Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia
Abstract
:1. Introduction
2. Materials and Methods
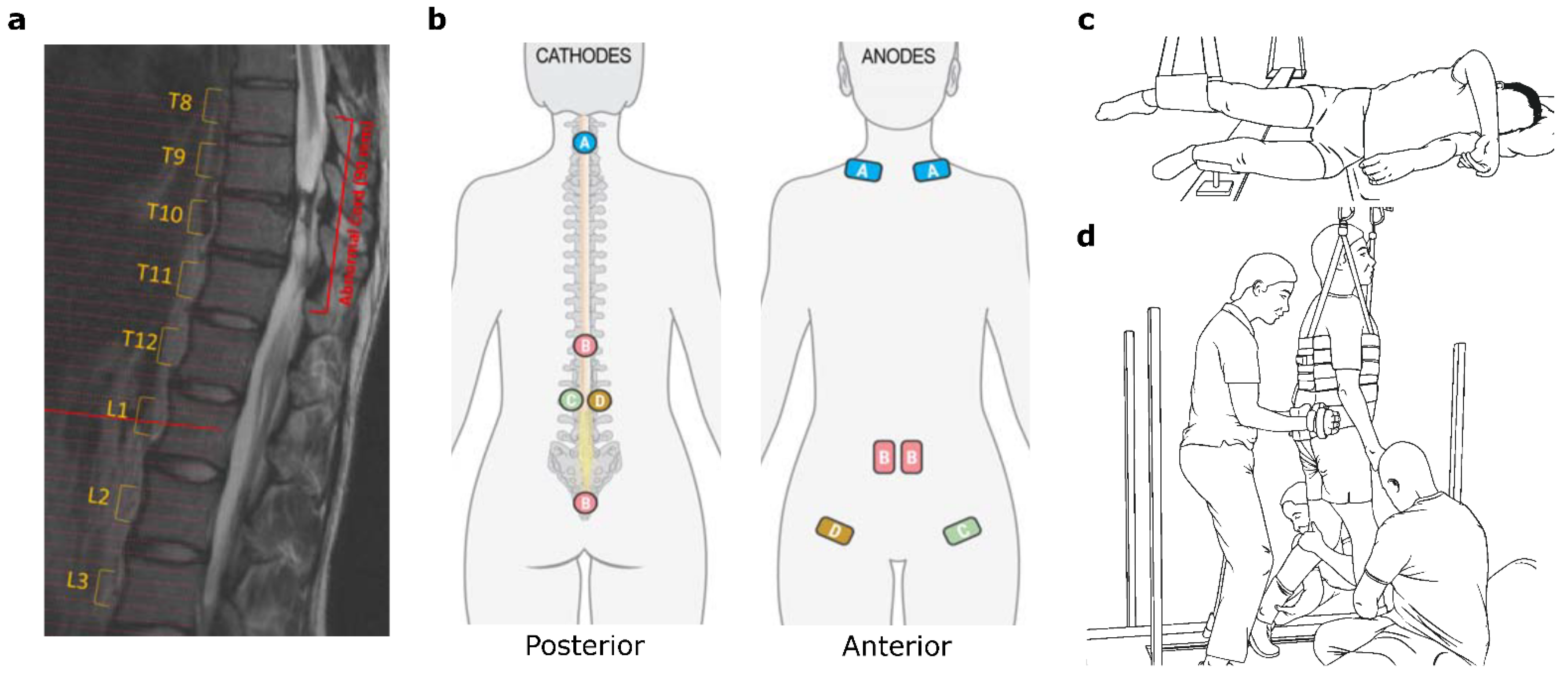
2.1. Spinal Cord Transcutaneous Electrical Stimulation
2.2. Locomotor Training
2.3. Kinematic Data and EMG Data Collection and Analysis
2.4. Supraspinal Conditioning of Motor Evoked Potentials
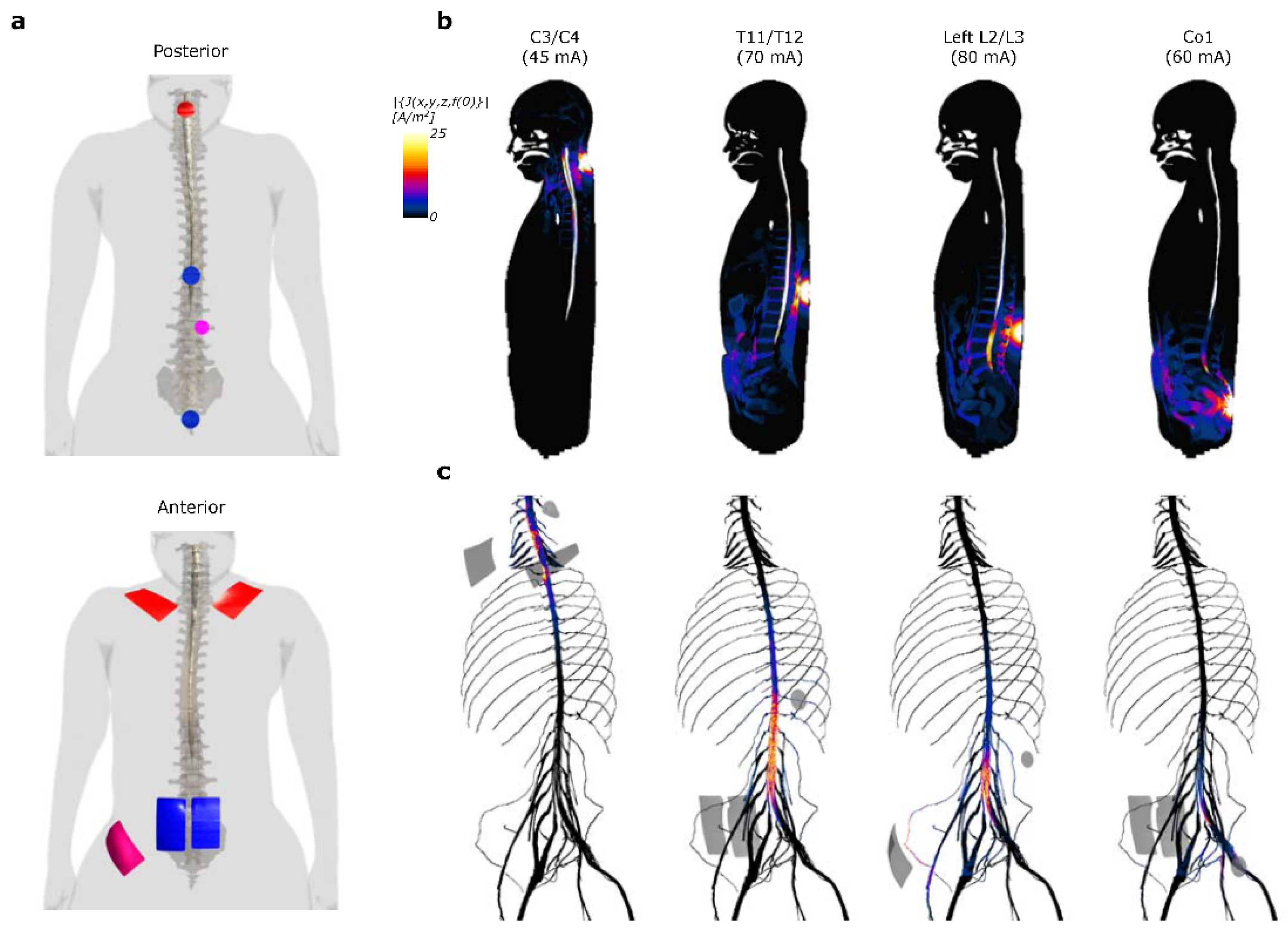
2.5. Computational Modeling
2.6. Statistical Analysis
3. Results
3.1. Computational Modeling of Current Density Induced by Multi-Site scTS
3.2. Improvement in Hip and Knee Kinematics during Non-Weight Bearing Stepping
3.3. Increase in EMG Power and Muscle Coordination during Weight-Bearing Stepping
3.4. Facilitation of Supraspinal Conditioning of Motor Evoked Potentials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, P.K.; Garcia-Alias, G.; Choe, J.; Gad, P.; Gerasimenko, Y.; Tillakaratne, N.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 2013, 136, 3362–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leon, R.D.; Hodgson, J.A.; Roy, R.R.; Edgerton, V.R. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 1999, 81, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubli, M.; Dietz, V. The physiological basis of neurorehabilitation-locomotor training after spinal cord injury. J. Neuroeng. Rehabil. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.L.; Evans, N.; Tefertiller, C.; Backus, D.; Sweatman, M.; Tansey, K.; Morrison, S. Activity-Based Therapy for Recovery of Walking in Individuals With Chronic Spinal Cord Injury: Results From a Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2014, 95, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.J.; Datta, S.; Harkema, S.J. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.R.; Harkema, S.J.; Edgerton, V.R. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.; van den Brand, R.; Marzendorfer, O.; Roy, R.R.; Gerasimenko, Y.; Edgerton, V.R.; Courtine, G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J. Neurosci. 2011, 31, 9264–9278. [Google Scholar] [CrossRef]
- Onifer, S.M.; Smith, G.M.; Fouad, K. Plasticity after spinal cord injury: Relevance to recovery and approaches to facilitate it. Neurotherapeutics 2011, 8, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [Green Version]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef]
- Musienko, P.E.; Pavlova, N.V.; Selionov, V.A.; Gerasimenko Iu, P. Locomotion induced by epidural stimulation in decerebrate cat after spinal cord injury. Biofizika 2009, 54, 293–300. [Google Scholar] [PubMed]
- Gad, P.; Lavrov, I.; Shah, P.; Zhong, H.; Roy, R.R.; Edgerton, V.R.; Gerasimenko, Y. Neuromodulation of motor-evoked potentials during stepping in spinal rats. J. Neurophysiol. 2013, 110, 1311–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, P.; Choe, J.; Nandra, M.S.; Zhong, H.; Roy, R.R.; Tai, Y.C.; Edgerton, V.R. Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J. Neuroeng. Rehabil. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Rejc, E.; Angeli, C.; Harkema, S. Effects of Lumbosacral Spinal Cord Epidural Stimulation for Standing after Chronic Complete Paralysis in Humans. PLoS ONE 2015, 10, e0133998. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.S.; Gill, M.L.; Linde, M.B.; Veith, D.D.; Thoreson, A.R.; Lopez, C.; Lee, K.H.; Gerasimenko, Y.P.; Edgerton, V.R.; Lavrov, I.A.; et al. Voluntary Modulation of Evoked Responses Generated by Epidural and Transcutaneous Spinal Stimulation in Humans with Spinal Cord Injury. J. Clin. Med. 2021, 10, 4898. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Reggie Edgerton, V. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Gad, P.N.; Gerasimenko, Y.P.; Zdunowski, S.; Sayenko, D.; Haakana, P.; Turner, A.; Lu, D.; Roy, R.R.; Edgerton, V.R. Iron ‘ElectriRx’ man: Overground stepping in an exoskeleton combined with noninvasive spinal cord stimulation after paralysis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 2015, 1124–1127. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Barbeau, H.; Ladouceur, M.; Mirbagheri, M.M.; Kearney, R.E. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: Walking and reflex studies. Brain Res. Brain Res. Rev. 2002, 40, 274–291. [Google Scholar] [CrossRef]
- Duffell, L.D.; Donaldson, N.N. A Comparison of FES and SCS for Neuroplastic Recovery After SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.; Gammage, K.; Chun, S.; Ditor, D. Effects of FES-Ambulation Training on Locomotor Function and Health-Related Quality of Life in Individuals With Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2014, 20, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.S.; Schwab, M.E. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr. Opin. Neurol. 2019, 32, 828–835. [Google Scholar] [CrossRef]
- Piazza, S.; Brand, J.; Escolano, C. Spinal Cord Plasticity and Neuromodulation. In Emerging Therapies in Neurorehabilitation; Biosystems & Biorobotics; Springer: Berlin/Heidelberg, Germany, 2014; Volume 4, pp. 67–87. [Google Scholar]
- Lynskey, J.V.; Belanger, A.; Jung, R. Activity-dependent plasticity in spinal cord injury. J. Rehabil. Res. Dev. 2008, 45, 229–240. [Google Scholar] [CrossRef]
- Atkinson, D.A.; Sayenko, D.G.; D’Amico, J.M.; Mink, A.; Lorenz, D.J.; Gerasimenko, Y.P.; Harkema, S. Interlimb conditioning of lumbosacral spinally evoked motor responses after spinal cord injury. Clin. Neurophysiol. 2020, 131, 1519–1532. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Puhov, A.; Moshonkina, T.; Savochin, A.; Selionov, V.; Roy, R.R.; Lu, D.C.; Edgerton, V.R. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 2015, 113, 834–842. [Google Scholar] [CrossRef]
- Alam, M.; Ling, Y.T.; Wong, A.Y.L.; Zhong, H.; Edgerton, V.R.; Zheng, Y.P. Reversing 21 years of chronic paralysis via non-invasive spinal cord neuromodulation: A case study. Ann. Clin. Transl. Neurol. 2020, 7, 829–838. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Barss, T.S.; Parhizi, B.; Mushahwar, V.K. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J. Neurophysiol. 2020, 123, 158–166. [Google Scholar] [CrossRef]
- Cherniak, M.; Anglister, L.; Lev-Tov, A. Shaping the Output of Lumbar Flexor Motoneurons by Sacral Neuronal Networks. J. Neurosci. 2017, 37, 1294–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlet, A.N.; Harnie, J.; Frigon, A. Inhibition and Facilitation of the Spinal Locomotor Central Pattern Generator and Reflex Circuits by Somatosensory Feedback From the Lumbar and Perineal Regions After Spinal Cord Injury. Front. Neurosci. 2021, 15, 720542. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.D.; Jin Jo, H.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Harkema, S.; Behrman, A.; Barbeau, H. Evidence-based therapy for recovery of function after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. The ABC of EMG—A Practical Introduction to Kinesiological Electromyography, version 1.4 ed.; Noraxon USA Inc.: Scottsdale, AZ, USA, 2006; Available online: https://www.noraxon.com/wp-content/uploads/2014/12/ABC-EMG-ISBN.pdf (accessed on 6 October 2021).
- Kim, M.; Moon, Y.; Hunt, J.; McKenzie, K.A.; Horin, A.; McGuire, M.; Kim, K.; Hargrove, L.J.; Jayaraman, A. A Novel Technique to Reject Artifact Components for Surface EMG Signals Recorded During Walking With Transcutaneous Spinal Cord Stimulation: A Pilot Study. Front. Hum. Neurosci. 2021, 15, 660583. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, S.; Gonnelli, F.; Angeli, C.A.; El-Baz, A.; Harkema, S.J.; Rejc, E. Neurophysiological markers predicting recovery of standing in humans with chronic motor complete spinal cord injury. Sci. Rep. 2019, 9, 14474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, T.A.; Do, K.P.; Rethlefsen, S.A.; Healy, B. Cross-correlation as a method for comparing dynamic electromyography signals during gait. J. Biomech. 2006, 39, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, D.H.; Alizadehkhaiyat, O.; Fisher, A.C.; Kemp, G.J.; Roebuck, M.M.; Frostick, S.P. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: An electromyographic study. J. Orthop. Res. 2012, 30, 53–60. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Mink, A.M.; Gurley, K.M.; Edgerton, V.R.; Harkema, S.J.; Gerasimenko, Y.P. Vestibulospinal and Corticospinal Modulation of Lumbosacral Network Excitability in Human Subjects. Front. Physiol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Kumru, H.; Vidal, J.; Kofler, M.; Benito, J.; Garcia, A.; Valls-Sole, J. Exaggerated auditory startle responses in patients with spinal cord injury. J. Neurol. 2008, 255, 703–709. [Google Scholar] [CrossRef]
- Sim4Life, 6.2 ed.; ZMT Zurich MedTech AG: Zurich, Switzerland, 2021.
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.M.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Muller, R.; Colombo, G. Locomotor activity in spinal man: Significance of afferent input from joint and load receptors. Brain 2002, 125, 2626–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, T.; Pearson, K.G. The role of proprioceptive feedback in the regulation and adaptation of locomotor activity. Adv. Exp. Med. Biol. 2002, 508, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; He, J.; D’Luzansky, S.; Willis, W.; Dilli, S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 2002, 40, 65–68. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, R.; Brown, E.H.; Mesbah, S.; Gonnelli, F.; Pisolkar, T.; Edgerton, V.R.; Ovechkin, A.V.; Gerasimenko, Y.P. Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia. J. Clin. Med. 2022, 11, 3670. https://doi.org/10.3390/jcm11133670
Siu R, Brown EH, Mesbah S, Gonnelli F, Pisolkar T, Edgerton VR, Ovechkin AV, Gerasimenko YP. Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia. Journal of Clinical Medicine. 2022; 11(13):3670. https://doi.org/10.3390/jcm11133670
Chicago/Turabian StyleSiu, Ricardo, Edward H. Brown, Samineh Mesbah, Federica Gonnelli, Tanvi Pisolkar, V. Reggie Edgerton, Alexander V. Ovechkin, and Yury P. Gerasimenko. 2022. "Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia" Journal of Clinical Medicine 11, no. 13: 3670. https://doi.org/10.3390/jcm11133670
APA StyleSiu, R., Brown, E. H., Mesbah, S., Gonnelli, F., Pisolkar, T., Edgerton, V. R., Ovechkin, A. V., & Gerasimenko, Y. P. (2022). Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia. Journal of Clinical Medicine, 11(13), 3670. https://doi.org/10.3390/jcm11133670






