Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR2E2 Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Imaging
2.3. Chest Radiographs
2.4. ECG Analysis
2.5. A Fortified Method to Screen for Left Ventricular Hypertrophy
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Associations between Radiographic and Basic CMR Variables
3.3. Characteristics of the Patients with Normal and Increased Chest Radiograph Indicators of Cardiac Size Enlargement
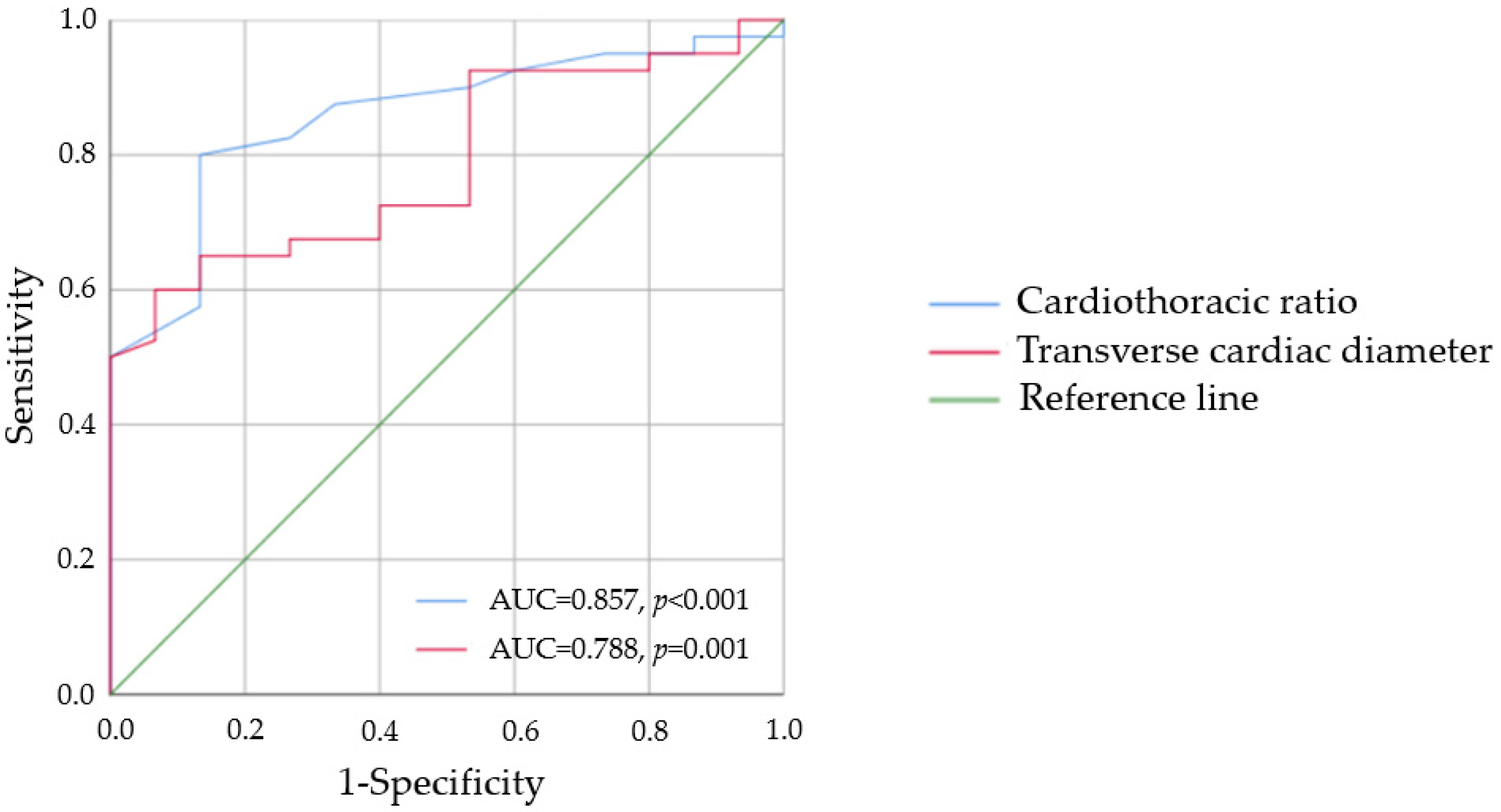
3.4. Radiographic and Electrocardiographic Criteria in the Screening for LVH
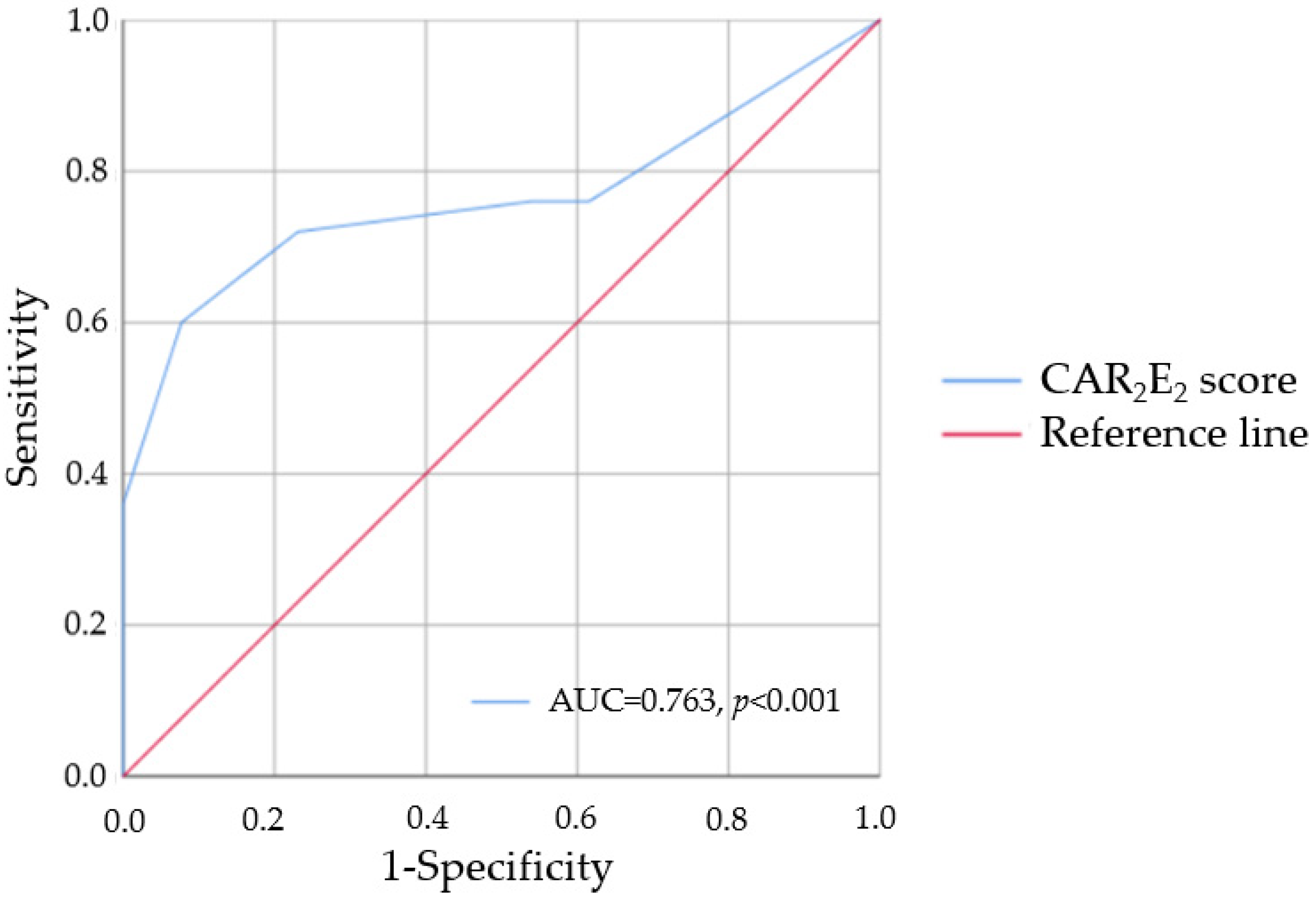
3.5. A Novel Screening Tool for LVH
3.6. Combined Criteria in the Screening for LVH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales, M.-A.; Prediletto, R.; Rossi, G.; Catapano, G.; Lombardi, M.; Rovai, D. Routine Chest X-ray: Still Valuable for the Assessment of Left Ventricular Size and Function in the Era of Super Machines? J. Clin. Imaging Sci. 2012, 2, 25. [Google Scholar] [CrossRef]
- Loomba, R.S.; Shah, P.H.; Nijhawan, K.; Aggarwal, S.; Arora, R. Cardiothoracic ratio for prediction of left ventricular dilation: A systematic review and pooled analysis. Futur. Cardiol. 2015, 11, 171–175. [Google Scholar] [CrossRef]
- Giamouzis, G.; Sui, X.; Love, T.E.; Butler, J.; Young, J.B.; Ahmed, A. A Propensity-Matched Study of the Association of Cardiothoracic Ratio with Morbidity and Mortality in Chronic Heart Failure. Am. J. Cardiol. 2008, 101, 343–347. [Google Scholar] [CrossRef]
- Vogl, T.J.; Reith, W.; Rummeny, E.J. Diagnostic and Interventional Radiology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Park, H.E.; Chon, S.-B.; Na, S.H.; Lee, H.; Choi, S.-Y. A Fortified Method to Screen and Detect Left Ventricular Hypertrophy in Asymptomatic Hypertensive Adults: A Korean Retrospective, Cross-Sectional Study. Int. J. Hypertens. 2018, 2018, 6072740. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzebska, A.; Sitkiewicz, D. MicroRNAs in the development of left ventricular remodeling and postmyocardial infarction heart failure. Pol. Arch. Intern. Med. 2020, 130, 59–65. [Google Scholar]
- Xu, L.; Chen, G.; Liang, Y.; Zhou, C.; Zhang, F.; Fan, T.; Chen, X.; Zhou, H.; Yuan, W. T helpers 17 cell responses induced cardiac hypertrophy and remodeling in essential hypertension. Pol. Arch. Intern. Med. 2021, 131, 257–265. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Gjesdal, O.; Almeida, A.; Nacif, M.; Wu, C.; Bluemke, D.A.; Brumback, L.; Lima, J.A.C. Left Ventricular Mass and Hypertrophy by Echocardiography and Cardiac Magnetic Resonance: The Multi-Ethnic Study of Atherosclerosis. Echocardiography 2013, 31, 12–20. [Google Scholar] [CrossRef]
- Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.; Liu, K.; Olson, J.; Burke, G.L.; Folsom, A.R. The Relationship of Left Ventricular Mass and Geometry to Incident Cardiovascular Events: The MESA (Multi-Ethnic Study of Atherosclerosis) Study. J. Am. Coll. Cardiol. 2008, 52, 2148–2155. [Google Scholar] [CrossRef]
- Alfakih, K.; Reid, S.; Hall, A.; Sivananthan, M.U. The assessment of left ventricular hypertrophy in hypertension. J. Hypertens. 2006, 24, 1223–1230. [Google Scholar] [CrossRef]
- Bacharova, L.; Schocken, D.; Estes, E.H.; Strauss, D. The Role of ECG in the Diagnosis of Left Ventricular Hypertrophy. Curr. Cardiol. Rev. 2014, 10, 257–261. [Google Scholar] [CrossRef]
- Pewsner, D.; Jüni, P.; Egger, M.; Battaglia, M.; Sundstrom, J.; Bachmann, L.M. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: Systematic review. BMJ 2007, 335, 711. [Google Scholar] [CrossRef]
- Peguero, J.G.; Presti, S.L.; Perez, J.; Issa, O.; Brenes, J.C.; Tolentino, A. Electrocardiographic Criteria for the Diagnosis of Left Ventricular Hypertrophy. J. Am. Coll. Cardiol. 2017, 69, 1694–1703. [Google Scholar] [CrossRef]
- Jalanko, M.; Heliö, T.; Mustonen, P.; Kokkonen, J.; Huhtala, H.; Laine, M.; Jääskeläinen, P.; Tarkiainen, M.; Lauerma, K.; Sipola, P.; et al. Novel electrocardiographic features in carriers of hypertrophic cardiomyopathy causing sarcomeric mutations. J. Electrocardiol. 2018, 51, 983–989. [Google Scholar] [CrossRef]
- Lim, D.Y.; Sng, G.; Ho, W.H.; Hankun, W.; Sia, C.-H.; Lee, J.S.; Shen, X.; Tan, B.Y.; Lee, E.C.; Dalakoti, M.; et al. Machine learning versus classic electrocardiographic criteria for the detection of echocardiographic left ventricular hypertrophy in a pre-participation cohort. Kardiologia Polska 2021, 79, 654–661. [Google Scholar] [CrossRef]
- Matusik, P.S.; Bryll, A.; Matusik, P.T.; Pac, A.; Popiela, T.J. Electrocardiography and cardiac magnetic resonance imaging in the detection of left ventricular hypertrophy: The impact of indexing methods. Kardiol. Pol. 2020, 78, 889–898. [Google Scholar] [CrossRef]
- Matusik, P.S.; Bryll, A.; Matusik, P.T.; Pac, A.; Popiela, T.J. Ischemic and non-ischemic patterns of late gadolinium enhancement in heart failure with reduced ejection fraction. Cardiol. J. 2021, 28, 67–76. [Google Scholar] [CrossRef]
- Petersen, S.E.; Aung, N.; Sanghvi, M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Zhu, X.; Wei, Y.; Yang, G.; Tang, L.; Xu, Y. Which can predict left ventricular size and systolic function: Cardiothoracic ratio or transverse cardiac diameter. J. X-ray Sci. Technol. 2015, 23, 557–565. [Google Scholar] [CrossRef]
- Chana, H.S.; Martin, C.A.; Cakebread, H.E.; Adjei, F.D.; Gajendragadkar, P.R. Diagnostic accuracy of cardiothoracic ratio on admission chest radiography to detect left or right ventricular systolic dysfunction: A retrospective study. J. R. Soc. Med. 2015, 108, 317–324. [Google Scholar] [CrossRef]
- Esmail, H.; Oni, T.; Thienemann, F.; Omar-Davies, N.; Wilkinson, R.; Ntsekhe, M. Cardio-Thoracic Ratio Is Stable, Reproducible and Has Potential as a Screening Tool for HIV-1 Related Cardiac Disorders in Resource Poor Settings. PLoS ONE 2016, 11, e0163490. [Google Scholar] [CrossRef]
- Baranowski, R.; Wojciechowski, D.; Kozłowski, D.; Kukla, P.; Kurpesa, M.; Lelakowski, J.; Maciejewska, M.; Średniawa, B.; Wranicz, J.K. Compendium for performing and describing the resting electrocardiogram. Diagnostic criteria describe rhythm, electrical axis of the heart, QRS voltage, automaticity and conduction disorders. Experts’ group statement of the Working Group on Noninvasive Ele. Kardiol. Pol. 2016, 74, 493–500. [Google Scholar] [CrossRef][Green Version]
- Buchner, S.; Debl, K.; Haimerl, J.; Djavidani, B.; Poschenrieder, F.; Feuerbach, S.; Riegger, G.A.; Luchner, A. Electrocardiographic diagnosis of left ventricular hypertrophy in aortic valve disease: Evaluation of ECG criteria by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2009, 11, 18. [Google Scholar] [CrossRef]
- Baranowski, R.; Wojciechowski, D.; Kozłowski, D.; Kukla, P.; Kurpesa, M.; Lelakowski, J.; Maciejewska, M.; Średniawa, B.; Wranicz, J.K. [Electrocardiographic criteria for diagnosis of the heart chamber enlargement, necrosis and repolarisation abnormalities including acute coronary syndromes. Experts’ group statement of the Working Group on Noninvasive Electrocardiology and Telemedicine of]. Kardiol. Pol. 2016, 74, 812–819. [Google Scholar] [CrossRef]
- Uygur, B.; Celik, O.; Demir, A.R.; Sahin, A.A.; Guner, A.; Avci, Y.; Bulut, U.; Tasbulak, O.; Demirci, G.; Uzun, F.; et al. A simplified acute kidney injury predictor following transcatheter aortic valve implantation: The ACEF score. Kardiologia Polska 2021, 79, 662–668. [Google Scholar] [CrossRef]
- Canepa, M.; Palmisano, P.; Dell’Era, G.; Ziacchi, M.; Ammendola, E.; Accogli, M.; Occhetta, E.; Biffi, M.; Nigro, G.; Ameri, P.; et al. Usefulness of the MAGGIC Score in Predicting the Competing Risk of Non-Sudden Death in Heart Failure Patients Receiving an Implantable Cardioverter-Defibrillator: A Sub-Analysis of the OBSERVO-ICD Registry. J. Clin. Med. 2021, 11, 121. [Google Scholar] [CrossRef]
- Yamada, S.; Kaneshiro, T.; Yoshihisa, A.; Nodera, M.; Amami, K.; Nehashi, T.; Takeishi, Y. Albumin-Bilirubin Score for Prediction of Outcomes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy. J. Clin. Med. 2021, 10, 5378. [Google Scholar] [CrossRef]
- Özdemir, E.; Esen, Ş.; Emren, S.V.; Karaca, M.; Nazlı, C. Association between Intermountain Risk Score and long-term mortality with the transcatheter aortic valve implantation procedure. Kardiologia Polska 2021, 79, 1215–1222. [Google Scholar] [CrossRef]
- Matusik, P.T. Biomarkers and Cardiovascular Risk Stratification. Eur. Heart J. 2019, 40, 1483–1485. [Google Scholar] [CrossRef]
- Okólska, M.; Skubera, M.; Matusik, P.; Płazak, W.; Pająk, J.; Róg, B.; Podolec, P.; Tomkiewicz-Pająk, L. Chronotropic incompetence causes multiple organ complications in adults after the Fontan procedure. Kardiologia Polska 2021, 79, 410–417. [Google Scholar] [CrossRef]
- Okólska, M.; Łach, J.; Matusik, P.T.; Pająk, J.; Mroczek, T.; Podolec, P.; Tomkiewicz-Pająk, L. Heart Rate Variability and Its Associations with Organ Complications in Adults after Fontan Operation. J. Clin. Med. 2021, 10, 4492. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chuang, Y.-T.; Huang, J.-Y.; Ueng, K.-C. Long-Term Prognosis of Febrile Individuals with Right Precordial Coved-Type ST-Segment Elevation Brugada Pattern: A 10-Year Prospective Follow-Up Study. J. Clin. Med. 2021, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.T.; Leśniak, W.J.; Heleniak, Z.; Undas, A. Thromboembolism and bleeding in patients with atrial fibrillation and stage 4 chronic kidney disease: Impact of biomarkers. Kardiologia Polska 2021, 79, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Rayner, B.L.; Goodman, H.; Opie, L.H. The chest radiographA useful investigation in the evaluation of hypertensive patients. Am. J. Hypertens. 2004, 17, 507–510. [Google Scholar] [CrossRef][Green Version]
- Buba, F.; Okeahialam, B.; Anjorin, C. The Value of Chest Radiogram and Electrocardiogram in the Assessment of Left Ventricular Hypertrophy among Adult Hypertensives. J. Med. Sci. 2008, 8, 298–301. [Google Scholar] [CrossRef][Green Version]
- Truszkiewicz, K.; Poręba, R.; Gać, P. Radiological Cardiothoracic Ratio in Evidence-Based Medicine. J. Clin. Med. 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bonow, R.O.; Mann, D.L.; Tomaselli, G.F.; Bhatt, D.; Solomon, S.D.; Braunwald, E. Braunwald’s Heart Disease. In A Textbook of Cardiovascular Medicine; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Okute, Y.; Shoji, T.; Hayashi, T.; Kuwamura, Y.; Sonoda, M.; Mori, K.; Shioi, A.; Tsujimoto, Y.; Tabata, T.; Emoto, M.; et al. Cardiothoracic Ratio as a Predictor of Cardiovascular Events in a Cohort of Hemodialysis Patients. J. Atheroscler. Thromb. 2017, 24, 412–421. [Google Scholar] [CrossRef]
- Perez, A.A.; Ribeiro, A.L.P.; Barros, M.V.L.; De Sousa, M.R.; Bittencourt, R.J.; Machado, F.S.; Rocha, M.O.C. Value of the radiological study of the thorax for diagnosing left ventricular dysfunction in Chagas’ disease. Arq. Bras. de Cardiol. 2003, 80, 208–213. [Google Scholar] [CrossRef]
- Grotenhuis, H.B.; Zhou, C.; Tomlinson, G.; Isaac, K.V.; Seed, M.; Grosse-Wortmann, L.; Yoo, S.-H. Cardiothoracic ratio on chest radiograph in pediatric heart disease: How does it correlate with heart volumes at magnetic resonance imaging? Pediatr. Radiol. 2015, 45, 1616–1623. [Google Scholar] [CrossRef]
- Fukuta, H.; Little, W.C. Contribution of Systolic and Diastolic Abnormalities to Heart Failure with a Normal and a Reduced Ejection Fraction. Prog. Cardiovasc. Dis. 2007, 49, 229–240. [Google Scholar] [CrossRef]
- Truszkiewicz, K.; Poręba, M.; Poręba, R.; Gać, P. Radiological Cardiothoracic Ratio as a Potential Predictor of Right Ventricular Enlargement in Patients with Suspected Pulmonary Embolism Due to COVID-19. J. Clin. Med. 2021, 10, 5703. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Morceli, J.; Gonçalves, R.S.; da Silva Franco, R.J.; Habermann, F.; Meira, D.A.; Matsubara, B.B. Accuracy of chest radiography plus electrocardiogram in diagnosis of hypertrophy in hypertension. Arq. Bras. Cardiol. 2012, 99, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Screaton, N. The cardiothoracic ratio—An inaccurate and outdated measurement: New data from CT. Eur. Radiol. 2010, 20, 1597–1598. [Google Scholar] [CrossRef] [PubMed]
- Simkus, P.; Gimeno, M.G.; Banisauskaite, A.; Noreikaite, J.; McCreavy, D.; Penha, D.; Arzanauskaite, M. Limitations of cardiothoracic ratio derived from chest radiographs to predict real heart size: Comparison with magnetic resonance imaging. Insights Imaging 2021, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Schlett, C.L.; Kwait, D.C.; Mahabadi, A.A.; Bamberg, F.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Simple area-based measurement for multidetector computed tomography to predict left ventricular size. Eur. Radiol. 2010, 20, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Gradman, A.H.; Alfayoumi, F. From Left Ventricular Hypertrophy to Congestive Heart Failure: Management of Hypertensive Heart Disease. Prog. Cardiovasc. Dis. 2006, 48, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, F.R.; Hohendanner, F.; Jin, G.; Sedej, S.; Edelmann, F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. J. Appl. Physiol. 2015, 119, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Meani, S.; Sala, C.; Valerio, C.; Negri, F.; Mancia, G. Age related prevalence of severe left ventricular hypertrophy in essential hypertension: Echocardiographic findings from the ETODH study. Blood Press. 2012, 21, 139–145. [Google Scholar] [CrossRef]
- Herman, C. What makes a screening exam “good”? Virtual Mentor 2006, 8, 34–37. [Google Scholar]
- Papadakis, M.; Carre, F.; Kervio, G.; Rawlins, J.; Panoulas, V.F.; Chandra, N.; Basavarajaiah, S.; Carby, L.; Fonseca, T.; Sharma, S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur. Heart J. 2011, 32, 2304–2313. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, S.Y.; Jeon, K.; Lee, Y.; Kim, K.; Park, J.; Oh, B.; Lee, M. Deep Learning–Based Algorithm for Detecting Aortic Stenosis Using Electrocardiography. J. Am. Heart Assoc. 2020, 9, e014717. [Google Scholar] [CrossRef]
- Mont, L.; Pelliccia, A.; Sharma, S.; Biffi, A.; Borjesson, M.; Terradellas, J.B.; Carré, F.; Guasch, E.; Heidbuchel, H.; La Gerche, A.; et al. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Eur. J. Prev. Cardiol. 2016, 24, 41–69. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.X.; Howard, L. Left Ventricular Hypertrophy in Athletes: Differentiating Physiology from Pathology. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Selvanayagam, J.B.; Francis, J.M.; Myerson, S.G.; Wiesmann, F.; Robson, M.D.; Östman-Smith, I.; Casadei, B.; Watkins, H.; Neubauer, S. Differentiation of athlete’s heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2005, 7, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.; Joshi, S.; Murigu, T.; Alpendurada, F.; Jabbour, A.; Melina, G.; Banya, W.; Gulati, A.; Roussin, I.; Raza, S.; et al. Midwall Fibrosis Is an Independent Predictor of Mortality in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2011, 58, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanaka, F.; Onoda, T.; Tanno, K.; Ohsawa, M.; Sakata, K.; Omama, S.; Ogasawara, K.; Ishibashi, Y.; Itai, K.; et al. Prognostic Value of Electrocardiographic Left Ventricular Hypertrophy on Cardiovascular Risk in a Non-Hypertensive Community-Based Population. Am. J. Hypertens. 2018, 31, 895–901. [Google Scholar] [CrossRef]
- D’Agostino Sr, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]

| Parameters | LVH; n = 40 | No LVH; n = 15 | p Value |
|---|---|---|---|
| Demographic characteristicsand anthropometric data | |||
| Age (years) | 51.5 (32.3–64.0) | 33.0 (25.0–47.0) | 0.04 |
| Age ≥40 years | 26 (65.0%) | 4 (26.7%) | 0.01 |
| Age ≥65 years | 11 (27.5%) | 2 (13.3%) | 0.48 * |
| Female sex, n (%) | 7 (17.5%) | 2 (13.3%) | 1 * |
| BMI (kg/m2) | 25.1 (22.1–29.0) | 26.8 (23.1–28.7) | 0.63 |
| BMI ≥ 25 kg/m2, n (%) | 20 (50.0%) | 9 (60.0%) | 0.5 |
| BMI ≥ 30 kg/m2, n (%) | 7 (17.5%) | 3 (20.0%) | 1 * |
| Cardiovascular diseases, comorbidities and risk factors, n (%) | |||
| Heart failure | 30 (75.0%) | 5 (33.3%) | 0.004 |
| CAD | 18 (45.0%) | 4 (26.7%) | 0.22 |
| Myocardial infarction | 10 (25.0%) | 2 (13.3%) | 0.35 |
| Diabetes mellitus | 7 (17.5%) | 1 (6.7%) | 0.42 * |
| Hypertension | 18 (45.0%) | 5 (33.3%) | 0.44 |
| Dyslipidemia | 17 (42.5%) | 4 (26.7%) | 0.28 |
| Smoking | 11 (27.5%) | 2 (13.3%) | 0.48 |
| Atrial fibrillation | 7 (17.5%) | 1 (6.7%) | 0.42 * |
| CKD | 5 (12.5%) | 1 (6.7%) | 1 * |
| CMR parameters | |||
| LVEF (%) | 29.7 (23.4–50.5) | 59.5 (50.9–66.2) | <0.001 |
| LVM (g) | 173.0 (152.9–234.0) | 126.0 (114.4–134.5) | <0.001 |
| LVEDV (mL) | 228.0 (169.4–322.4) | 169.5 (123.0–189.6) | 0.002 |
| LVEDV/BSA > 117 (M), >101 (F), n (%) | 20 (50.0%) | 0 (0.0%) | 0.001 |
| LVESV (mL) | 124.6 (84.7–261.7) | 65.6 (58.3–74.1) | <0.001 |
| Chest radiograph data | |||
| CTR | 0.50 ± 0.07 | 0.42 ± 0.04 | <0.001 |
| CTR > 0.5, n (%) | 17 (42.5%) | 0 (0.0%) | 0.002 * |
| TCD (mm) | 167.7 ± 26.9 | 141.4 ± 16.9 | 0.001 |
| TCD ≥ 155 mm (M), ≥145 mm (F), n (%) | 28 (70.0%) | 3 (20.0%) | 0.001 |
| Parameters | CTR > 0.5 (n = 17) | CTR ≤ 0.5 (n = 38) | p Value | TCD ≥ 155 mm (M) or ≥ 145 mm (F) (n = 31) | TCD < 155 mm (M) or < 145 mm (F) (n = 24) | p Value |
|---|---|---|---|---|---|---|
| Demographic characteristicsand anthropometric data | ||||||
| Age (years) | 60.0 (40.5–69.5) | 36.0 (27.0–60.5) | 0.009 | 60.0 (37.0–70.0) | 32.5 (27.0–44.3) | <0.001 |
| Age ≥40 years | 14 (82.4%) | 16 (42.1%) | 0.006 | 23 (74.2%) | 7 (29.2%) | 0.001 |
| Age ≥65 years | 6 (35.3%) | 7 (18.4%) | 0.19 * | 12 (38.7%) | 1 (4.2%) | 0.003 |
| Female sex, n (%) | 2 (11.8%) | 7 (18.4%) | 0.71 * | 3 (9.7%) | 6 (25.0%) | 0.16 * |
| BMI (kg/m2) | 29.0 ± 5.6 | 24.8 ± 3.7 | 0.002 | 27.0 (24.0–30.2) | 23.8 (22.1–27.5) | 0.08 |
| BMI ≥ 25 kg/m2, n (%) | 14 (82.4%) | 15 (39.5%) | 0.003 | 20 (64.5%) | 9 (37.5%) | 0.047 |
| BMI ≥ 30 kg/m2, n (%) | 6 (35.3%) | 4 (10.5%) | 0.05 * | 7 (22.6%) | 3 (12.5%) | 0.49 * |
| Cardiovascular diseases, comorbidities and risk factors, n (%) | ||||||
| Heart failure | 16 (94.1%) | 19 (50.0%) | 0.002 | 25 (80.6%) | 10 (41.7%) | 0.005 |
| CAD | 10 (58.8%) | 12 (31.6%) | 0.06 | 16 (51.6%) | 6 (25.0%) | 0.06 |
| Myocardial infarction | 4 (23.5%) | 8 (21.1%) | 1 * | 9 (29.0%) | 3 (12.5%) | 0.19 |
| Diabetes mellitus | 5 (29.4%) | 3 (7.9%) | 0.09 * | 6 (19.4%) | 2 (8.3%) | 0.44 * |
| Hypertension | 8 (47.1%) | 15 (39.5%) | 0.6 | 17 (54.8%) | 6 (25.0%) | 0.03 |
| Dyslipidemia | 9 (52.9%) | 12 (31.6%) | 0.13 | 16 (51.6%) | 5 (20.8%) | 0.03 |
| Smoking | 3 (17.6%) | 10 (26.3%) | 0.73 * | 7 (22.6%) | 6 (25.0%) | 1 |
| Atrial fibrillation | 6 (35.3%) | 2 (5.3%) | 0.008 * | 6 (19.4%) | 2 (8.3%) | 0.44 * |
| CKD | 2 (11.8%) | 4 (10.5%) | 1 * | 5 (16.1%) | 1 (4.2%) | 0.22 * |
| CMR parameters | ||||||
| LVEF (%) | 25.6 ± 9.1 | 49.2 ± 16.7 | <0.001 | 29.5 (21.0–46.7) | 58.2 (42.7–63.0) | <0.001 |
| LVM (g) | 233.6 (181.9–271.3) | 134.7 (120.9–167.0) | <0.001 | 201.6 ± 62.8 | 136.8 ± 29.1 | <0.001 |
| LVM/BSA > 72 g/m2 (M) or >55 g/m2 (F), n (%) | 17 (100.0%) | 23 (60.5%) | 0.002 * | 28 (90.3%) | 12 (50.0%) | 0.001 |
| LVEDV (mL) | 317.3 (239.7–392.0) | 175.2 (140.2–199.5) | <0.001 | 228.8 (168.7–355.6) | 173.9 (136.3–194.6) | 0.005 |
| LVEDV/BSA > 117 (M), >101 (F), n (%) | 14 (82.4%) | 6 (15.8%) | <0.001 | 17 (54.8%) | 3 (12.5%) | 0.002 |
| LVESV (mL) | 239.4 (152.4–313.1) | 82.4 (63.3–112.4) | <0.001 | 173.9 (82.1–271.3) | 71.7 (58.0–104.9) | 0.001 |
| Chest radiograph data | ||||||
| CTR | 0.57 ± 0.05 | 0.44 ± 0.04 | <0.001 | 0.53 ± 0.06 | 0.42 ± 0.04 | <0.001 |
| TCD (mm) | 192.3 ± 17.9 | 146.3 ± 16.3 | <0.001 | 171.0 (161.8–194.3) | 140.7 (130.3–149.5) | <0.001 |
| TCD ≥ 155 mm (M), ≥145 mm (F), n (%) | 17 (100%) | 14 (36.8%) | <0.001 | 17 (54.8%) | 0 (0.0%) | <0.001 |
| Parameters | LVH; n = 25 | No LVH; n = 13 | McNemar Test | ||
|---|---|---|---|---|---|
| TP | FN | FP | TN | ||
| Chest radiograph indicators of cardiac size enlargement | |||||
| CTR > 0.5 | 7 (28.0%) | 18 (72.0%) | 0 (0.0%) | 13 (100.0%) | <0.001 |
| TCD ≥ 155 mm (M), ≥145 mm (F) | 14 (56.0%) | 11 (44.0%) | 3 (23.1%) | 76.9%) | 0.06 |
| ECG-LVH criteria | |||||
| Cornell voltage | 1 (4.0%) | 24 (96.0%) | 0 (0.0%) | 13 (100.0%) | <0.001 |
| Cornell product | 2 (8.0%) | 23 (92.0%) | 0 (0.0%) | 13 (100.0%) | <0.001 |
| Peguero-Lo Presti criterion | 14 (56.0%) | 11 (44.0%) | 2 (15.4%) | 11 (84.6%) | 0.02 |
| Sokolow-Lyon voltage | 3 (12.0%) | 22 (88.0%) | 1 (7.7%) | 12 (92.3%) | <0.001 |
| At least one positive ECG-LVH criterion | 14 (56.0%) | 11 (44.0%) | 3 (23.1%) | 10 (76.9%) | 0.06 |
| Parameters | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Accuracy (%) (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|---|---|---|---|---|---|---|
| Chest radiograph indicators of cardiac size enlargement | |||||||
| CTR > 0.5 | 28.0 (12.1–49.4) | 100.0 (75.3–100.0) | 100.0 * | 41.9 (36.1–48.0) | 52.6 (35.8–69.0) | * | 0.7 (0.6–0.9) |
| TCD ≥ 155 mm (M), ≥145 mm (F) | 56.0 (34.9–75.6) | 76.9 (46.2–95.0) | 82.4 (62.0–93.0) | 47.6 (34.8–60.8) | 63.2 (46.0–78.2) | 2.4 (0.9–7.0) | 0.6 (0.3–1.0) |
| ECG-LVH criteria | |||||||
| Cornell voltage | 4.0 (0.1–20.4) | 100.0 (75.3–100.0) | 100.0 * | 35.1 (33.3–37.0) | 36.8 (21.8–54.0) | * | 1.0 (0.9–1.0) |
| Cornell product | 8.0 (1.0–26.0) | 100.0 (75.3–100.0) | 100.0 * | 36.1 (33.5–38.8) | 39.5 (24.0–56.6) | * | 0.9 (0.8–1.0) |
| Peguero-Lo Presti criterion | 56.0 (34.9–75.6) | 84.6 (54.6–98.1) | 87.5 (65.1–96.3) | 50.0 (37.8–62.2) | 65.8 (48.7–80.4) | 3.6 (1.0–13.6) | 0.5 (0.3–0.9) |
| Sokolow-Lyon voltage | 12.0 (2.6–31.2) | 92.3 (64.0–99.8) | 75.0 (25.7–96.3) | 35.3 (30.6–40.3) | 39.5 (24.0–56.6) | 1.6 (0.2–13.6) | 1.0 (0.8–1.2) |
| At least one positive ECG-LVH criterion | 56.0 (34.9–75.6) | 76.9 (46.2–95.0) | 82.4 (62.0–93.0) | 47.6 (34.8–60.8) | 63.2 (46.0–78.2) | 2.4 (0.9–7.0) | 0.6 (0.3–1.0) |
| Parameters | LVH; n = 25 | No LVH; n = 13 | McNemar Test | ||
|---|---|---|---|---|---|
| TP | FN | FP | TN | ||
| Combined Rules with Use the Clinical Risk Factors, Chest Radiograph and ECG-LVH Criteria | |||||
| Model proposed by Park et al. [5] at score ≥2 † | 10 (40.0%) | 15 (60.0%) | 2 (15.4%) | 11 (84.6%) | 0.002 |
| Model 1 at score ≥2 ‡ | 15 (60.0%) | 10 (40.0%) | 4 (30.8%) | 9 (69.2%) | 0.18 |
| Model 2 at score ≥2 # | 15 (60.0%) | 10 (40.0%) | 3 (23.1%) | 10 (76.9%) | 0.09 |
| CAR2E2 score ≥3 ## | 18 (72.0%) | 7 (28.0%) | 3 (23.1%) | 10 (76.9%) | 0.344 |
| Parameters | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Accuracy (%) (95% CI) | PLR (95% CI) | NLR (95% CI) |
| Combined Rules with Use the Clinical Risk Factors, Chest Radiograph and ECG-LVH Criteria | |||||||
| Model proposed by Park et al. [5] score ≥2 † | 40.0 (21.1–61.3) | 84.6 (54.6–98.1) | 83.3 (56.2–95.1) | 42.3 (33.1–52.1) | 55.3 (38.3–71.4) | 2.6 (0.7–10.2) | 0.7 (0.5–1.1) |
| Model 1 score ≥2 ‡ | 60.0 (38.7–78.9) | 69.2 (38.6–90.9) | 79.0 (61.0–90.0) | 47.4 (33.0–62.2) | 63.2 (46.0–78.2) | 2.0 (0.8–4.7) | 0.6 (0.3–1.1) |
| Model 2 score ≥2 # | 60.0 (38.7–78.9) | 76.9 (46.2–95.0) | 83.3 (63.8–93.4) | 50.0 (36.2–63.8) | 65.8 (48.7–80.4) | 2.6 (0.9–77.4) | 0.5 (0.3–0.9) |
| CAR2E2 score ≥3 ## | 72.0 (50.6–87.9) | 76.9 (46.2–95.0) | 85.7 (68.3–94.3) | 58.8 (41.6–74.1) | 73.7 (57.0–86.6) | 3.1 (1.1–8.7) | 0.4 (0.2–0.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusik, P.S.; Bryll, A.; Pac, A.; Popiela, T.J.; Matusik, P.T. Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR2E2 Score. J. Clin. Med. 2022, 11, 3585. https://doi.org/10.3390/jcm11133585
Matusik PS, Bryll A, Pac A, Popiela TJ, Matusik PT. Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR2E2 Score. Journal of Clinical Medicine. 2022; 11(13):3585. https://doi.org/10.3390/jcm11133585
Chicago/Turabian StyleMatusik, Patrycja S., Amira Bryll, Agnieszka Pac, Tadeusz J. Popiela, and Paweł T. Matusik. 2022. "Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR2E2 Score" Journal of Clinical Medicine 11, no. 13: 3585. https://doi.org/10.3390/jcm11133585
APA StyleMatusik, P. S., Bryll, A., Pac, A., Popiela, T. J., & Matusik, P. T. (2022). Clinical Data, Chest Radiograph and Electrocardiography in the Screening for Left Ventricular Hypertrophy: The CAR2E2 Score. Journal of Clinical Medicine, 11(13), 3585. https://doi.org/10.3390/jcm11133585






