The Role of Wilms’ Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia
Abstract
1. Introduction
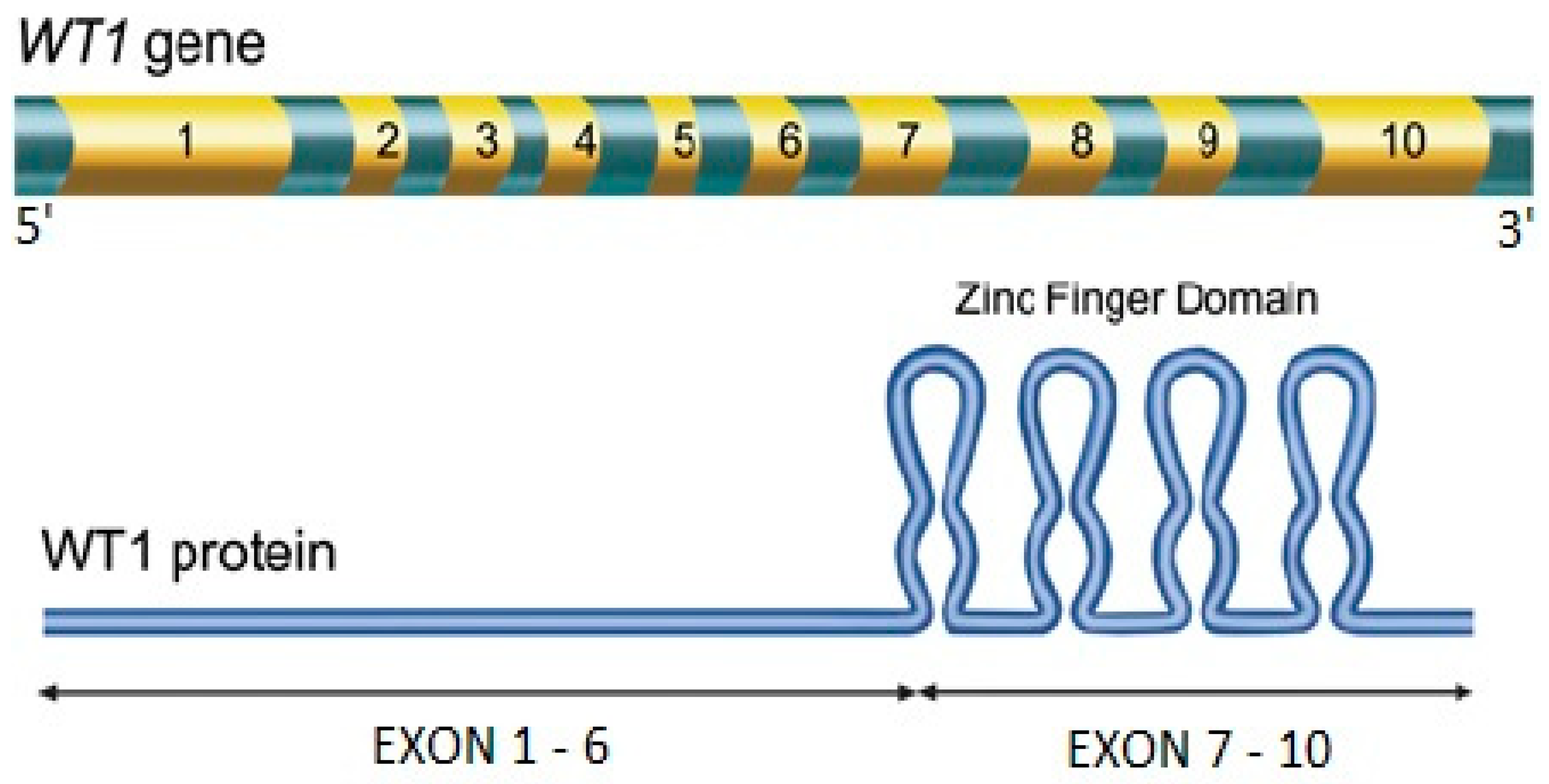
2. The WT1 Gene in AML
3. The Crucial Role of MRD in AML
4. European LeukemiaNet Standardized Method for Quantitative Evaluation of WT1 Expression
5. Role of WT1 Expression Monitoring in Bone Marrow as MRD Marker
| WT1-MRD Monitoring PRE-Allo-SCT) | |||
|---|---|---|---|
| Study (Reference) | WT1 Threshold | N° of Patients | Summary of Results |
| Cilloni et al. [4] | 250 copies /104 ABL | 91 | WT1 log reduction post-induction → independent predictor of relapse. WT1-MRD-positive post-consolidation → increased risk of relapse (67% vs. 42% at 5 years; p = 0.004). |
| Candoni et al. [36] | 250 copies /104 ABL | 122 | Better OS (median not reached vs. 9 months, p < 0.0001), DFS (median not reached vs. 8 months, p < 0.0001), and relapse rate (15% vs. 44%, p = 0.00073) for WT1-MRD-negative before Allo-SCT. |
| Candoni et al. [37] | 250 copies /104 ABL | 62 FLT3 pos | Better OS (median not reached vs. 10.5 months, p = 0.0005) and DFS (median not reached vs. 5.5 months, p = 0.0001) for WT1-MRD-negative before Allo-SCT. Same outcome after Allo-SCT for WT1-MRD-positive and cases with active disease at the time of transplant. |
| Frairia et al. [38] | 350 copies /104 ABLpost induction 150 copies /104 ABL pre-Allo-SCT | 255 | Shorter OS and DFS for WT1-MRD-positive after induction (HR for mortality 2.13, p = 0.018 and HR for relapse 2.81, p = 0.025). 2-year CIR after Allo-SCT higher for WT1-positive pre-Allo-SCT (HR 4.61, p = 0.002). |
| Lambert et al. [39] | 250 copies /104 ABL | 341 (ALFA-0702 trial) | Better 4-year CIR (29% vs. 61%, p < 0.0001), RFS (60% vs. 26%, p < 0.0001), and OS (71% vs. 44%, p = 0.0005) for patients WT1-MRD-negative after induction. |
| Nomdedéu et al. [40] | 3 groups post-induction (<17.5, 17.6 to 170.5, >170.6 /104 ABL) and post-consolidation (<10, 10.1 to 100, >100 /104 ABL) | 365 (CETLAM protocol) | Median OS and RFS of the 3 post-induction groups: 59 and 59 months, 48 and 41 months, and 23 and 19 months, respectively. Median OS and RFS of the 3 post-consolidation groups: 72 and 65 months, 59 and 46 months, and 30 and 27 months, respectively. |
| WT1-MRD monitoring POST-Allo-SCT | |||
| Candoni et al. [41] | 250 copies /104 ABL | 38 | Rapid decline in WT1 levels in patients in CR after SCT. WT1 increased before o at morphologic relapse in all patients. |
| Candoni et al. [42] | 250 copies /104 ABL | 25 | WT1 increased before relapse in all patients. WT1 increase anticipated loss of molecular chimerism in 50% of cases. |
| Pozzi et al. [43] | 100 copies /104 ABL | 122 | Higher incidence of relapse in WT1-MRD-positive at any time post-Allo-SCT. 5-year OS 40% for WT1-MRD-positive vs. 63% for WT1-MRD-negative cases (p = 0.03). |
| Nomdedéu et al. [44] | 100 copies /104 ABL | 193 | WT1-MRD-negative at first evaluation post-Allo-SCT had better OS, PFS, and CIR. Sustained WT1-MRD negativity → excellent outcomes. |
| Duléry et al. [45] | 250 copies /104 ABL | 139 | Worse CIR, EFS, and OS for WT1-MRD-positive than WT1-MRD-negative patients (90%, 10%, and 21.4% vs. 14.7%, 72.3%, and 75.4%, respectively). RIC associated with higher rate of WT1-MRD positivity at 3 months in univariate analysis (not in multivariate). |
6. Role of WT1 Expression Monitoring in Peripheral Blood as MRD Marker
| WT1-MRD Monitoring PRE-Allo-SCT) | |||
|---|---|---|---|
| Study (Reference) | WT1 Threshold | N° of Patients | Summary of Results |
| Cilloni et al. [4] | 50 copies /104 ABL | 91 (15 only PB) | See Table 1. No differences between log reduction in PB compared to BM in patients with paired analysis available. |
| Lambert et al. [39] | 50 copies /104 ABL | 341 (231 paired samples) | See Table 1. In paired samples, observed 9% of discrepancies. Discrepant patients were designed as WT1-MRD-positive. |
| Rautenberg et al. [46] | 50 copies /104 ABL | 64 AML/MDS (50 AML) | Better 2-year CIR (10% vs. 61%, p < 0.01), RFS (89% vs. 37%, p < 0.01), and OS (90% vs. 54%, p = 0.03) for WT1-MRD-negative pre-Allo-SCT. Same outcome after Allo-SCT for WT1-MRD-positive and patients with active disease at the time of transplant. |
| Malagola et al. [47] | 5 copies /104 ABL | 24 | Better OS for WT1-MRD-negative (1-year: 81% vs. 60%, 2-year: 81% vs. 0%, 3-year: 54% vs. 0%; p = 0.03) and better RFS (1-year: 63% vs. 20%, p = 0.02). Relapse rate was 31% for WT1-MRD-negative patients pre-Allo-SCT vs. 73% for WT1-MRD-positive. |
| WT1-MRD monitoring POST-Allo-SCT | |||
| Duléry et al. [45] | 50 copies /104 ABL | 139 (106 PB) | See Table 1. Similar outcome for patients tested in PB and BM alone. Better correlation between relapse and WT1-MRD positivity in PB than in BM. |
| Malagola et al. [47] | 5 copies /104 ABL | 24 | Relapse rate higher in patients with WT1 ≥ 5 at 3 months (56% vs. 38%; p = 0.43) and 6 months (71% vs. 20%; p = 0.03) after Allo-SCT. |
| Israyelyan et al. [48] | 50 copies /104 ABL | 82 (39 AML) | Specificity 100% with a positive predictive value of 100%, and sensitivity 75% for the method. |
| Polak et al. [49] | 50 copies /104 ABL | 32 | Absolute correlation with MFC, chimerism, and fusion transcripts. WT1 increase anticipated positivity by MFC and chimerism by 1 month. |
7. WT1 Expression Assessment in Peripheral Blood Stem Cells Harvest
8. Possible Pitfalls of WT1 Overexpression as MRD Marker
9. Conclusions
- WT1 is a non-specific panleukemic marker whose overexpression is detectable at diagnosis in about 80% of AML. WT1 can serve as a longitudinal MRD quantitative monitoring, alone (in AML without other biological markers) or in combination with other MRD markers (MFC and/or other specific markers if available).
- To evaluate the WT1 expression, it is recommended to use the standardized method of the European LeukemiaNet and its proposed cut-off values of 250 WT1 copies/104 ABL on BM and 50 WT1 copies/104 ABL on PB, which has been validated in large and multicenter cohorts of patients and normal controls (optimal sensitivity and specificity) [4].
- It is necessary to have the value of WT1 expression at diagnosis of AML, to exclude from the dynamic monitoring the cases of AML that do not overexpress WT1. WT1 monitoring should not be performed in subsequent treatment phases if WT1 expression is unknown at the time of AML diagnosis.
- During treatment, the most important and informative time points for WT1-MRD evaluation are post-induction, to assess both the log reduction and achievement of WT1-MRD negativity, and before Allo-SCT, or at the end of consolidation, if Allo-SCT is not planned [4,36,37,38,39,40,46,47]. A recent large study (including 425 patients) conducted by Cho et al. supports this recommendation [53]. It is clear, from this study, that the most informative time point for WT1-MRD assessment was before Allo-SCT, and that the 250 copies/104 ABL was the most predictive threshold for post-transplant relapse [53].
- In the post-transplant (or post-consolidation) setting, WT1-MRD evaluation can detect relapse earlier compared to other MRD methods (such as MFC and chimerism) and allow early intervention [42,49]. In this regard, some small studies, involving MRD-triggered ciclosporin withdrawal with or without donor lymphocyte infusions (DLI) or even hypomethylating agents, reported interesting results that need to be confirmed in prospective and larger clinical trials [43,54,55].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- Cilloni, D.; Gottardi, E.; De Micheli, D.; Serra, A.; Volpe, G.; Messa, F.; Rege-Cambrin, G.; Guerrasio, A.; Divona, M.; Lo Coco, F.; et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002, 16, 2115–2121. [Google Scholar] [CrossRef]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T.; et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European LeukemiaNet study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Suarez Saiz, F.; Minden, M.D. A tumor suppressor and oncogene: The WT1 story. Leukemia 2007, 21, 868–876. [Google Scholar] [CrossRef]
- Gessler, M.; Poustka, A.; Cavenee, W.; Neve, R.L.; Orkin, S.H.; Bruns, G.A.P. Homozygous deletion in Wilms tumours of a zinc-finge gene identifie by chromosome jumping. Nature 1990, 343, 774–778. [Google Scholar] [CrossRef]
- Hossain, A.; Saunders, G.F. The human sex-determining gene SRY is a direct target of WT1. J. Biol. Chem. 2001, 276, 16817–16823. [Google Scholar] [CrossRef]
- Haber, D.A.; Sohn, R.L.; Buckler, A.J.; Pelletier, J.; Call, K.M.; Housman, D.E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc. Natl. Acad. Sci. USA 1991, 88, 9618–9622. [Google Scholar] [CrossRef]
- Englert, C.; Vidal, M.; Maheswaran, S.; Ge, Y.; Ezzell, R.M.; Isselbacher, K.J.; Haber, D.A. Truncated WT1 mutants alter the subnuclear localization of the wild-type protein. Proc. Natl. Acad. Sci. USA 1995, 92, 11960–11964. [Google Scholar] [CrossRef]
- Inoue, K.; Tamaki, H.; Ogawa, H.; Oka, Y.; Soma, T.; Tatekawa, T.; Oji, Y.; Tsuboi, A.; Kim, E.H.; Kawakami, M.; et al. Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood 1998, 91, 2969–2976. [Google Scholar] [CrossRef]
- Gashler, A.L.; Bonthron, D.T.; Madden, S.L.; Rauscher, F.J., III.; Collins, T.; Sukhatme, V.P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc. Natl. Acad. Sci. USA 1992, 89, 10984. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.A.; Konicek, B.; Song, A.; Xia, X.-L.; Fredericks, W.J.; Rauscher, F.J., III. Inhibition of colony-stimulating factor-1 promoter activity by the product of the Wilms’ tumor locus. J. Biol. Chem. 1993, 268, 21271. [Google Scholar] [CrossRef]
- Drummond, I.A.; Madden, S.L.; Rohwer-Nutter, P.; Bell, G.I.; Sukhatme, V.P.; Rauscher, F.J., III. Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science 1992, 257, 674. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Re, G.G.; Drummond, I.A.; Sukhatme, V.P.; Rauscher, F.J., III.; Sens, D.A.; Garvin, A.J.; LeRoith, D.; Roberts, C.T., Jr. Increased expression of the insulin-like growth factor I receptor gene, IGFIR, in Wilms tumor is correlated with modulation of IGFIR promoter activity by the WT1 Wilms tumor gene product. Proc. Natl. Acad. Sci. USA 1993, 90, 5828. [Google Scholar] [CrossRef]
- Godyer, P.; Dehbi, M.; Torban, E.; Bruening, W.; Pelletier, J. Repression of the retinoic acid receptor-a gene by the Wilms tumor suppressor gene product, wt1. Oncogene 1995, 10, 1125. [Google Scholar]
- Hosen, N.; Sonoda, Y.; Oji, Y.; Kimura, T.; Minamiguchi, H.; Tamaki, H.; Kawakami, M.; Asada, M.; Kanato, K.; Motomura, M.; et al. Very low frequencies of human normal CD34+ haematopoietic progenitor cells express the Wilms’ tumour gene WT1 at levels similar to those in leukaemia cells. Br. J. Haematol. 2002, 116, 409–420. [Google Scholar] [CrossRef]
- Ellisen, L.W.; Carlesso, N.; Cheng, T.; Scadden, D.T.; Haber, D.A. The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells. EMBO J. 2001, 20, 1897–1909. [Google Scholar] [CrossRef]
- Svensson, E.; Eriksson, H.; Gekas, C.; Olofsson, T.; Richter, J.; Gullberg, U. DNA-binding dependent and independent functions of WT1 protein during human hematopoiesis. Exp. Cell Res. 2005, 308, 211–221. [Google Scholar] [CrossRef]
- Miwa, H.; Beran, M.; Saunders, G.F. Expression of the Wilms’ tumor gene (WT1) in human leukemias. Leukemia 1992, 6, 405–409. [Google Scholar]
- Menssen, H.D.; Renkl, H.J.; Rodeck, U.; Maurer, J.; Notter, M.; Schwartz, S.; Reinhardt, R.; Thiel, E. Presence of Wilms’ tumor gene (wt1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia 1995, 9, 1060–1067. [Google Scholar]
- Miyagi, T.; Ahuja, H.; Kubota, T.; Kubonishi, I.; Koeffler, H.P.; Miyoshi, I. Expression of the candidate Wilm’s tumor gene, WT1, in human leukemia cells. Leukemia 1993, 7, 970–977. [Google Scholar] [PubMed]
- Østergaard, M.; Olesen, L.H.; Hasle, H.; Kjeldsen, E.; Hokland, P. WT1 gene expression: An excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients—results from a single-centre study. Br. J. Haematol. 2004, 125, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Brieger, J.; Weidmann, E.; Fenchel, K.; Mitrou, P.S.; Hoelzer, D.; Bergmann, L. The expression of the Wilms’ tumor gene in acute myelocytic leukemias as a possible marker for leukemic blast cells. Leukemia 1994, 8, 2138–2143. [Google Scholar] [PubMed]
- Schmid, D.; Heinze, G.; Linnerth, B.; Tisljar, K.; Kusec, R.; Geissler, K.; Sillaber, C.; Laczika, K.; Mitterbauer, M.; Zöchbauer, S.; et al. Prognostic significance of WT1 gene expression at diagnosis in adult de novo acute myeloid leukemia. Leukemia 1997, 11, 639–643. [Google Scholar] [CrossRef][Green Version]
- Boyapati, A.; Kanbe, E.; Zhang, D.E. p53 alterations in myeloid leukemia. Acta Haematol. 2004, 111, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Valk, P.J.; Verhaak, R.G.; Beijen, M.A.; Erpelinck, C.A.; Barjesteh van Waalwijk van Doorn-Khosrovani, S.; Boer, J.M.; Beverloo, H.B.; Moorhouse, M.J.; van der Spek, P.J.; Löwenberg, B.; et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N. Engl. J. Med. 2004, 350, 1617–1628. [Google Scholar] [CrossRef]
- Inoue, K.; Sugiyama, H.; Ogawa, H.; Nakagawa, M.; Yamagami, T.; Miwa, H.; Kita, K.; Hiraoka, A.; Masaoka, T.; Nasu, K.; et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 1994, 84, 3071–3079. [Google Scholar] [CrossRef]
- Bergmann, L.; Miething, C.; Maurer, U.; Brieger, J.; Karakas, T.; Weidmann, E.; Hoelzer, D. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood 1997, 90, 1217–1225. [Google Scholar] [CrossRef]
- Barragán, E.; Cervera, J.; Bolufer, P.; Ballester, S.; Martín, G.; Fernández, P.; Collado, R.; Sayas, M.J.; Sanz, M.A. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica 2004, 89, 926–933. [Google Scholar]
- Ghannam, J.; Dillon, L.W.; Hourigan, C.S. Next-generation sequencing for measurable residual disease detection in acute myeloid leukaemia. Br. J. Haematol. 2020, 188, 77–85. [Google Scholar] [CrossRef]
- Ossenkoppele, G.; Schuurhuis, G.J. MRD in AML: Does it already guide therapy decision-making? Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.D.; Virgo, P.; Couzens, S.; Grimwade, D.; Russell, N.; Hills, R.K.; Burnett, A.K. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J. Clin. Oncol. 2013, 31, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Minervini, M.M.; Carella, A.M.; de Waure, C.; di Nardo, F.; Melillo, L.; D’Arena, G.; Zini, G.; Cascavilla, N. Comparison between multiparameter flow cytometry and WT1-RNA quantification in monitoring minimal residual disease in acute myeloid leukemia without specific molecular targets. Leuk. Res. 2012, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Malagola, M.; Skert, C.; Borlenghi, E.; Chiarini, M.; Cattaneo, C.; Morello, E.; Cancelli, V.; Cattina, F.; Cerqui, E.; Pagani, C.; et al. Postremission sequential monitoring of minimal residual disease by WT1 Q-PCR and multiparametric flow cytometry assessment predicts relapse and may help to address risk-adapted therapy in acute myeloid leukemia patients. Cancer Med. 2016, 5, 265–274. [Google Scholar] [CrossRef]
- Guolo, F.; Minetto, P.; Clavio, M.; Miglino, M.; Galaverna, F.; Raiola, A.M.; Di Grazia, C.; Colombo, N.; Pozzi, S.; Ibatici, A.; et al. Combining flow cytometry and WT1 assessment improves the prognostic value of pre-transplant minimal residual disease in acute myeloid leukemia. Haematologica 2017, 102, e348–e351. [Google Scholar] [CrossRef]
- Candoni, A.; De Marchi, F.; Zannier, M.E.; Lazzarotto, D.; Filì, C.; Dubbini, M.V.; Rabassi, N.; Toffoletti, E.; Lau, B.W.; Fanin, R. High prognostic value of pre-allogeneic stem cell transplantation minimal residual disease detection by WT1 gene expression in AML transplanted in cytologic complete remission. Leuk. Res. 2017, 63, 22–27. [Google Scholar] [CrossRef]
- Candoni, A.; De Marchi, F.; Zanini, F.; Zannier, M.E.; Simeone, E.; Toffoletti, E.; Chiarvesio, A.; Cerno, M.; Filì, C.; Patriarca, F.; et al. Predictive value of pretransplantation molecular minimal residual disease assessment by WT1 gene expression in FLT3-positive acute myeloid leukemia. Exp. Hematol. 2017, 49, 25–33. [Google Scholar] [CrossRef]
- Frairia, C.; Aydin, S.; Audisio, E.; Riera, L.; Aliberti, S.; Allione, B.; Busca, A.; D’Ardia, S.; Dellacasa, C.M.; Demurtas, A.; et al. Post-remissional and pre-transplant role of minimal residual disease detected by WT1 in acute myeloid leukemia: A retrospective cohort study. Leuk. Res. 2017, 61, 10–17. [Google Scholar] [CrossRef]
- Lambert, J.; Lambert, J.; Thomas, X.; Marceau-Renaut, A.; Micol, J.B.; Renneville, A.; Clappier, E.; Hayette, S.; Récher, C.; Raffoux, E.; et al. Early detection of WT1 measurable residual disease identifies high-risk patients, independent of transplantation in AML. Blood Adv. 2021, 5, 5258–5268. [Google Scholar] [CrossRef]
- Nomdedéu, J.F.; Hoyos, M.; Carricondo, M.; Bussaglia, E.; Estivill, C.; Esteve, J.; Tormo, M.; Duarte, R.; Salamero, O.; de Llano, M.P.; et al. Bone marrow WT1 levels at diagnosis, post-induction and post-intensification in adult de novo AML. Leukemia 2013, 27, 2157–2164. [Google Scholar] [CrossRef]
- Candoni, A.; Tiribelli, M.; Toffoletti, E.; Cilloni, D.; Chiarvesio, A.; Michelutti, A.; Simeone, E.; Pipan, C.; Saglio, G.; Fanin, R. Quantitative assessment of WT1 gene expression after allogeneic stem cell transplantation is a useful tool for monitoring minimal residual disease in acute myeloid leukemia. Eur. J. Haematol. 2009, 82, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Toffoletti, E.; Gallina, R.; Simeone, E.; Chiozzotto, M.; Volpetti, S.; Fanin, R. Monitoring of minimal residual disease by quantitative WT1 gene expression following reduced intensity conditioning allogeneic stem cell transplantation in acute myeloid leukemia. Clin. Transpl. 2011, 25, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, S.; Geroldi, S.; Tedone, E.; Luchetti, S.; Grasso, R.; Colombo, N.; Di Grazia, C.; Lamparelli, T.; Gualandi, F.; Ibatici, A.; et al. Leukaemia relapse after allogeneic transplants for acute myeloid leukaemia: Predictive role of WT1 expression. Br. J. Haematol. 2013, 160, 503–509. [Google Scholar] [CrossRef]
- Nomdedéu, J.F.; Esquirol, A.; Carricondo, M.; Pratcorona, M.; Hoyos, M.; Garrido, A.; Rubio, M.; Bussaglia, E.; García-Cadenas, I.; Estivill, C.; et al. Bone Marrow WT1 Levels in Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplasia: Clinically Relevant Time Points and 100 Copies Threshold Value. Biol. Blood Marrow Transpl. 2018, 24, 55–63. [Google Scholar] [CrossRef]
- Duléry, R.; Nibourel, O.; Gauthier, J.; Elsermans, V.; Behal, H.; Coiteux, V.; Magro, L.; Renneville, A.; Marceau, A.; Boyer, T.; et al. Impact of Wilms’ tumor 1 expression on outcome of patients undergoing allogeneic stem cell transplantation for AML. Bone Marrow Transpl. 2017, 52, 539–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rautenberg, C.; Lauseker, M.; Kaivers, J.; Jäger, P.; Fischermanns, C.; Pechtel, S.; Haas, R.; Kobbe, G.; Germing, U.; Schroeder, T. Prognostic impact of pretransplant measurable residual disease assessed by peripheral blood WT1-mRNA expression in patients with AML and MDS. Eur. J. Haematol. 2021, 107, 283–292. [Google Scholar] [CrossRef]
- Malagola, M.; Skert, C.; Ruggeri, G.; Turra, A.; Ribolla, R.; Cancelli, V.; Cattina, F.; Alghisi, E.; Bernardi, S.; Perucca, S.; et al. Peripheral blood WT1 expression predicts relapse in AML patients undergoing allogeneic stem cell transplantation. Biomed. Res. Int. 2014, 2014, 123079. [Google Scholar] [CrossRef]
- Israyelyan, A.; Goldstein, L.; Tsai, W.; Aquino, L.; Forman, S.J.; Nakamura, R.; Diamond, D.J. Real-time assessment of relapse risk based on the WT1 marker in acute leukemia and myelodysplastic syndrome patients after hematopoietic cell transplantation. Bone Marrow Transpl. 2015, 50, 26–33. [Google Scholar] [CrossRef]
- Polak, J.; Hajkova, H.; Haskovec, C.; Cechova, H.; Marinov, I.; Mikulenkova, D.; Markova, J.; Markova, M.; Vitek, A.; Valkova, V. Quantitative monitoring of WT1 expression in peripheral blood before and after allogeneic stem cell transplantation for acute myeloid leukemia—A useful tool for early detection of minimal residual disease. Neoplasma 2013, 60, 74–82. [Google Scholar] [CrossRef]
- Messina, C.; Candoni, A.; Carrabba, M.G.; Tresoldi, C.; Sala, E.; Tassara, M.; Crippa, A.; Peccatori, J.; Assanelli, A.; Gattillo, S.; et al. Wilms’ tumor gene 1 transcript levels in leukapheresis of peripheral blood hematopoietic cells predict relapse risk in patients autografted for acute myeloid leukemia. Biol. Blood Marrow Transpl. 2014, 20, 1586–1591. [Google Scholar] [CrossRef]
- Mulé, M.P.; Mannis, G.N.; Wood, B.L.; Radich, J.P.; Hwang, J.; Ramos, N.R.; Andreadis, C.; Damon, L.; Logan, A.C.; Martin, T.G.; et al. Multigene Measurable Residual Disease Assessment Improves Acute Myeloid Leukemia Relapse Risk Stratification in Autologous Hematopoietic Cell Transplantation. Biol. Blood Marrow Transpl. 2016, 22, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- King-Underwood, L.; Renshaw, J.; Pritchard-Jones, K. Mutations in the Wilms’ tumor gene WT1 in leukemias. Blood 1996, 87, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Min, G.J.; Park, S.S.; Shin, S.H.; Yahng, S.A.; Jeon, Y.W.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; Kim, Y.J.; et al. WT1 Measurable Residual Disease Assay in Patients With Acute Myeloid Leukemia Who Underwent Allogeneic Hematopoietic Stem Cell Transplantation: Optimal Time Points, Thresholds, and Candidates. Biol. Blood Marrow Transpl. 2019, 25, 1925–1932. [Google Scholar] [CrossRef]
- Di Grazia, C.; Pozzi, S.; Geroldi, S.; Grasso, R.; Miglino, M.; Colombo, N.; Tedone, E.; Luchetti, S.; Lamparelli, T.; Gualandi, F.; et al. Wilms Tumor 1 Expression and Pre-emptive Immunotherapy in Patients with Acute Myeloid Leukemia Undergoing an Allogeneic Hemopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2016, 22, 1242–1246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rautenberg, C.; Bergmann, A.; Pechtel, S.; Fischermanns, C.; Haas, R.; Germing, U.; Kobbe, G.; Schroeder, T. Wilm’s Tumor 1-guided preemptive treatment with hypomethylating agents for molecular relapse of AML and MDS after allogeneic transplantation. Bone Marrow Transpl. 2021, 56, 442–450. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzarotto, D.; Candoni, A. The Role of Wilms’ Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia. J. Clin. Med. 2022, 11, 3306. https://doi.org/10.3390/jcm11123306
Lazzarotto D, Candoni A. The Role of Wilms’ Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia. Journal of Clinical Medicine. 2022; 11(12):3306. https://doi.org/10.3390/jcm11123306
Chicago/Turabian StyleLazzarotto, Davide, and Anna Candoni. 2022. "The Role of Wilms’ Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia" Journal of Clinical Medicine 11, no. 12: 3306. https://doi.org/10.3390/jcm11123306
APA StyleLazzarotto, D., & Candoni, A. (2022). The Role of Wilms’ Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia. Journal of Clinical Medicine, 11(12), 3306. https://doi.org/10.3390/jcm11123306






