matter to white matter ratio for predicting neurological outcomes in patients treated with target temperature management after cardiac arrest: A systematic review and meta-analysis. Resuscitation 2018, 132, 21–28. [Google Scholar] [CrossRef]
- Keijzer, H.M.; Hoedemaekers, C.W.E.; Meijer, F.J.A.; Tonino, B.A.R.; Klijn, C.J.M.; Hofmeijer, J. Brain imaging in comatose survivors of cardiac arrest: Pathophysiological correlates and prognostic properties. Resuscitation 2018, 133, 124–136. [Google Scholar] [CrossRef]
- Lee, B.K.; Jeung, K.W.; Song, K.H.; Jung, Y.H.; Choi, W.J.; Kim, S.H.; Youn, C.S.; Cho, I.S.; Lee, D.H.; Korean Hypothermia Network Investigators. Prognostic values of gray matter to white matter ratios on early brain computed tomography in adult comatose patients after out-of-hospital cardiac arrest of cardiac etiology. Resuscitation 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.P.; Park, K.N.; Youn, C.S.; Oh, S.H.; Choi, S.M. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 57. [Google Scholar] [CrossRef] [Green Version]
- Scarpino, M.; Lolli, F.; Lanzo, G.; Carrai, R.; Spalletti, M.; Valzania, F.; Lombardi, M.; Audenino, D.; Celani, M.G.; Marrelli, A.; et al. Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation 2019, 143, 115–123. [Google Scholar] [CrossRef]
- Ryu, J.A.; Chung, C.R.; Cho, Y.H.; Sung, K.; Suh, G.Y.; Park, T.K.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Gwon, H.C.; et al. The association of findings on brain computed tomography with neurologic outcomes following extracorporeal cardiopulmonary resuscitation. Crit. Care 2017, 21, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Lee, J.H.; Hong, C.K.; Cho, K.W.; Yeo, J.H.; Kang, M.J.; Kim, Y.W.; Lee, K.Y.; Kim, J.J.; Hwang, S.Y. Feasibility of optic nerve sheath diameter measured on initial brain computed tomography as an early neurologic outcome predictor after cardiac arrest. Acad. Emerg. Med. 2014, 21, 1121–1128. [Google Scholar]
- Chae, M.K.; Ko, E.; Lee, J.H.; Lee, T.R.; Yoon, H.; Hwang, S.Y.; Cha, W.C.; Shin, T.G.; Sim, M.S.; Jo, I.J.; et al. Better prognostic value with combined optic nerve sheath diameter and grey-to-white matter ratio on initial brain computed tomography in post-cardiac arrest patients. Resuscitation 2016, 104, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Zhang, S.; Gao, H.; Li, C.; Zhang, M.X. Prognostic Role of Optic Nerve Sheath Diameter for Neurological Outcomes in Post-Cardiac Arrest Patients: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 5219367. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, S.J. Diagnostic performance of optic nerve sheath diameter for predicting neurologic outcome in post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2019, 138, 59–67. [Google Scholar] [CrossRef]
- Kimberly, H.H.; Noble, V.E. Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit. Care 2008, 12, 181. [Google Scholar] [CrossRef] [Green Version]
- Maetani, K.; Namiki, J.; Matsumoto, S.; Matsunami, K.; Narumi, A.; Tsuneyoshi, T.; Kishikawa, M. Routine Head Computed Tomography for Patients in the Emergency Room with Trauma Requires Both Thick- and Thin-Slice Images. Emerg. Med. Int. 2016, 2016, 5781790. [Google Scholar] [CrossRef] [Green Version]
- Bello, H.R.; Graves, J.A.; Rohatgi, S.; Vakil, M.; McCarty, J.; Van Hemert, R.L.; Geppert, S.; Peterson, R.B. Skull Base-related Lesions at Routine Head CT from the Emergency Department: Pearls, Pitfalls, and Lessons Learned. Radiographics 2019, 39, 1161–1182. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Park, K.N.; Choi, S.P.; Lee, B.K.; Park, K.; Kim, J.; Kim, J.H.; Chung, S.P.; Hwang, S.O. Part 4. Post-cardiac arrest care: 2015 Korean Guidelines for Cardiopulmonary Resuscitation. Clin. Exp. Emerg. Med. 2016, 3, S27–S38. [Google Scholar] [CrossRef]
- Lim, J.Y.; Oh, S.H.; Park, K.N.; Choi, S.P.; Oh, J.S.; Youn, C.S.; Kim, H.J.; Kim, H.J.; Song, H. Prognostic value of brainstem auditory and visual evoked potentials in cardiac arrest patients with targeted temperature management. Resuscitation 2021, 164, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, S.H.; Oh, J.H.; Cho, I.S.; Lee, Y.H.; Han, C.; Choi, W.J.; Sohn, Y.D.; KORHN investigators. Optic nerve sheath diameter measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation 2018, 128, 144–150. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Song, H.; Kang, C.; Park, J.; You, Y.; In, Y.; Min, J.; Jeong, W.; Cho, Y.; Ahn, H.; Kim, D. Intracranial Pressure Patterns and Neurological Outcomes in Out-of-Hospital Cardiac Arrest Survivors after Targeted Temperature Management: A Retrospective Observational Study. J. Clin. Med. 2021, 10, 5697. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Park, J.S.; Yoo, I.S.; You, Y.H.; Min, J.H.; Jeong, W.J.; Cho, Y.C.; Cho, S.U.; Oh, S.K.; Ahn, H.J.; et al. Usefulness of Intracranial Pressure and Mean Arterial Pressure for Predicting Neurological Prognosis in Cardiac Arrest Survivors Who Undergo Target Temperature Management. Ther. Hypothermia Temp. Manag. 2020, 10, 165–170. [Google Scholar] [CrossRef]
- Wang, L.J.; Chen, L.M.; Chen, Y.; Bao, L.Y.; Zheng, N.N.; Wang, Y.Z.; Xing, Y.Q. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a Noninvasive and Dynamic Method of Detecting Changes in Intracranial Pressure. JAMA Ophthalmol. 2018, 136, 250–256. [Google Scholar] [CrossRef]
- You, Y.; Park, J.; Min, J.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. Relationship between time related serum albumin concentration, optic nerve sheath diameter, cerebrospinal fluid pressure, and neurological prognosis in cardiac arrest survivors. Resuscitation 2018, 131, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; You, Y.; Min, J.H.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.W.; Cho, S.U.; et al. Study on the timing of severe blood-brain barrier disruption using cerebrospinal fluid-serum albumin quotient in post cardiac arrest patients treated with targeted temperature management. Resuscitation 2019, 135, 118–123. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, Y.; You, Y.; Min, J.H.; Jeong, W.; Ahn, H.J.; Kang, C.; Yoo, I.; Ryu, S.; Lee, J.; et al. Optimal timing to measure optic nerve sheath diameter as a prognostic predictor in post-cardiac arrest patients treated with targeted temperature management. Resuscitation 2019, 143, 173–179. [Google Scholar] [CrossRef]
- Hickman, S.J.; Miszkiel, K.A.; Plant, G.T.; Miller, D.H. The optic nerve sheath on MRI in acute optic neuritis. Neuroradiology 2005, 47, 51–55. [Google Scholar] [CrossRef]
- Peyster, R.G.; Hoover, E.D.; Hershey, B.L.; Haskin, M.E. High-resolution CT of lesions of the optic nerve. AJR Am. J. Roentgenol. 1983, 140, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, J.; Shin, H.; Kim, C.; Choi, H.J.; Kang, B.S. Predictive Utility of Changes in Optic Nerve Sheath Diameter after Cardiac Arrest for Neurologic Outcomes. Int. J. Environ. Res. Public Health 2021, 18, 6567. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, C.; D’Arrigo, S.; Cacciola, S.; Hoedemaekers, C.W.E.; Kamps, M.J.A.; Oddo, M.; Taccone, F.S.; Di Rocco, A.; Meijer, F.J.A.; Westhall, E.; et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: A systematic review. Intensive Care Med. 2020, 46, 1803–1851. [Google Scholar] [CrossRef]
- Oh, J.H.; Choi, S.P.; Zhu, J.H.; Kim, S.H.; Park, K.N.; Youn, C.S.; Oh, S.H.; Kim, H.J.; Park, S.H. Differences in the gray-to-white matter ratio according to different computed tomography scanners for outcome prediction in post-cardiac arrest patients receiving target temperature management. PLoS ONE 2021, 16, e0258480. [Google Scholar] [CrossRef] [PubMed]
- Streitberger, K.J.; Endisch, C.; Ploner, C.J.; Stevens, R.; Scheel, M.; Kenda, M.; Storm, C.; Leithner, C. Timing of brain computed tomography and accuracy of outcome prediction after cardiac arrest. Resuscitation 2019, 145, 8–14. [Google Scholar] [CrossRef]
- Wang, G.N.; Chen, X.F.; Lv, J.R.; Sun, N.N.; Xu, X.Q.; Zhang, J.S. The prognostic value of gray-white matter ratio on brain computed tomography in adult comatose cardiac arrest survivors. J. Chin. Med. Assoc. 2018, 81, 599–604. [Google Scholar] [CrossRef]
- Lee, B.K.; Callaway, C.W.; Coppler, P.J.; Rittenberger, J.C.; Pittsburgh Post-Cardiac Arrest Service. The prognostic performance of brain ventricular characteristic differ according to sex, age, and time after cardiac arrest in comatose out-of-hospital cardiac arrest survivors. Resuscitation 2020, 154, 69–76. [Google Scholar] [CrossRef]
- Moseby-Knappe, M.; Pellis, T.; Dragancea, I.; Friberg, H.; Nielsen, N.; Horn, J.; Kuiper, M.; Roncarati, A.; Siemund, R.; Undén, J.; et al. Head computed tomography for prognostication of poor outcome in comatose patients after cardiac arrest and targeted temperature management. Resuscitation 2017, 119, 89–94. [Google Scholar] [CrossRef]
- Wang, W.J.; Cui, J.; Lv, G.W.; Feng, S.Y.; Zhao, Y.; Zhang, S.L.; Li, Y. Prognostic Values of the Gray-to-White Matter Ratio on Brain Computed Tomography Images for Neurological Outcomes after Cardiac Arrest: A Meta-Analysis. BioMed Res. Int. 2020, 2020, 7949516. [Google Scholar] [CrossRef]
- Callaway, C.W.; Coppler, P.J.; Faro, J.; Puyana, J.S.; Solanki, P.; Dezfulian, C.; Doshi, A.A.; Elmer, J.; Frisch, A.; Guyette, F.X.; et al. Association of Initial Illness Severity and Outcomes After Cardiac Arrest With Targeted Temperature Management at 36 °C or 33 °C. JAMA Netw. Open. 2020, 3, e208215. [Google Scholar] [CrossRef]
- Hanning, U.; Sporns, P.B.; Lebiedz, P.; Niederstadt, T.; Zoubi, T.; Schmidt, R.; Knecht, S.; Heindel, W.; Kemmling, A. Automated assessment of early hypoxic brain edema in non-enhanced CT predicts outcome in patients after cardiac arrest. Resuscitation 2016, 104, 91–94. [Google Scholar] [CrossRef] [PubMed]

| Good Outcome (n = 46) | Poor Outcome (n = 113) | p-Value | |
|---|---|---|---|
| Male | 33 (71.7) | 82 (72.6) | 0.916 |
| Age, years, mean ± SD | 49.9 ± 16.1 | 61.1 ± 16.2 | <0.001 |
| Comorbidity | |||
| Coronary artery disease | 6 (13.0) | 14 (12.4) | 0.910 |
| Hypertension | 17 (37.0) | 49 (43.4) | 0.457 |
| Diabetes mellitus | 6 (13.0) | 35 (31.0) | 0.019 |
| Chronic renal failure | 3 (6.5) | 8 (7.1) | 0.900 |
| Resuscitation variables | |||
| Witnessed | 32 (69.6) | 77 (68.1) | 0.861 |
| Bystander CPR | 34 (73.9) | 70 (61.9) | 0.150 |
| Shockable rhythm | 37 (80.4) | 22 (19.5) | <0.001 |
| Cardiac origin | 44 (95.7) | 59 (52.2) | <0.001 |
| Time from arrest to ROSC, min (IQR) | 15.0 (10.0–22.8) | 34.0 (23.0–42.0) | <0.001 |
| Variables immediately after ROSC | |||
| Absent brainstem reflex | 12 (26.1) | 92 (81.4) | <0.001 |
| Motor grade < 3 | 29 (63.0) | 106 (93.8) | <0.001 |
| Absent spontaneous respiration | 19 (42.2) | 92 (81.4) | <0.001 |
| STEMI | 12 (26.1) | 19 (16.8) | 0.181 |
| STEMI or new onset LBBB | 14 (30.4) | 23 (20.4) | 0.173 |
| ROSC-to-CT interval, min (IQR) | 24.5 (14.8–33.5) | 15.0 (10.0–24.0) | 0.003 |
| GWR variables | |||
| GWR-average | 1.21 ± 0.05 | 1.18 ± 0.06 | <0.001 |
| GWR-basal ganglia | 1.24 ± 0.07 | 1.19 ± 0.07 | <0.001 |
| GWR-cerebrum | 1.19 ± 0.06 | 1.17 ± 0.08 | 0.068 |
| ONSD variables | |||
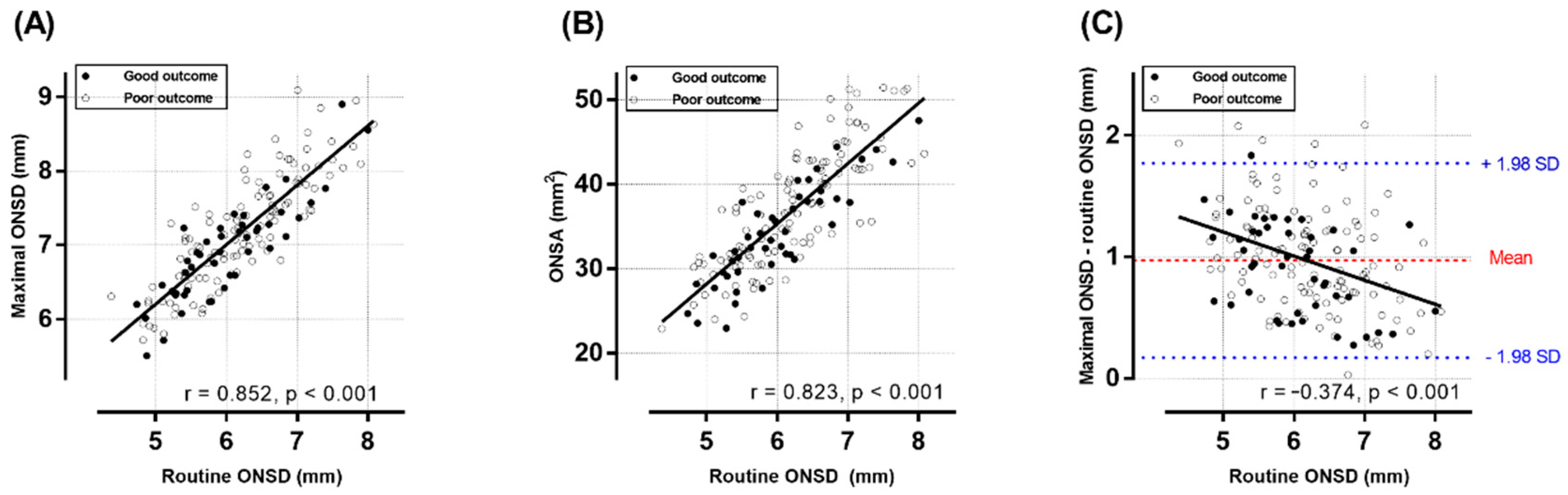
| Routine ONSD a, mm | 6.01 ± 0.75 | 6.22 ± 0.79 | 0.116 |
| Maximal ONSD b, mm | 6.92 ± 0.67 | 7.22 ± 0.74 | 0.018 |
| Difference of ONSDs, mm | 0.91 ± 0.37 | 1.00 ± 0.43 | 0.224 |
| Percentage difference, % | 14.43 ± 6.24 | 15.17 ± 6.78 | 0.521 |
| ONSA b, mm2 | 34.20 ± 5.81 | 37.42 ± 6.93 | 0.006 |
| Target temperature, 33 °C | 42 (91.3) | 103 (91.2) | 0.975 |
| Shock during the initiation of TTM | 11 (23.9) | 60 (53.1) | 0.001 |
| Cutoff | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|---|---|
| GWR-AV | <1.20 a | 80 | 15 | 31 | 33 | 70.8 (61.5–79.0) | 67.4 (52.0–80.5) |
| <1.11 b,c | 14 | 0 | 46 | 99 | 12.4 (6.9–19.9) | 100.0 (92.3–100.0) | |
| rONSD (mm) | >6.45 a | 47 | 11 | 35 | 66 | 41.6 (32.4–51.2) | 76.1 (61.2–87.4) |
| >7.40 b | 8 | 2 | 44 | 105 | 7.1 (3.1–13.5) | 95.7 (85.2–99.5) | |
| >8.00 c | 1 | 0 | 46 | 112 | 0.9 (0.0–4.8) | 100.0 (92.3–100.0) | |
| mONSD (mm) | >7.28 a | 54 | 10 | 36 | 59 | 47.8 (38.3–57.4) | 78.3 (63.5–89.1) |
| >7.89 b | 23 | 2 | 44 | 90 | 20.4 (13.4–29.0) | 95.7 (85.2–99.5) | |
| >8.90 c | 2 | 0 | 46 | 111 | 1.8 (0.2–6.3) | 100.0 (92.3–100.0) | |
| ONSA (mm2) | >39.21 a | 47 | 8 | 38 | 66 | 41.6 (32.4–51.2) | 82.6 (68.6–92.2) |
| >44.13 b | 19 | 2 | 44 | 94 | 16.8 (10.4–25.0) | 95.7 (85.2–99.5) | |
| >47.54 c | 9 | 0 | 46 | 104 | 8.0 (3.7–14.6) | 100.0 (92.3–100.0) | |
| GWR-AV + rONSD (mm) | <1.11 b,c or >7.40 b | 22 | 2 | 44 | 91 | 19.5 (12.6–28.0) | 95.7 (85.2–99.5) |
| <1.11 b,c or >8.00 c | 15 | 0 | 46 | 98 | 13.3 (7.6–21.0) | 100.0 (92.3–100.0) | |
| GWR-AV + mONSD (mm) | <1.11 b,c or >7.89 b | 35 | 2 | 44 | 78 | 31.0 (22.6–40.4) | 95.7 (85.2–99.5) |
| <1.11 b,c or >8.90 c | 15 | 0 | 46 | 98 | 13.3 (7.6–21.0) | 100.0 (92.3–100.0) | |
| GWR-AV + ONSA (mm2) | <1.11 b,c or >44.13 b | 31 | 2 | 44 | 82 | 27.4 (19.5–36.6) | 95.7 (85.2–99.5) |
| <1.11 b,c or >47.54 c | 22 | 0 | 46 | 91 | 19.5 (12.6–28.0) | 100.0 (92.3–100.0) |
| Crude a | Model 1 b | Model 2 c | Model 3 d | p a,b | p a,c | p a,d | |
|---|---|---|---|---|---|---|---|
| AUC of the resuscitation variable model (95% CI) | 0.90 (0.83–0.96) | 0.90 (0.83–0.96) | 0.90 (0.84–0.96) | 0.90 (0.84–0.96) | 1.000 | 0.638 | 0.557 |
| AUC of the neurological examination model (95% CI) | 0.87 (0.82–0.93) | 0.87 (0.82–0.93) | 0.87 (0.82–0.93) | 0.87 (0.82–0.93) | 0.653 | 0.757 | 0.537 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.H.; Oh, S.H.; Jang, J.; Kim, S.H.; Park, K.N.; Youn, C.S.; Kim, H.J.; Lim, J.Y.; Kim, H.J.; Bang, H.J. Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors? J. Clin. Med. 2022, 11, 3677. https://doi.org/10.3390/jcm11133677
Kwon SH, Oh SH, Jang J, Kim SH, Park KN, Youn CS, Kim HJ, Lim JY, Kim HJ, Bang HJ. Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors? Journal of Clinical Medicine. 2022; 11(13):3677. https://doi.org/10.3390/jcm11133677
Chicago/Turabian StyleKwon, Sung Ho, Sang Hoon Oh, Jinhee Jang, Soo Hyun Kim, Kyu Nam Park, Chun Song Youn, Han Joon Kim, Jee Yong Lim, Hyo Joon Kim, and Hyo Jin Bang. 2022. "Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors?" Journal of Clinical Medicine 11, no. 13: 3677. https://doi.org/10.3390/jcm11133677
APA StyleKwon, S. H., Oh, S. H., Jang, J., Kim, S. H., Park, K. N., Youn, C. S., Kim, H. J., Lim, J. Y., Kim, H. J., & Bang, H. J. (2022). Can Optic Nerve Sheath Images on a Thin-Slice Brain Computed Tomography Reconstruction Predict the Neurological Outcomes in Cardiac Arrest Survivors? Journal of Clinical Medicine, 11(13), 3677. https://doi.org/10.3390/jcm11133677






