The Frequency of Primary Healthcare Contacts Preceding the Diagnosis of Lower-Extremity Arterial Disease: Do Women Consult General Practice Differently?
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Data Extraction
2.4. Variables of Interest
2.5. Statistical Analyses
3. Results
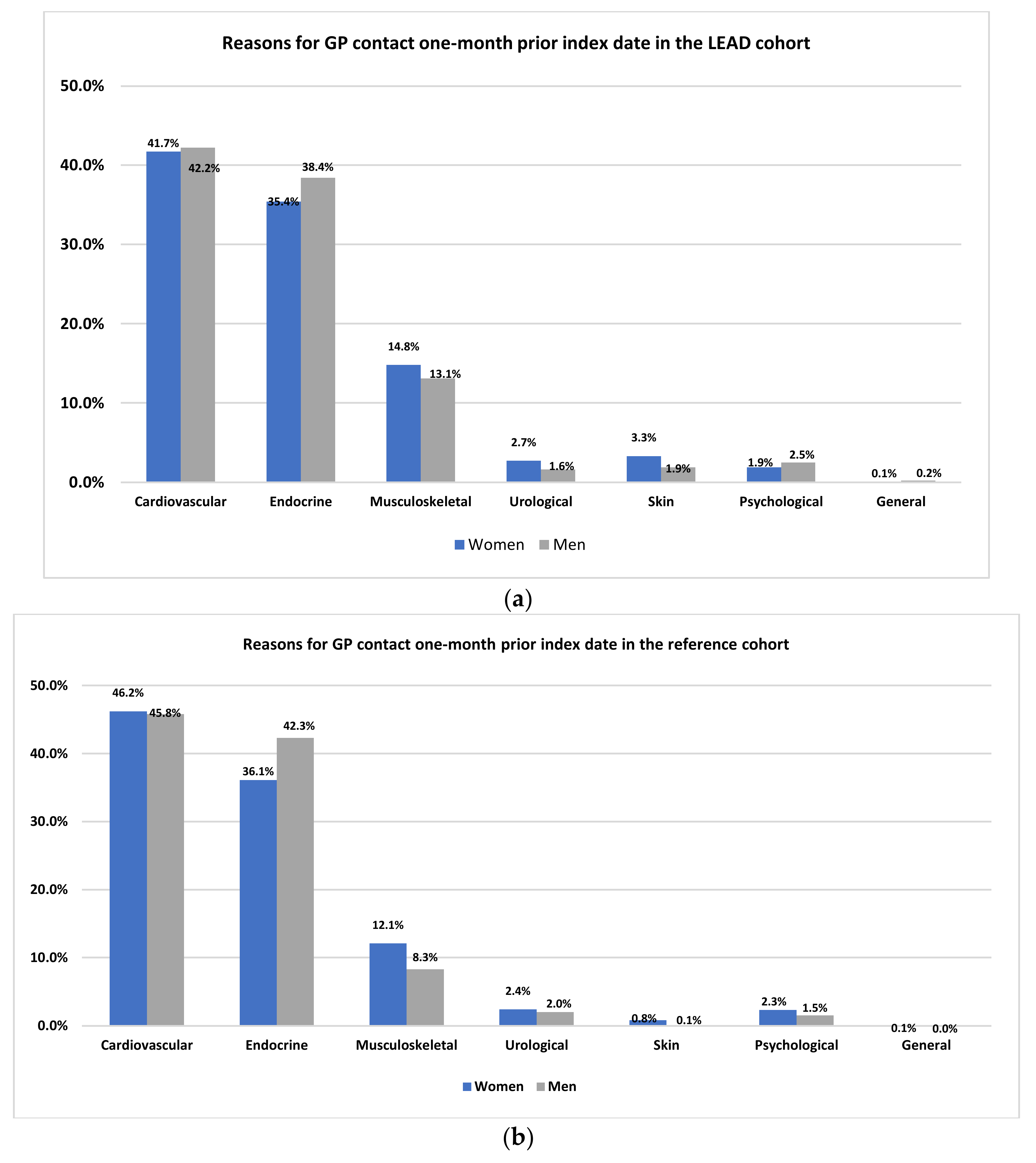
3.1. GP Contacts Six Months Preceding the Index Date
3.2. ZINB Model
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, H.B. An extension of the Alma-Ata vision for primary health care in light of twenty-first century evidence and realities. Gates Open Res. 2018, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Velasco Garrido, M.; Zentner, A.; Busse, R. The effects of gatekeeping: A systematic review of the literature. Scand. J. Prim. Health Care 2011, 29, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Skarshaug, L.J.; Svedahl, E.R.; Bjørngaard, J.H.; Steinsbekk, A.; Pape, K. Contact with primary health care physicians before an acute hospitalisation. Scand. J. Prim. Health Care 2019, 37, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zylyftari, N.; Møller, S.G.; Wissenberg, M.; Folke, F.; Barcella, C.A.; Møller, A.L.; Gnesin, F.; Mills, E.H.A.; Jensen, B.; Lee, C.J.-Y.; et al. Contacts With the Health Care System Before Out-of-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2021, 10, e021827. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Vouyouka, A.G.; Kent, K.C. Arterial vascular disease in women. J. Vasc. Surg. 2007, 46, 1295–1302. [Google Scholar] [CrossRef][Green Version]
- Hultgren, R.; Olofsson, P.; Wahlberg, E. Sex-related differences in outcome after vascular interventions for lower limb ischemia. J. Vasc. Surg. 2002, 35, 510–516. [Google Scholar] [CrossRef]
- Egorova, N.; Vouyouka, A.G.; Quin, J.; Guillerme, S.; Moskowitz, A.; Marin, M.; Faries, P.L. Analysis of gender-related differences in lower extremity peripheral arterial disease. J. Vasc. Surg. 2010, 51, 372–378.e371. [Google Scholar] [CrossRef]
- Porras, C.P.; Bots, M.L.; Teraa, M.; van Doorn, S.; Vernooij, R.W.M. Differences in Symptom Presentation in Women and Men with Confirmed Lower Limb Peripheral Artery Disease: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 602–612. [Google Scholar] [CrossRef]
- Vural, T.; Tan, M.N.; Kartal, M.; Güldal, A.D. Detecting Peripheral Arterial Disease in Primary Care: A Population Based Study. Korean J. Fam. Med. 2020, 41, 61–67. [Google Scholar] [CrossRef]
- Lecouturier, J.; Scott, J.; Rousseau, N.; Stansby, G.; Sims, A.; Allen, J. Peripheral arterial disease diagnosis and management in primary care: A qualitative study. BJGP Open 2019, 3, bjgpopen19X101659. [Google Scholar] [CrossRef]
- Smeets, H.M.; Kortekaas, M.F.; Rutten, F.H.; Bots, M.L.; van der Kraan, W.; Daggelders, G.; Smits-Pelser, H.; Helsper, C.W.; Hoes, A.W.; de Wit, N.J. Routine primary care data for scientific research, quality of care programs and educational purposes: The Julius General Practitioners’ Network (JGPN). BMC Health Serv. Res. 2018, 18, 735. [Google Scholar] [CrossRef]
- Hajek, A.; König, H.-H. Which factors lead to frequent attendance in the outpatient sector among individuals in the second half of life? Evidence from a population-based longitudinal study in Germany. BMC Health Serv Res. 2018, 18, 673. [Google Scholar] [CrossRef]
- Sauver, J.L.S.; Warner, D.O.; Yawn, B.P.; Jacobson, D.J.; McGree, M.E.; Pankratz, J.J.; Melton, L.J.; Roger, V.L.; Ebbert, J.O.; Rocca, W.A. Why patients visit their doctors: Assessing the most prevalent conditions in a defined American population. Mayo Clin. Proc. 2013, 88, 56–67. [Google Scholar] [CrossRef]
- Coxe, S.; West, S.G.; Aiken, L.S. The Analysis of Count Data: A Gentle Introduction to Poisson Regression and Its Alternatives. J. Personal. Assess. 2009, 91, 121–136. [Google Scholar] [CrossRef]
- Perumean-Chaney, S.; Morgan, C.; McDowall, D.; Aban, I. Zero-inflated and overdispersed: What’s one to do? J. Stat. Comput. Simul. 2013, 83, 1671–1683. [Google Scholar] [CrossRef]
- Mwalili, S.; Lesaffre, E.; Declerck, D. The zero-inflated negative binomial regression model with correction for misclassification: An example in caries research. Stat. Methods Med. Res. 2008, 17, 123–139. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 September 2021).
- Statistiek CBvd. Health and Health Care; Personal Characteristics. 2021. Available online: https://www.cbs.nl/en-gb/figures/detail/83005ENG?q=Medical%20contacts (accessed on 20 January 2022).
- Forsling, E.; Lundqvist, R.; Eliasson, M.; Isaksson, R.-M. Health care contact is higher in the week preceding a first myocardial infarction: A review of medical records in Northern Sweden in 2007. Eur. J. Cardiovasc. Nurs. 2015, 14, 450–456. [Google Scholar] [CrossRef]
- Hunt, K.; Ford, G.; Harkins, L.; Wyke, S. Are Women More Ready to Consult than Men? Gender Differences in Family Practitioner Consultation for Common Chronic Conditions. J. Health Serv. Res. Policy 1999, 4, 96–100. [Google Scholar] [CrossRef]
- Wang, Y.; Hunt, K.; Nazareth, I.; Freemantle, N.; Petersen, I. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open 2013, 3, e003320. [Google Scholar] [CrossRef]
- McDermott, M.M. Lower extremity manifestations of peripheral artery disease: The pathophysiologic and functional implications of leg ischemia. Circ. Res. 2015, 116, 1540–1550. [Google Scholar] [CrossRef]
- McDermott, M.M.; Fried, L.; Simonsick, E.; Ling, S.; Guralnik, J.M. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The women’s health and aging study. Circulation 2000, 101, 1007–1012. [Google Scholar] [CrossRef]
- Gerritsen, A.A.M.; Devillé, W.L. Gender differences in health and health care utilisation in various ethnic groups in the Netherlands: A cross-sectional study. BMC Public Health 2009, 9, 109. [Google Scholar] [CrossRef]
| LEAD Cohort 1 | Reference Cohort | |||||
|---|---|---|---|---|---|---|
| Women | Men | p Value | Women | Men | p Value | |
| N | 1761 | 2283 | 4851 | 5635 | ||
| Age (mean (SD)) | 69.23 (13.74) | 67.55 (11.67) | <0.001 | 67.08 (14.25) | 65.22 (12.24) | <0.001 |
| Age group | <0.001 | <0.001 | ||||
| <50 years (%) | 159 (9.0) | 159 (7.0) | 561 (11.6) | 589 (10.5) | ||
| ≥50 <70 years (%) | 677 (38.4) | 1107 (48.5) | 2095 (43.2) | 2990 (53.1) | ||
| ≥70 <85 years (%) | 736 (41.8) | 895 (39.2) | 1730 (35.7) | 1826 (32.4) | ||
| ≥85 years (%) | 189 (10.7) | 122 (5.3) | 465 (9.6) | 230 (4.1) | ||
| Hypertension (%) | 1111 (63.1) | 1308 (57.3) | <0.001 | 2163 (44.6) | 2193 (38.9) | <0.001 |
| Diabetes mellitus (%) | 494 (28.1) | 823 (36.0) | <0.001 | 815 (16.8) | 1035 (18.4) | 0.038 |
| Hyperlipidemia (%) | 487 (27.7) | 624 (27.3) | 0.848 | 856 (17.6) | 888 (15.8) | 0.010 |
| Renal impairment (%) | 262 (14.9) | 307 (13.4) | 0.211 | 367 (7.6) | 335 (5.9) | 0.001 |
| Rheumatic disease (%) | 105 (6.0) | 67 (2.9) | <0.001 | 193 (4.0) | 139 (2.5) | <0.001 |
| Vascular disease 2 (%) | 225 (12.8) | 307 (13.4) | 0.563 | 310 (6.4) | 405 (7.2) | 0.115 |
| MI 3 (%) | 182 (10.3) | 450 (19.7) | <0.001 | 202 (4.2) | 500 (8.9) | <0.001 |
| Musculoskeletal (%) | 1094 (62.1) | 1180 (51.7) | <0.001 | 2525 (52.1) | 2421 (43.0) | <0.001 |
| Tobacco abuse 4 (%) | 523 (29.7) | 670 (29.3) | 0.835 | 467 (9.6) | 577 (10.2) | 0.311 |
| Predictor | LEAD Cohort | Reference Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative Binomial Model 1 (Count Model) | Zero-Inflated Model 2 (Logit Model) | Negative Binomial Model 1 (Count Model) | Zero-Inflated Model 2 (Logit Model) | |||||
| Exp (β) * | CI | Exp (β) ** | CI | Exp (β) * | CI | Exp (β) ** | CI | |
| Intercept + | 2.70 | 2.42–3.02 | 2.70 | 1.97–3.68 | 1.77 | 1.62–1.94 | 6.96 | 5.80–8.36 |
| Sex (men) | 0.94 | 0.87–1.01 | 0.94 | 0.70–1.26 | 0.92 | 0.87–0.98 | 1.16 | 0.97–1.38 |
| Diabetes | 1.77 | 1.65–1.91 | 0.04 | 0.01–0.11 | 2.01 | 1.88–2.14 | 0.01 | 0.00–0.03 |
| Hypertension | 1.20 | 1.10–1.30 | 0.11 | 0.07–0.17 | 1.31 | 1.22–1.40 | 0.06 | 0.05–0.08 |
| Hyperlipidemia | 1.08 | 1.00–1.16 | 0.35 | 0.22–0.58 | 1.09 | 1.02–1.16 | 0.23 | 0.17–0.32 |
| Musculoskeletal | 1.08 | 1.01–1.17 | 0.39 | 0.29–0.52 | 1.08 | 1.01–1.15 | 0.34 | 0.28–0.42 |
| Rheumatic disease | 1.09 | 0.92–1.29 | 0.62 | 0.25–1.50 | 1.25 | 1.10–1.43 | 0.17 | 0.09–0.33 |
| Vascular disease 3 | 1.17 | 1.07–1.29 | 0.14 | 0.04–0.45 | 1.20 | 1.09–1.32 | 0.18 | 0.09–0.33 |
| MI 4 | 1.21 | 1.11–1.32 | 0.10 | 0.04–0.26 | 1.22 | 1.11–1.34 | 0.04 | 0.01–0.12 |
| Tobacco abuse 5 | 1.22 | 1.13–1.32 | 0.66 | 0.48–0.92 | 1.19 | 1.09–1.31 | 0.37 | 0.27–0.50 |
| Age 6 | 1.01 | 1.00–1.01 | 0.98 | 0.97–0.99 | 1.00 | 1.00–1.01 | 0.96 | 0.95–0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras, C.P.; Teraa, M.; Bots, M.L.; de Boer, A.R.; Peters, S.A.E.; van Doorn, S.; Vernooij, R.W.M. The Frequency of Primary Healthcare Contacts Preceding the Diagnosis of Lower-Extremity Arterial Disease: Do Women Consult General Practice Differently? J. Clin. Med. 2022, 11, 3666. https://doi.org/10.3390/jcm11133666
Porras CP, Teraa M, Bots ML, de Boer AR, Peters SAE, van Doorn S, Vernooij RWM. The Frequency of Primary Healthcare Contacts Preceding the Diagnosis of Lower-Extremity Arterial Disease: Do Women Consult General Practice Differently? Journal of Clinical Medicine. 2022; 11(13):3666. https://doi.org/10.3390/jcm11133666
Chicago/Turabian StylePorras, Cindy P., Martin Teraa, Michiel L. Bots, Annemarijn R. de Boer, Sanne A. E. Peters, Sander van Doorn, and Robin W. M. Vernooij. 2022. "The Frequency of Primary Healthcare Contacts Preceding the Diagnosis of Lower-Extremity Arterial Disease: Do Women Consult General Practice Differently?" Journal of Clinical Medicine 11, no. 13: 3666. https://doi.org/10.3390/jcm11133666
APA StylePorras, C. P., Teraa, M., Bots, M. L., de Boer, A. R., Peters, S. A. E., van Doorn, S., & Vernooij, R. W. M. (2022). The Frequency of Primary Healthcare Contacts Preceding the Diagnosis of Lower-Extremity Arterial Disease: Do Women Consult General Practice Differently? Journal of Clinical Medicine, 11(13), 3666. https://doi.org/10.3390/jcm11133666