Menstrual Cycle Changes Joint Laxity in Females—Differences between Eumenorrhea and Oligomenorrhea
Abstract
:1. Introduction
2. Materials and Methods
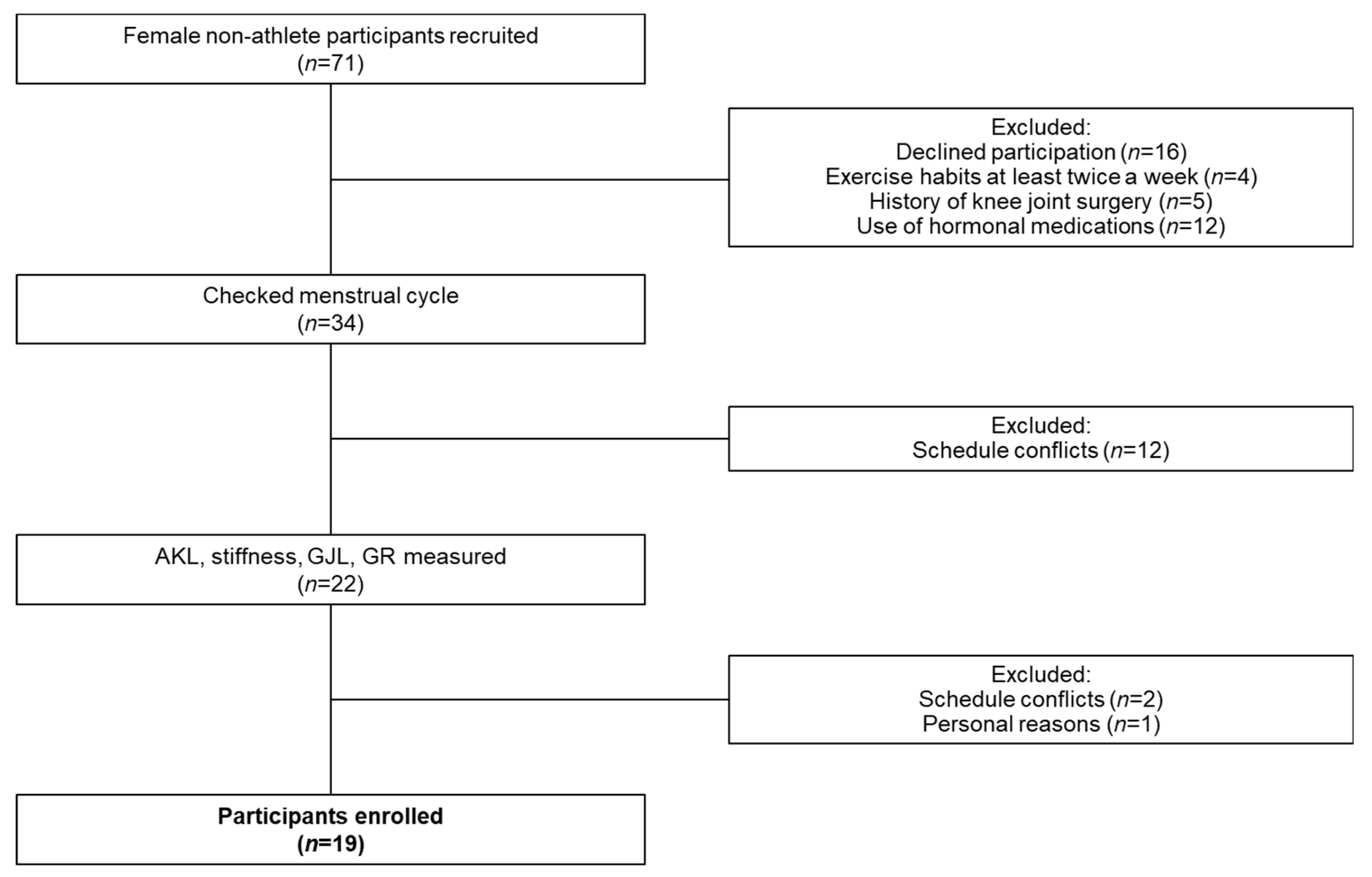
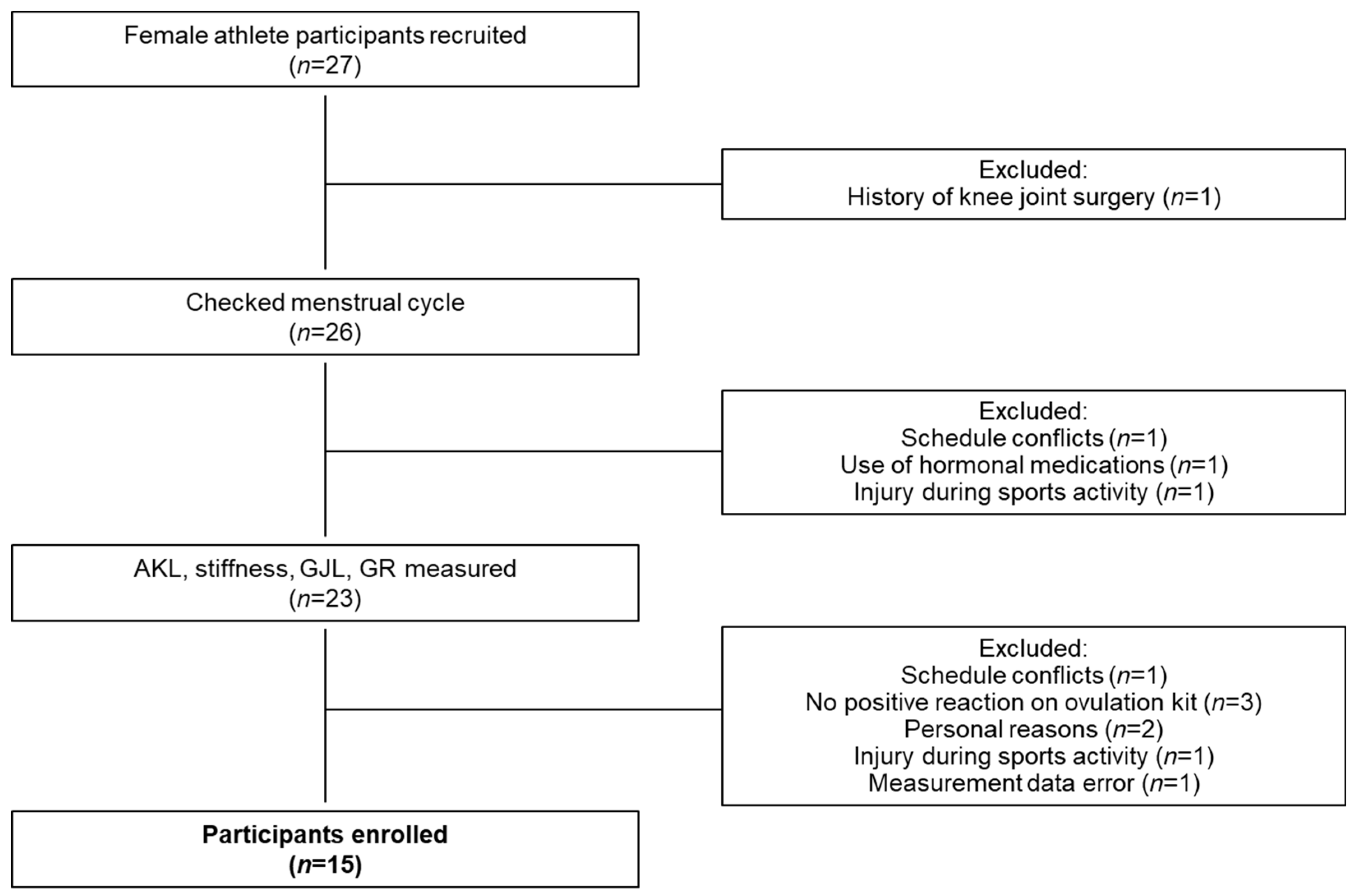
2.1. Participants
2.2. Classification of the Menstrual Cycle
2.3. Menstrual Cycle Recording
2.4. Timing of Measurements
2.5. Measurement Methods
2.5.1. Hormone Level Measurement
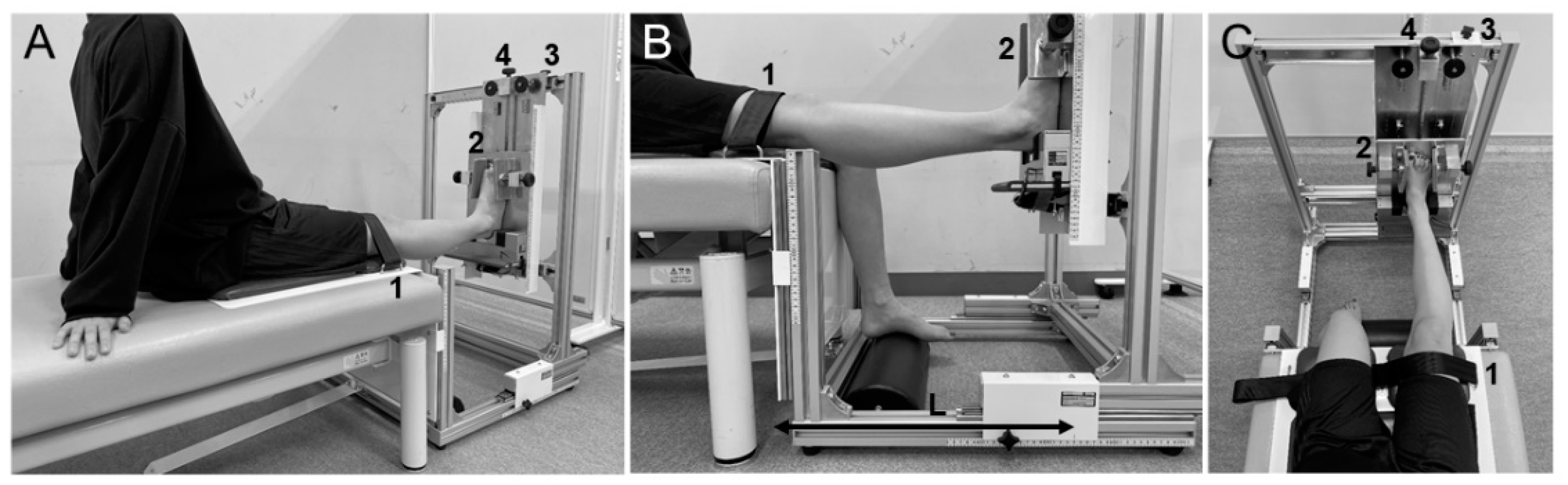
2.5.2. Laxity Measurement
2.6. Reliability of GR Measurements
2.7. Statistical Analysis
3. Results
3.1. Female Non-Athletes
3.2. Female Athletes
3.3. Reliability of GR Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beynnon, B.D.; Vacek, P.M.; Newell, M.K.; Tourville, T.W.; Smith, H.C.; Shultz, S.J.; Slauterbeck, J.R.; Johnson, R.J. The Effects of Level of Competition, Sport, and Sex on the Incidence of First-Time Noncontact Anterior Cruciate Ligament Injury. Am. J. Sports Med. 2014, 42, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am. J. Sports Med. 2006, 34, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Beynnon, B.D.; Johnson, R.J.; Braun, S.; Sargent, M.; Bernstein, I.M.; Skelly, J.M.; Vacek, P.M. The relationship between menstrual cycle phase and anterior cruciate ligament injury: A case-control study of recreational alpine skiers. Am. J. Sports Med. 2006, 34, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Huston, L.J.; Boynton, M.D.; Spindler, K.P.; Lindenfeld, T.N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am. J. Sports Med. 2002, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Vacek, P.M.; Slauterbeck, J.R.; Tourville, T.W.; Sturnick, D.R.; Holterman, L.A.; Smith, H.C.; Shultz, S.J.; Johnson, R.J.; Tourville, K.J.; Beynnon, B.D. Multivariate Analysis of the Risk Factors for First-Time Noncontact ACL Injury in High School and College Athletes: A Prospective Cohort Study With a Nested, Matched Case-Control Analysis. Am. J. Sports Med. 2016, 44, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Uhorchak, J.M.; Scoville, C.R.; Williams, G.N.; Arciero, R.A.; St Pierre, P.; Taylor, D.C. Risk factors associated with noncontact injury of the anterior cruciate ligament: A prospective four-year evaluation of 859 West Point cadets. Am. J. Sports Med. 2003, 31, 831–842. [Google Scholar] [CrossRef]
- Kramer, L.C.; Denegar, C.R.; Buckley, W.E.; Hertel, J. Factors associated with anterior cruciate ligament injury: History in female athletes. J. Sports Med. Phys. Fit. 2007, 47, 446–454. [Google Scholar]
- Beynnon, B.D.; Bernstein, I.M.; Belisle, A.; Brattbakk, B.; Devanny, P.; Risinger, R.; Durant, D. The effect of estradiol and progesterone on knee and ankle joint laxity. Am. J. Sports Med. 2005, 33, 1298–1304. [Google Scholar] [CrossRef]
- Shultz, S.J.; Sander, T.C.; Kirk, S.E.; Perrin, D.H. Sex differences in knee joint laxity change across the female menstrual cycle. J. Sports Med. Phys. Fit. 2005, 45, 594–603. [Google Scholar]
- Jansson, A.; Saartok, T.; Werner, S.; Renström, P. General joint laxity in 1845 Swedish school children of different ages: Age- and gender-specific distributions. Acta Paediatr. 2004, 93, 1202–1206. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Shultz, S.J. Sex differences in clinical measures of lower extremity alignment. J. Orthop. Sports Phys. Ther. 2007, 37, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J.; Levine, B.J.; Nguyen, A.D.; Kim, H.; Montgomery, M.M.; Perrin, D.H. A comparison of cyclic variations in anterior knee laxity, genu recurvatum, and general joint laxity across the menstrual cycle. J. Orthop. Res. 2010, 28, 1411–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, S.J.; Schmitz, R.J.; Nguyen, A.D.; Levine, B.; Kim, H.; Montgomery, M.M.; Shimokochi, Y.; Beynnon, B.D.; Perrin, D.H. Knee joint laxity and its cyclic variation influence tibiofemoral motion during weight acceptance. J. Orthop. Res. 2011, 43, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Stefanyshyn, D.J.; Loitz-Ramage, B.; Hart, D.A.; Ronsky, J.L. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am. J. Sports Med. 2009, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Eiling, E.; Bryant, A.L.; Petersen, W.; Murphy, A.; Hohmann, E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Karageanes, S.J.; Blackburn, K.; Vangelos, Z.A. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin. J. Sport Med. 2000, 10, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Yamazaki, T.; Sato, Y.; Suzuki, Y.; Shimizu, S.; Ikezu, M.; Kaneko, F.; Matsuzawa, K.; Hirabayashi, R.; Edama, M. Relationship Between Anterior Knee Laxity and General Joint Laxity During the Menstrual Cycle. Orthop. J. Sports Med. 2021, 9, 2325967121993045. [Google Scholar] [CrossRef]
- Shagawa, M.; Maruyama, S.; Sekine, C.; Yokota, H.; Hirabayashi, R.; Hirata, A.; Yokoyama, M.; Edama, M. Comparison of anterior knee laxity, stiffness, genu recurvatum, and general joint laxity in the late follicular phase and the ovulatory phase of the menstrual cycle. BMC Musculoskelet. Disord. 2021, 22, 886. [Google Scholar] [CrossRef]
- Lee, H.; Petrofsky, J.S.; Daher, N.; Berk, L.; Laymon, M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: Oral contraceptive users versus non-oral contraceptive users. Eur. J. Appl. Physiol. 2014, 114, 285–294. [Google Scholar] [CrossRef]
- Nose-Ogura, S. Advancement in female sports medicine and preventive medicine. J. Obstet. Gynaecol. Res. 2021, 47, 476–485. [Google Scholar] [CrossRef]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand. J. Med. Sci. Sports 2019, 29, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Nose-Ogura, S.; Yoshino, O.; Dohi, M.; Kigawa, M.; Harada, M.; Hiraike, O.; Onda, T.; Osuga, Y.; Fujii, T.; Saito, S. Risk factors of stress fractures due to the female athlete triad: Differences in teens and twenties. Scand. J. Med. Sci. Sports 2019, 29, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J.; Schmitz, R.J.; Nguyen, A.D.; Levine, B.J. Joint laxity is related to lower extremity energetics during a drop jump landing. Med. Sci. Sports. Exerc. 2010, 42, 771–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, J.C.; Nattiv, A.; Barrack, M.T.; Williams, N.I.; Rauh, M.J.; Nichols, J.F.; De Souza, M.J. Low bone density risk is higher in exercising women with multiple triad risk factors. Med. Sci. Sports Exerc. 2014, 46, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K.S. Prediction and detection of ovulation. Fertil. Steril. 1980, 34, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Salimetrics LLC.; Salivabio LLC. Saliva Collection and Handling Advice, 3rd ed.; Salimetrics: State College, PA, USA; Salivabio: Carlsbad, CA, USA, 2015; Available online: https://fnkprddata.blob.core.windows.net/domestic/download/pdf/SAL_handbook3.pdf (accessed on 20 December 2021).
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Maitland, M.E.; Bell, G.D.; Mohtadi, N.G.; Herzog, W. Quantitative analysis of anterior cruciate ligament instability. Clin. Biomech. 1995, 10, 93–97. [Google Scholar] [CrossRef]
- Davey, A.P.; Vacek, P.M.; Caldwell, R.A.; Slauterbeck, J.R.; Gardner-Morse, M.G.; Tourville, T.W.; Beynnon, B.D. Risk Factors Associated With a Noncontact Anterior Cruciate Ligament Injury to the Contralateral Knee After Unilateral Anterior Cruciate Ligament Injury in High School and College Female Athletes: A Prospective Study. Am. J. Sports Med. 2019, 47, 3347–3355. [Google Scholar] [CrossRef]
- Herzberg, S.D.; Motu’apuaka, M.L.; Lambert, W.; Fu, R.; Brady, J.; Guise, J.M. The Effect of Menstrual Cycle and Contraceptives on ACL Injuries and Laxity: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2017, 5, 2325967117718781. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Al-Shaikh, R.; Panossian, V.; Yang, R.S.; Nelson, S.D.; Soleiman, N.; Finerman, G.A.; Lane, J.M. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J. Orthop. Res. 1996, 14, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Ekman, M.; Morgan, G.; Johansson, O.; Jansson, E.; Esbjörnsson, M. Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem. Cell. Biol. 2005, 124, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Myrick, M.P.; Blackburn, J.T.; Shultz, S.J.; Guskiewicz, K.M.; Padua, D.A. The effect of menstrual-cycle phase on hamstring extensibility and muscle stiffness. J. Sport. Rehabil. 2009, 18, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.S.; Kim, J.H. The difference effect of estrogen on muscle tone of medial and lateral thigh muscle during ovulation. J. Exerc. Rehabil. 2018, 14, 419–423. [Google Scholar] [CrossRef]
- Avrillon, S.; Lacourpaille, L.; Hug, F.; Le Sant, G.; Frey, A.; Nordez, A.; Guilhem, G. Hamstring muscle elasticity differs in specialized high-performance athletes. Scand. J. Med. Sci. Sports 2020, 30, 83–91. [Google Scholar] [CrossRef]
- Zweers, M.C.; Hakim, A.J.; Grahame, R.; Schalkwijk, J. Joint hypermobility syndromes: The pathophysiologic role of tenascin-X gene defects. Arthritis Rheum. 2004, 50, 2742–2749. [Google Scholar] [CrossRef]
- Saccomanno, M.F.; Fodale, M.; Capasso, L.; Cazzato, G.; Milano, G. Generalized joint laxity and multidirectional instability of the shoulder. Joints 2013, 1, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Thompson, B.M.; Drover, K.B.; Stellmaker, R.J.; Sculley, D.V.; Janse de Jonge, X.A.K. The Effect of the Menstrual Cycle and Oral Contraceptive Cycle on Muscle Performance and Perceptual Measures. Int. J. Environ. Res. Public Health 2021, 18, 10565. [Google Scholar] [CrossRef]
- Fouladi, R.; Rajabi, R.; Naseri, N.; Pourkazemi, F.; Geranmayeh, M. Menstrual cycle and knee joint position sense in healthy female athletes. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1647–1652. [Google Scholar] [CrossRef]
- Zyroul, R.; Hossain, M.G.; Azura, M.; Abbas, A.A.; Kamarul, T. Knee laxity of Malaysian adults: Gender differentials, and association with age and anthropometric measures. Knee 2014, 21, 557–562. [Google Scholar] [CrossRef]
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | Total | |
|---|---|---|---|---|---|
| Estradiol [pg/mL] | |||||
| Female non-athletes (n = 19) | 1.1 ± 0.3 | 1.3 ± 0.4 | 1.5 ± 0.4 a | 1.4 ± 0.4 b | |
| Eumenorrhea group (n = 11) | 1.1 ± 0.3 | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.3 ± 0.4 |
| Oligomenorrhea group (n = 8) | 1.0 ± 0.3 | 1.2 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.3 | 1.3 ± 0.4 |
| Female athletes (n = 15) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.6 f | |
| Eumenorrhea group (n = 8) | 0.8 ± 0.2 | 0.9 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.6 | 1.0 ± 0.4 |
| Oligomenorrhea group (n = 7) | 1.2 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.5 ± 0.4 | 1.3 ± 0.3 |
| Progesterone [pg/mL] | |||||
| Female non-athletes (n = 19) | 132.0 ± 75.3 | 161.1 ± 77.4 | 164.5 ± 94.5 | 343.7 ± 280.2 | |
| Eumenorrhea group (n = 11) | 146.7 ± 81.3 | 167.1 ± 85.4 | 172.1 ± 104.0 | 477.6 ± 324.4 c,d,e | 233.4 ± 148.8 |
| Oligomenorrhea group (n = 8) | 111.7 ± 65.9 | 152.9 ± 69.7 | 154.1 ± 85.3 | 200.7 ± 105.8 | 154.8 ± 81.7 |
| Female athletes (n = 15) | 127.6 ± 61.0 | 141.2 ± 60.3 | 147.2 ± 73.1 | 231.4 ± 108.6 g,h,i | |
| Eumenorrhea group (n = 8) | 101.1 ± 66.2 | 146.7 ± 70.3 | 172.1 ± 83.1 | 242.1 ± 128.5 | 165.5 ± 87.0 |
| Oligomenorrhea group (n = 7) | 157.8 ± 39.8 | 135.0 ± 51.3 | 118.8 ± 51.5 | 219.2 ± 89.0 | 157.7 ± 57.9 |
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | Total | ||
|---|---|---|---|---|---|---|
| Anterior knee laxity [mm] | ||||||
| Female non-athletes (n = 19) | ||||||
| 67 N | Eumenorrhea group (n = 11) | 4.6 ± 1.3 | 4.6 ± 1.4 | 4.4 ± 1.2 | 4.2 ± 1.2 | 4.5 ± 1.3 |
| Oligomenorrhea group (n = 8) | 4.5 ± 2.1 | 4.4 ± 1.7 | 4.5 ± 1.4 | 4.3 ± 1.5 | 4.4 ± 1.7 | |
| 89 N | Eumenorrhea group (n = 11) | 5.7 ± 1.5 | 5.6 ± 1.6 | 5.3 ± 1.3 | 5.2 ± 1.2 | 5.5 ± 1.4 |
| Oligomenorrhea group (n = 8) | 5.4 ± 2.2 | 5.3 ± 1.8 | 5.4 ± 1.5 | 5.1 ± 1.4 | 5.3 ± 1.7 | |
| 111 N | Eumenorrhea group (n = 11) | 6.6 ± 1.6 | 6.5 ± 1.8 | 6.2 ± 1.4 | 6.0 ± 1.2 | 6.3 ± 1.5 |
| Oligomenorrhea group (n = 8) | 6.0 ± 2.3 | 6.0 ± 1.8 | 6.1 ± 1.5 | 5.8 ± 1.5 | 6.0 ± 1.8 | |
| 133 N | Eumenorrhea group (n = 11) | 7.5 ± 1.7 | 7.4 ± 1.9 | 7.0 ± 1.5 | 6.8 ± 1.3 | 7.2 ± 1.6 |
| Oligomenorrhea group (n = 8) | 6.7 ± 2.3 | 6.7 ± 1.8 | 6.8 ± 1.5 | 6.4 ± 1.5 | 6.6 ± 1.8 | |
| Female athletes (n = 15) | ||||||
| 67 N | Eumenorrhea group (n = 8) | 3.6 ± 1.2 | 3.8 ± 1.0 | 3.8 ± 1.0 | 3.4 ± 0.9 | 3.6 ± 1.0 |
| Oligomenorrhea group (n = 7) | 4.0 ± 2.0 | 4.5 ± 1.3 | 4.0 ± 1.7 | 4.0 ± 1.5 | 4.1 ± 1.6 | |
| 89 N | Eumenorrhea group (n = 8) | 4.5 ± 1.4 | 4.7 ± 1.3 | 4.7 ± 1.1 | 4.1 ± 1.1 | 4.5 ± 1.2 |
| Oligomenorrhea group (n = 7) | 4.8 ± 2.2 | 5.4 ± 1.6 | 4.8 ± 1.9 | 4.9 ± 1.7 | 5.0 ± 1.9 | |
| 111 N | Eumenorrhea group (n = 8) | 5.2 ± 1.5 | 5.4 ± 1.5 | 5.4 ± 1.2 | 4.8 ± 1.2 | 5.2 ± 1.4 |
| Oligomenorrhea group (n = 7) | 5.5 ± 2.2 | 6.1 ± 1.8 | 5.5 ± 2.1 | 5.7 ± 2.0 | 5.7 ± 2.0 | |
| 133 N | Eumenorrhea group (n = 8) | 5.9 ± 1.6 | 6.0 ± 1.7 | 6.1 ± 1.4 | 5.4 ± 1.2 | 5.9 ± 1.5 |
| Oligomenorrhea group (n = 7) | 6.2 ± 2.4 | 6.8 ± 2.0 | 6.1 ± 2.1 | 6.4 ± 2.2 | 6.4 ± 2.2 | |
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | Total | ||
|---|---|---|---|---|---|---|
| Stiffness [N/mm] | ||||||
| Female non-athletes (n = 19) | ||||||
| 67–89 N | Eumenorrhea group (n = 11) | 21.6 ± 4.3 | 23.4 ± 5.6 | 24.5 ± 6.3 | 24.6 ± 2.9 | 23.5 ± 4.8 |
| Oligomenorrhea group (n = 8) | 27.5 ± 7.0 | 27.1 ± 5.7 | 25.2 ± 5.8 | 28.4 ± 6.0 | 27.1 ± 6.1 | |
| 89–111 N | Eumenorrhea group (n = 11) | 25.6 ± 5.5 | 26.5 ± 6.6 | 28.4 ± 7.7 | 27.6 ± 3.5 | 27.0 ± 5.8 |
| Oligomenorrhea group (n = 8) | 34.3 ± 9.6 | 33.6 ± 9.0 | 30.6 ± 5.4 | 35.6 ± 3.9 | 33.6 ± 7.0 a | |
| 111–133 N | Eumenorrhea group (n = 11) | 26.7 ± 6.9 | 27.0 ± 5.1 | 26.9 ± 4.5 | 28.1 ± 4.3 | 27.2 ± 5.2 |
| Oligomenorrhea group (n = 8) | 35.5 ± 8.9 | 35.9 ± 10.7 | 32.3 ± 6.6 | 38.0 ± 7.4 | 35.4 ± 8.4 b | |
| Female athletes (n = 15) | ||||||
| 67–89 N | Eumenorrhea group (n = 8) | 26.8 ± 8.1 | 28.8 ± 7.8 | 27.9 ± 5.1 | 28.3 ± 12.4 | 28.0 ± 0.4 |
| Oligomenorrhea group (n = 7) | 31.5 ± 10.3 | 29.1 ± 12.8 | 31.9 ± 14.3 | 27.3 ± 11.4 | 29.9 ± 12.2 | |
| 89–111 N | Eumenorrhea group (n = 8) | 32.8 ± 9.0 | 34.2 ± 9.2 | 32.5 ± 7.7 | 36.2 ± 10.6 | 33.9 ± 9.1 |
| Oligomenorrhea group (n = 7) | 33.2 ± 7.8 | 36.3 ± 15.7 | 36.7 ± 11.4 | 32.8 ± 14.3 | 34.8 ± 12.3 | |
| 111–133 N | Eumenorrhea group (n = 8) | 37.1 ± 11.5 | 38.1 ± 11.9 | 36.7 ± 11.5 | 38.0 ± 7.1 | 37.5 ± 10.5 |
| Oligomenorrhea group (n = 7) | 34.6 ± 9.3 | 40.0 ± 21.9 | 43.6 ± 18.9 | 32.3 ± 13.1 | 37.7 ± 15.8 | |
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | Total | |
|---|---|---|---|---|---|
| General joint laxity [points] | |||||
| Female non-athletes (n = 19) | |||||
| Eumenorrhea group (n = 11) | 1.7 ± 1.4 | 1.6 ± 1.4 | 1.4 ± 1.2 | 1.4 ± 1.3 | 1.5 ± 1.3 |
| Oligomenorrhea group (n = 8) | 1.6 ± 1.3 | 1.6 ± 1.1 | 1.3 ± 1.3 | 1.5 ± 1.0 | 1.5 ± 1.2 |
| Female athletes (n = 15) | |||||
| Eumenorrhea group (n = 8) | 2.2 ± 1.7 | 2.3 ± 1.5 | 2.5 ± 1.4 | 2.6 ± 1.6 | 2.4 ± 1.5 |
| Oligomenorrhea group (n = 7) | 1.6 ± 2.5 | 1.9 ± 2.6 | 1.7 ± 2.9 | 1.6 ± 2.6 | 1.7 ± 2.6 |
| Early Follicular Phase | Late Follicular Phase | Ovulation Phase | Luteal Phase | Total | |
|---|---|---|---|---|---|
| Genu recurvatum [°] | |||||
| Female non-athletes (n = 19) | 7.0 ± 3.9 | 7.2 ± 3.9 | 7.3 ± 4.1 | 7.7 ± 4.1 | |
| Eumenorrhea group (n = 11) | 6.2 ± 3.9 | 6.3 ± 3.8 | 6.3 ± 3.9 | 6.6 ± 4.1 | 6.4 ± 3.9 |
| Oligomenorrhea group (n = 8) | 8.1 ± 3.9 | 8.4 ± 3.8 | 8.6 ± 4.2 | 9.3 ± 3.9 | 8.6 ± 3.9 |
| Female athletes (n = 15) | 7.7 ± 3.7 | 8.3 ± 3.7 a | 8.7 ± 3.8 b | 9.0 ± 3.8 c | |
| Eumenorrhea group (n = 8) | 6.1 ± 3.7 | 7.0 ± 4.1 | 7.4 ± 4.4 | 7.6 ± 4.3 | 7.0 ± 4.1 |
| Oligomenorrhea group (n = 7) | 9.5 ± 2.8 | 9.8 ± 2.8 | 10.2 ± 2.6 | 10.7 ± 2.4 | 10.1 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, S.; Sekine, C.; Shagawa, M.; Yokota, H.; Hirabayashi, R.; Togashi, R.; Yamada, Y.; Hamano, R.; Ito, A.; Sato, D.; et al. Menstrual Cycle Changes Joint Laxity in Females—Differences between Eumenorrhea and Oligomenorrhea. J. Clin. Med. 2022, 11, 3222. https://doi.org/10.3390/jcm11113222
Maruyama S, Sekine C, Shagawa M, Yokota H, Hirabayashi R, Togashi R, Yamada Y, Hamano R, Ito A, Sato D, et al. Menstrual Cycle Changes Joint Laxity in Females—Differences between Eumenorrhea and Oligomenorrhea. Journal of Clinical Medicine. 2022; 11(11):3222. https://doi.org/10.3390/jcm11113222
Chicago/Turabian StyleMaruyama, Sae, Chie Sekine, Mayuu Shagawa, Hirotake Yokota, Ryo Hirabayashi, Ryoya Togashi, Yuki Yamada, Rena Hamano, Atsushi Ito, Daisuke Sato, and et al. 2022. "Menstrual Cycle Changes Joint Laxity in Females—Differences between Eumenorrhea and Oligomenorrhea" Journal of Clinical Medicine 11, no. 11: 3222. https://doi.org/10.3390/jcm11113222







