Procedural Outcome Following Stent-Assisted Coiling for Wide-Necked Aneurysms Using Three Different Stent Models: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Antiplatelet and Anticoagulation Therapy
2.3. Endovascular Procedure
2.4. Stent Models
2.5. Aneurysm Details and Procedural Outcome
2.6. Statistical Methods
3. Results
3.1. Study Population
3.2. Procedural Outcome and Complications
3.3. Logistic Regression Analysis for Complete Occlusion
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Han, H.; Lee, W.; Park, S.; Chung, J.; Kim, Y.; Park, K. Safety and Efficacy of Stent-Assisted Coiling of Unruptured Intracranial Aneurysms Using Low-Profile Stents in Small Parent Arteries. Am. J. Neuroradiol. 2021, 42, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Primiani, C.T.; Ren, Z.; Piper, K.; Fiorella, D.J.; Rai, A.T.; Orlov, K.; Kislitsin, D.; Gorbatykh, A.; Mocco, J.; et al. Stent-assisted coiling of cerebral aneurysms: Multi-center analysis of radiographic and clinical outcomes in 659 patients. J. NeuroInterv. Surg. 2019, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Jabbour, P.; Singhal, S.; Drueding, R.; Starke, R.M.; Dalyai, R.T.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; et al. Stent-Assisted Coiling of Intracranial Aneurysms. Stroke 2013, 44, 1348–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotin, M.; Blanc, R. Balloons and Stents in the Endovascular Treatment of Cerebral Aneurysms: Vascular Anatomy Remodeled. Front. Neurol. 2014, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, G.; Maus, V.; Weber, W.; Berlis, A.; Maurer, C.; Fischer, S. Two-center experience with Neuroform Atlas stent-assisted coil occlusion of broad-based intracranial aneurysms. Neuroradiology 2021, 63, 1093–1101. [Google Scholar] [CrossRef]
- Krischek, Ö.; Miloslavski, E.; Fischer, S.; Shrivastava, S.; Henkes, H. A Comparison of Functional and Physical Properties of Self-Expanding Intracranial Stents [Neuroform3, Wingspan, Solitaire, Leo(+), Enterprise]. Minim. Invasive Neurosurg. 2011, 54, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Jankowitz, B.T.; Hanel, R.; Jadhav, A.P.; Loy, D.N.; Frei, D.; Siddiqui, A.H.; Puri, A.S.; Khaldi, A.; Turk, A.S.; Malek, A.M.; et al. Neuroform Atlas Stent System for the treatment of intracranial aneurysm: Primary results of the Atlas Humanitarian Device Exemption cohort. J. NeuroInterv. Surg. 2019, 11, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Kis, B.; Weber, W.; Berlit, P.; Kühne, D. Elective Treatment of Saccular and Broad-necked Intracranial Aneurysms using a Closed-cell Nitinol Stent (Leo). Neurosurgery 2006, 58, 443–450. [Google Scholar] [CrossRef]
- Pumar, J.M.; Blanco, M.; Vázquez, F.; Castiñeira, J.A.; Guimaraens, L.; Garcia-Allut, A. Preliminary Experience with Leo Self-Expanding Stent for the Treatment of Intracranial Aneurysms. Am. J. Neuroradiol. 2005, 26, 2573–2577. [Google Scholar]
- Higashida, R.T.; Halbach, V.V.; Dowd, C.F.; Juravsky, L.; Meagher, S. Initial Clinical Experience with a New Self-Expanding Nitinol Stent for the Treatment of Intracranial Cerebral Aneurysms: The Cordis Enterprise Stent. Am. J. Neuroradiol. 2005, 26, 1751–1756. [Google Scholar]
- Roy, D.; Milot, G.; Raymond, J. Endovascular Treatment of Unruptured Aneurysms. Stroke 2001, 32, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Kallmes, D.F.; Cloft, H.J. Effect of Age on Outcomes of Treatment of Unruptured Cerebral Aneurysms. Stroke 2011, 42, 1320–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.N.; Brown, R.D., Jr. Management of unruptured intracranial aneurysms. Neurol. Clin. Pract. 2013, 3, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, Y.; Koji, T.; Kashimura, H.; Otawara, Y.; Ogawa, A.; Ogasawara, K. Female sex as a risk factor for the growth of asymptomatic unruptured cerebral saccular aneurysms in elderly patients: Clinical article. J. Neurosurg. JNS 2014, 121, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Smietana, J.; Hauck, E.; Hoh, B.; Hopkins, N.; Siddiqui, A.; Levy, E.I.; Meng, H.; Mocco, J. Size Ratio Correlates with Intracranial Aneurysm Rupture Status. Stroke 2010, 41, 916–920. [Google Scholar] [CrossRef] [Green Version]
- Weir, B.; Amidei, C.; Kongable, G.; Findlay, J.M.; Kassell, N.F.; Kelly, J.; Dai, L.; Karrison, T.G. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J. Neurosurg. 2003, 99, 447–451. [Google Scholar] [CrossRef]
- Zhao, B.; Yin, R.; Lanzino, G.; Kallmes, D.; Cloft, H.; Brinjikji, W. Endovascular Coiling of Wide-Neck and Wide-Neck Bifurcation Aneurysms: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2016, 37, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Henkes, H.; Bose, A.; Felber, S.; Miloslavski, E.; Berg-Dammer, E.; Kühne, D. Endovascular Coil Occlusion of Intracranial Aneurysms Assisted by a Novel Self-Expandable Nitinol Microstent (Neuroform). Interv. Neuroradiol. 2002, 8, 107–119. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Dong, L.; Liu, P.; Jia, L.; Zhang, Y.; Lv, M. Clinical and Angiographic Outcomes after Stent-Assisted Coiling of Cerebral Aneurysms with Laser-Cut and Braided Stents: A Comparative Analysis of the Literatures. Front. Neurol. 2021, 12, 666481. [Google Scholar] [CrossRef]
- Lubicz, B.; Kadou, A.; Morais, R.F.; Mine, B. Leo stent for endovascular treatment of intracranial aneurysms: Very long-term results in 50 patients with 52 aneurysms and literature review. Neuroradiology 2017, 59, 271–276. [Google Scholar] [CrossRef]
- Luecking, H.; Struffert, T.; Goelitz, P.; Engelhorn, T.; Brandner, S.; Kuramatsu, J.B.; Lang, S.; Schmidt, M.; Doerfler, A. Stent-Assisted Coiling Using Leo+ Baby Stent: Immediate and Mid-Term Results. Clin. Neuroradiol. 2021, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Olthuis, S.G.H.; Hartog, S.J.D.; van Kuijk, S.M.J.; Staals, J.; Benali, F.; van der Leij, C.; Beumer, D.; Nijeholt, G.J.L.; Uyttenboogaart, M.; Martens, J.M.; et al. Influence of the interventionist’s experience on outcomes of endovascular thrombectomy in acute ischemic stroke: Results from the MR CLEAN Registry. J. NeuroInterv. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Pumar, J.M.; Sucasas, P.; Mosqueira, A.; Vega, P.; Murias, E. Five-Years Angiographic Follow-Up of Wide-Neck Intracranial Aneurysms Treated with LEO Plus Stent. Front. Neurol. 2021, 12, 744962. [Google Scholar] [CrossRef] [PubMed]
- Júnior, J.R.; Telles, J.; Da Silva, S.A.; Iglesio, R.F.; Brigido, M.M.; Caldas, J.G.M.P.; Teixeira, M.J.; Figueiredo, E.G. Epidemiological analysis of 1404 patients with intracranial aneurysm followed in a single Brazilian institution. Surg. Neurol. Int. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Harrod, C.G.; Batjer, H.H.; Bendok, B.R. Deficiencies in estrogen-mediated regulation of cerebrovascular homeostasis may contribute to an increased risk of cerebral aneurysm pathogenesis and rupture in menopausal and postmenopausal women. Med. Hypotheses 2006, 66, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, C.C.; Molenberg, R.; Mensing, L.A.; Wermer, M.J.; Juvela, S.; Lindgren, A.E.; Jääskeläinen, J.E.; Koivisto, T.; Yamazaki, T.; Uyttenboogaart, M.; et al. Sex Difference and Rupture Rate of Intracranial Aneurysms: An Individual Patient Data Meta-Analysis. Stroke 2022, 53, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Jeong, H.W.; Jeong, Y.G.; Heo, Y.J.; Seo, J.H.; Paeng, S.H. A Self-expanding Nitinol Stent (Enterprise) for the Treatment of Wide-necked Intracranial Aneurysms: Angiographic and Clinical Results in 40 Aneurysms. J. Cerebrovasc. Endovasc. Neurosurg. 2013, 15, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Chalouhi, N.; Drueding, R.; Starke, R.M.; Jabbour, P.; Dumont, A.S.; Gonzalez, L.F.; Rosenwasser, R.; Tjoumakaris, S. In-Stent Stenosis after Stent-Assisted Coiling: Incidence, Predictors and Clinical Outcomes of 435 Cases. Neurosurgery 2012, 72, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Izar, B.; Rai, A.; Raghuram, K.; Rotruck, J.; Carpenter, J. Comparison of Devices Used for Stent-Assisted Coiling of Intracranial Aneurysms. PLoS ONE 2011, 6, e24875. [Google Scholar] [CrossRef] [Green Version]
- Geyik, S.; Yavuz, K.; Yurttutan, N.; Saatci, I.; Cekirge, H. Stent-Assisted Coiling in Endovascular Treatment of 500 Consecutive Cerebral Aneurysms with Long-Term Follow-Up. Am. J. Neuroradiol. 2013, 34, 2157–2162. [Google Scholar] [CrossRef] [Green Version]

| Baseline Characteristics | All Patients | LEO+ | Enterprise | Atlas | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| (n = 156) | (n = 93) | (n = 19) | (n = 44) | ||||
| ▪ Age, years; median (IQR) | 62 (55–71) | 62 (56–73) | 59 (48–67) | 61 (53.5–68) | 0.235 | 0.276 | 0.549 |
| ▪ Female sex, % (n) | 73.7 (115) | 72 (67) | 73.7 (14) | 77.3 (34) | 0.884 | 0.516 | 0.759 |
| ▪ Arterial hypertension, % (n) | 45.5 (71) | 47.3 (44) | 47.4 (9) | 59.1 (26) | 0.996 | 0.197 | 0.390 |
| ▪ History of multiple Aneurysms, % (n) | 44.9 (70) | 51.6 (48) | 21.1 (4) | 40.9 (18) | 0.015 * | 0.242 | 0.129 |
| Aneurysm detection, % (n) | |||||||
| ▪ Incidental | 82 (128) | 75.3 (70) | 73.7 (14) | 90.9 (40) | |||
| ▪ Previously treated | 19.9 (31) | - | - | - | - | - | - |
| - Clipped | 1.3 (2) | 2.2 (2) | - | - | - | - | - |
| - Coiled | 18.6 (29) | 21.5 (20) | 26.3 (5) | 9.1 (4) | - | - | - |
| Aneurysm location, % (n) | |||||||
| ▪ Anterior circulation | 85.3 (133) | 86 (80) | 94.7 (18) | 79.5 (5) | 0.457 | <0.001 | <0.001 |
| - Internal carotid artery | 20.5 (32) | 19.4 (18) | 21.1 (4) | 22.7 (10) | - | - | - |
| - Middle cerebral artery | 41.7 (65) | 45.2 (42) | 31.6 (6) | 38.6 (17) | - | - | - |
| - Anterior communicating artery | 19.9 (31) | 19.4 (18) | 42.1 (8) | 11.4 (5) | - | - | - |
| - Pericallosal artery | 3.2 (5) | 2.2 (2) | - | 6.8 (3) | - | - | - |
| ▪ Posterior circulation | 14.7 (23) | 14 (13) | 5.3 (1) | 20.5 (9) | 0.457 | 0.332 | 0.258 |
| - Vertebral artery | 1.9 (3) | 3.2 (2) | - | - | - | - | - |
| - Posterior inferior cerebellar artery | 1.9 (3) | 3.2 (2) | - | - | - | - | - |
| - Basilar artery | 6.4 (10) | 2.2 (2) | - | 18.2 (8) | - | - | - |
| - Basilar tip | 3.8 (6) | 4.3 (3) | 5.3 (1) | 2.3 (1) | - | - | - |
| - Posterior cerebral artery | 0.6 (1) | 1.1 (1) | - | - | - | - | - |
| Aneurysm size, mm; mean (±SD) | |||||||
| ▪ Dome | 5.9 (3) | 5.5 (2.7) | 5.5 (2.6) | 6.7 (3.7) | 0.969 | 0.049 | 0.109 |
| ▪ Neck | 4 (2.1) | 3.8 (2.1) | 3.7 (1.5) | 4.6 (2.4) | 0.901 | 0.109 | 0.214 |
| ▪ Dome/Neck ratio | 1.5 (0.57) | 1.5 (0.54) | 1.6 (0.83) | 1.5 (0.49) | 0.664 | 0.895 | 0.626 |
| ▪ Vessel diameter pre-target | 3 (0.78) | 2.9 (0.8) | 3 (0.5) | 3.1 (0.9) | 0.252 | 0.205 | 0.851 |
| ▪ Vessel diameter post-target | 2.4 (0.67) | 2.4 (0.7) | 2.4 (0.6) | 2.5 (0.7) | 0.938 | 0.414 | 0.559 |
| Procedural details, median (IQR) | |||||||
| Implanted number of coils | 6 (4–8) | 6 (3–8) | 7 (4–9) | 7 (4–9) | 0.544 | 0.084 | 0.741 |
| Periprocedural Complications | All Patients | LEO+ | Enterprise | Atlas |
|---|---|---|---|---|
| % (n) | (n = 156) | (n = 93) | (n = 19) | (n = 44) |
| Technical | ||||
| 1.3 (2) | 2 | - | - |
| 3.2 (5) | 2 | 2 | 1 |
| Imaging | ||||
| 16 (25) | 7 | 2 | 16 |
| 3.2 (5) | 4 | 1 | - |
| 0.6 (1) | 1 | - | - |
| 0.6 (1) | 1 | - | - |
| 1.3 (2) | - | - | 2 |
| 5.1 (8) | 6 | 2 | - |
| 6.8 (9) | 7 | - | 2 |
| Clinical | ||||
| 0.6 (1) | 1 | - | - |
| 0.6 (1) | 1 | - | - |
| Univariable Logistic Regression Analysis | ||||||
|---|---|---|---|---|---|---|
| First Follow-Up | Last Available Follow-Up | |||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.0 | 0.96–1.04 | 0.942 | 0.99 | 0.9–1.03 | 0.851 |
| (years) | ||||||
| Sex | 5.9 | 2.26–15.32 | 0.001 * | 5 | 2.95–12.16 | 0.001 * |
| (female) | ||||||
| Aneurysm | 2.56 | 0.76–8.68 | 0.13 | 1.72 | 0.55–5.44 | 0.353 |
| location | ||||||
| (anterior) | ||||||
| Leo | 1.94 | 0.79–4.78 | 0.149 | 2.24 | 0.97–5.18 | 0.059 |
| (yes) | ||||||
| Atlas | 1.76 | 0.66–4.69 | 0.259 | 0.58 | 0.23–1.45 | 0.241 |
| (yes) | ||||||
| Enterprise | 0.66 | 0.19–2.30 | 0.511 | 0.51 | 0.17–1.151 | 0.224 |
| (yes) | ||||||
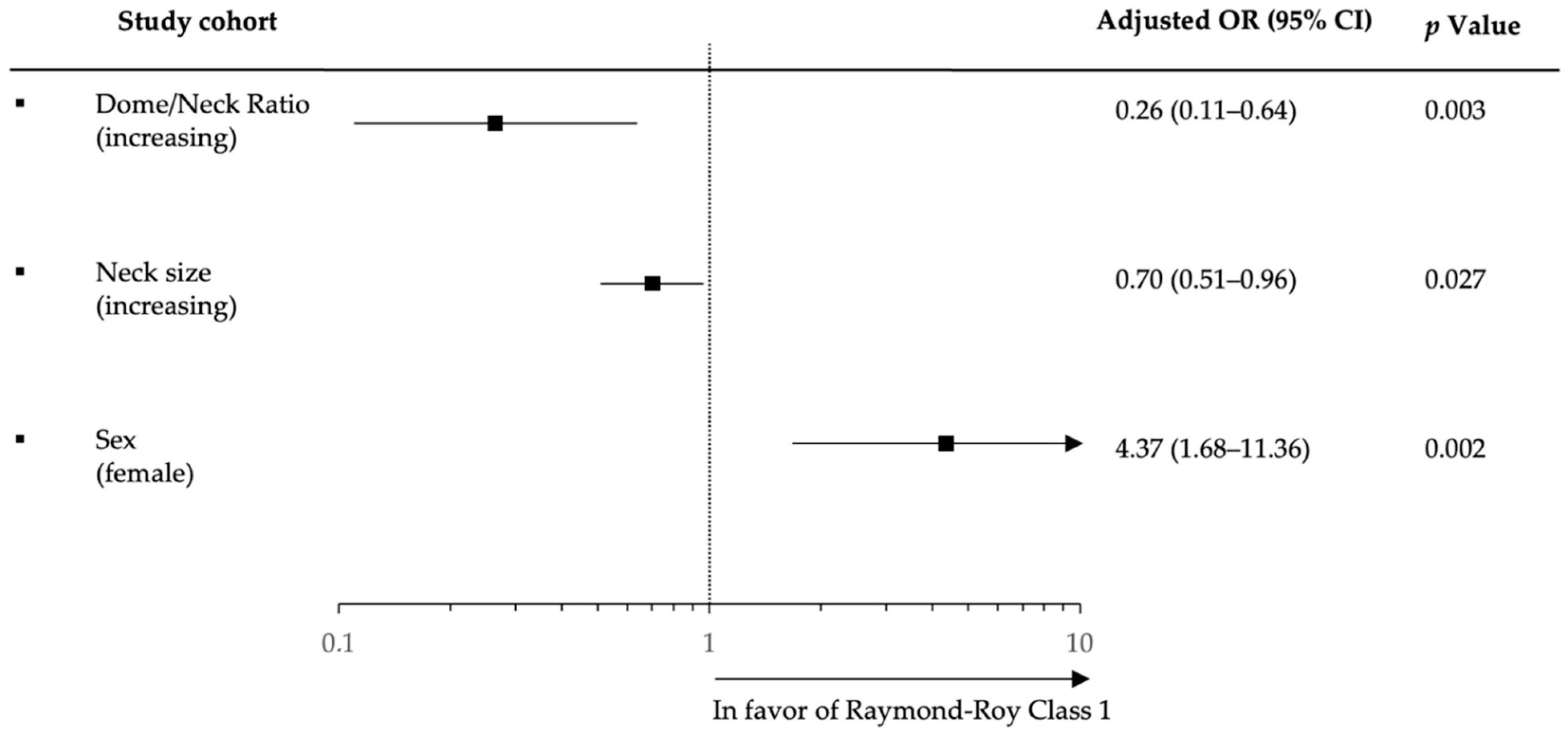
| Dome size (increasing) | 0.78 | 0.66–0.93 | 0.006 * | 0.75 | 0.63–0.89 | 0.001 * |
| Neck size | 0.73 | 0.56–0.95 | 0.021 * | 0.74 | 0.58–0.95 | 0.017 * |
| (increasing) | ||||||
| Dome/Neck Ratio | 0.61 | 0.29–1.27 | 0.187 | 0.45 | 0.22–9.23 | 0.029 * |
| (increasing) | ||||||
| Vessel diameter pre-target (increasing) | 0.41 | 0.22–0.77 | 0.006 * | 0.52 | 0.29–9.24 | 0.026 * |
| Vessel diameter post-target (increasing) | 0.707 | 0.37–1.35 | 0.292 | 0.75 | 0.41–1.41 | 0.376 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strittmatter, C.; Meyer, L.; Broocks, G.; Alexandrou, M.; Politi, M.; Boutchakova, M.; Henssler, A.; Reinges, M.; Simgen, A.; Papanagiotou, P.; et al. Procedural Outcome Following Stent-Assisted Coiling for Wide-Necked Aneurysms Using Three Different Stent Models: A Single-Center Experience. J. Clin. Med. 2022, 11, 3469. https://doi.org/10.3390/jcm11123469
Strittmatter C, Meyer L, Broocks G, Alexandrou M, Politi M, Boutchakova M, Henssler A, Reinges M, Simgen A, Papanagiotou P, et al. Procedural Outcome Following Stent-Assisted Coiling for Wide-Necked Aneurysms Using Three Different Stent Models: A Single-Center Experience. Journal of Clinical Medicine. 2022; 11(12):3469. https://doi.org/10.3390/jcm11123469
Chicago/Turabian StyleStrittmatter, Catherine, Lukas Meyer, Gabriel Broocks, Maria Alexandrou, Maria Politi, Maria Boutchakova, Andreas Henssler, Marcus Reinges, Andreas Simgen, Panagiotis Papanagiotou, and et al. 2022. "Procedural Outcome Following Stent-Assisted Coiling for Wide-Necked Aneurysms Using Three Different Stent Models: A Single-Center Experience" Journal of Clinical Medicine 11, no. 12: 3469. https://doi.org/10.3390/jcm11123469
APA StyleStrittmatter, C., Meyer, L., Broocks, G., Alexandrou, M., Politi, M., Boutchakova, M., Henssler, A., Reinges, M., Simgen, A., Papanagiotou, P., & Roth, C. (2022). Procedural Outcome Following Stent-Assisted Coiling for Wide-Necked Aneurysms Using Three Different Stent Models: A Single-Center Experience. Journal of Clinical Medicine, 11(12), 3469. https://doi.org/10.3390/jcm11123469






