Development and Validation of Survival Nomograms in Patients with Primary Bladder Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
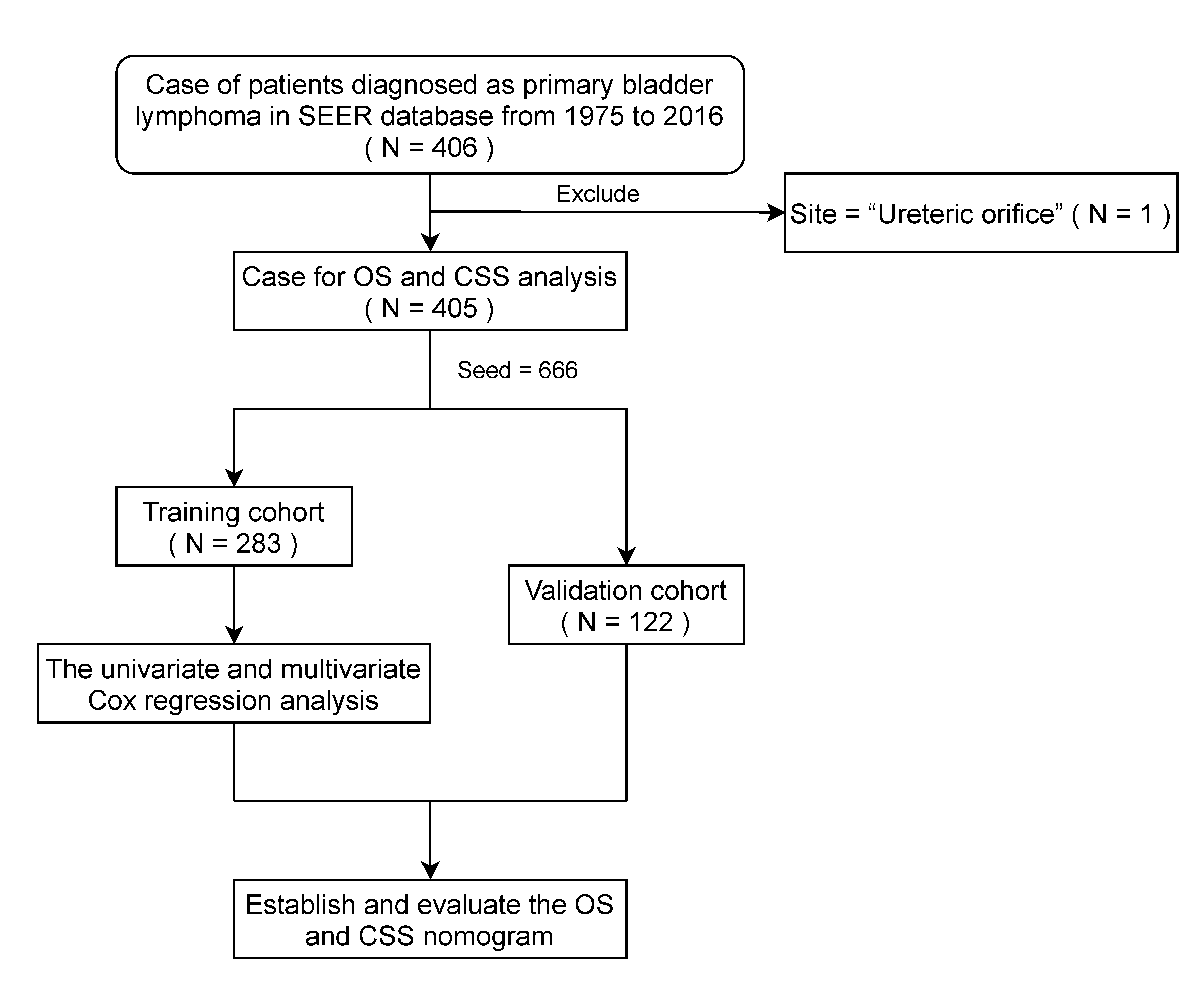
2.1. Patients and Data Collection
2.2. Model Development
2.3. Performance Assessment and Validation of the Model
2.4. Survival Risk Classification Based on the Nomogram
2.5. Clinical Usefulness of the Nomogram
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Total Patients with PBL
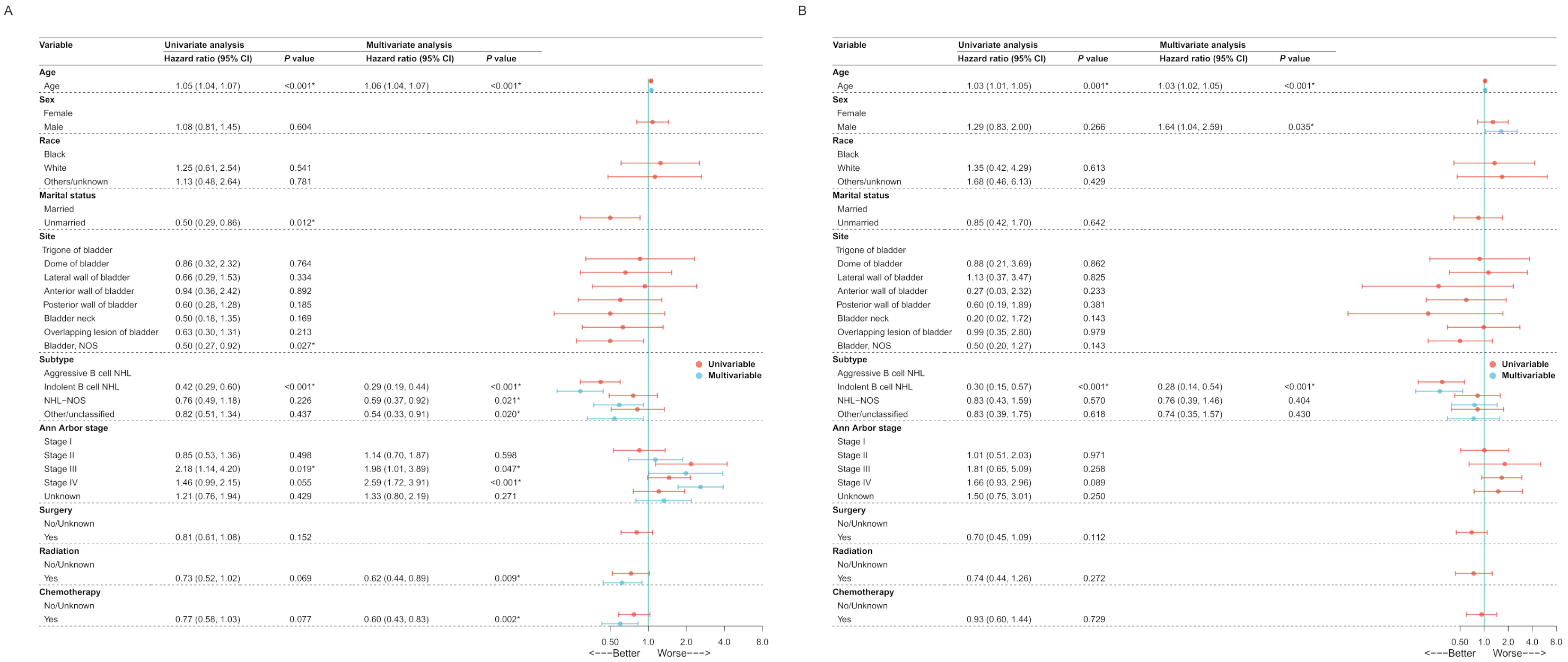
3.2. Model Development
3.3. Performance Assessment and Validation of The Nomogram
3.4. Survival Risk Classification Based on the Nomogram and Prognosis Analysis
3.5. Clinical Usefulness of the Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D.A.; Moore, A.T. The first case of vesico-vaginal fistula in a patient with primary lymphoma of the bladder—A case report. J. Med. Case Rep. 2007, 1, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maninderpal, K.G.; Amir, F.H.; Azad, H.A.; Mun, K.S. Imaging findings of a primary bladder maltoma. Br. J. Radiol. 2011, 84, e186–e190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, I.; Zozumi, M.; Tsuchida, Y.A.; Kimura, N.; Liu, N.N.; Fujimori, Y.; Okada, M.; Hashimoto, T.; Yamamoto, S.; Hirota, S. Primary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type with malakoplakia in the urinary bladder: A case report. Int. J. Clin. Exp. Pathol. 2014, 7, 5280–5284. [Google Scholar] [PubMed]
- Venyo, A.K. Lymphoma of the urinary bladder. Adv. Urol. 2014, 2014, 327917. [Google Scholar] [CrossRef] [Green Version]
- Kempton, C.L.; Kurtin, P.J.; Inwards, D.J.; Wollan, P.; Bostwick, D.G. Malignant lymphoma of the bladder: Evidence from 36 cases that low-grade lymphoma of the MALT-type is the most common primary bladder lymphoma. Am. J. Surg. Pathol. 1997, 21, 1324–1333. [Google Scholar] [CrossRef]
- Mourad, W.A.; Khalil, S.; Radwi, A.; Peracha, A.; Ezzat, A. Primary T-cell lymphoma of the urinary bladder. Am. J. Surg. Pathol. 1998, 22, 373–377. [Google Scholar] [CrossRef]
- Khaitan, A.; Gupta, N.P.; Goel, A.; Safaya, R.; Kumar, L. Primary non-Hodgkin’s lymphoma of urinary bladder. Report of a case and review of the literature. Urol. Int. 2004, 72, 82–84. [Google Scholar] [CrossRef]
- Ohsawa, M.; Aozasa, K.; Horiuchi, K.; Kanamaru, A. Malignant lymphoma of bladder. Report of three cases and review of the literature. Cancer 1993, 72, 1969–1974. [Google Scholar] [CrossRef]
- Leite, K.R.; Bruschini, H.; Camara-Lopes, L.H. Primary lymphoma of the bladder. Int. Braz. J. Urol. 2004, 30, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Al-Maghrabi, J.; Kamel-Reid, S.; Jewett, M.; Gospodarowicz, M.; Wells, W.; Banerjee, D. Primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type arising in the urinary bladder: Report of 4 cases with molecular genetic analysis. Arch. Pathol. Lab. Med. 2001, 125, 332–336. [Google Scholar] [CrossRef]
- Simpson, R.H.; Bridger, J.E.; Anthony, P.P.; James, K.A.; Jury, I. Malignant lymphoma of the lower urinary tract. A clinicopathological study with review of the literature. Br. J. Urol. 1990, 65, 254–260. [Google Scholar] [CrossRef]
- Bates, A.W.; Norton, A.J.; Baithun, S.I. Malignant lymphoma of the urinary bladder: A clinicopathological study of 11 cases. J. Clin. Pathol. 2000, 53, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.; Morrison, A.; Jackson, R. Primary bladder lymphoma: Management and outcome of 12 patients with a review of the literature. Leuk. Lymphoma 2005, 46, 873–877. [Google Scholar] [CrossRef]
- Sellman, D.P.; Simpson, W.G.; Klaassen, Z.; Jen, R.P.; DiBianco, J.M.; Reinstatler, L.; Li, Q.; Madi, R.; Terris, M.K. Characterization and outcomes of local treatment for primary bladder lymphoma: A population-based cohort analysis. Urol. Ann. 2018, 10, 249–253. [Google Scholar] [CrossRef]
- Oh, K.C.; Zang, D.Y. Primary non-Hodgkin’s lymphoma of the bladder with bone marrow involvement. Korean J. Intern. Med. 2003, 18, 40–44. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Horesh, N.; Horowitz, N.A. Does gender matter in non-hodgkin lymphoma? Differences in epidemiology, clinical behavior, and therapy. Rambam Maimonides Med. J. 2014, 5, e0038. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, D.; Myśliwska, J.; Suchecka-Rachon, K.; Wieckiewicz, J.; Myśliwski, A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 2002, 172, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández Aceñero, M.J.; Martín Rodilla, C.; López García-Asenjo, J.; Coca Menchero, S.; Sanz Esponera, J. Primary malignant lymphoma of the bladder. Report of three cases. Pathol. Res. Pract. 1996, 192, 160–163; discussion 164–165. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [Green Version]


| Training Cohort, N = 283 | Validation Cohort, N = 122 | p Value | |
|---|---|---|---|
| Age (median [IQR]) | 75.00 [64.50, 83.00] | 77.00 [64.00, 84.00] | 0.727 |
| Sex | 0.464 | ||
| Female | 175 (61.84%) | 70 (57.38%) | |
| Male | 108 (38.16%) | 52 (42.62%) | |
| Race | 0.236 | ||
| White | 237 (83.75%) | 105 (86.06%) | |
| Black | 14 (4.95%) | 9 (7.38%) | |
| Other/Unknown | 32 (11.30%) | 8 (6.56%) | |
| Marital status | 1.000 | ||
| Married/Unknown | 250 (88.34%) | 108 (88.52%) | |
| Unmarried | 33 (11.66%) | 14 (11.48%) | |
| Site | 0.231 | ||
| Trigone of bladder | 14 (4.95%) | 4 (3.28%) | |
| Dome of bladder | 8 (2.83%) | 6 (4.92%) | |
| Lateral wall of bladder | 15 (5.30%) | 11 (9.02%) | |
| Anterior wall of bladder | 9 (3.18%) | 3 (2.46%) | |
| Posterior wall of bladder | 22 (7.77%) | 15 (12.29%) | |
| Bladder neck | 10 (3.53%) | 6 (4.92%) | |
| Overlapping lesion of bladder | 27 (9.54%) | 5 (4.10%) | |
| Bladder, NOS | 178 (62.90%) | 72 (59.01%) | |
| Subtype | 0.324 | ||
| Aggressive B cell NHL | 144 (50.88%) | 59 (48.36%) | |
| Indolent B cell NHL | 79 (27.92%) | 44 (36.06%) | |
| NHL-NOS | 35 (12.37%) | 12 (9.84%) | |
| Other/unclassified | 25 (8.83%) | 7 (5.74%) | |
| Ann Arbor stage | 0.828 | ||
| Stage I | 147 (51.94%) | 66 (54.10%) | |
| Stage II | 41 (14.49%) | 20 (16.39%) | |
| Stage III | 11 (3.89%) | 6 (4.92%) | |
| Stage IV | 49 (17.31%) | 19 (15.57%) | |
| Unknown | 35 (12.37%) | 11 (9.02%) | |
| Surgery | |||
| Yes | 139 (49.12%) | 58 (47.54%) | 0.855 |
| No/Unknown | 144 (50.88%) | 64 (52.6%) | |
| Radiation | |||
| Yes | 215 (75.97%) | 102 (83.61%) | 0.115 |
| No/Unknown | 68 (24.03%) | 20 (16.39%) | |
| Chemotherapy | |||
| Yes | 150 (53.00%) | 63 (51.64%) | 0.886 |
| No/Unknown | 133 (47.00%) | 59 (48.36%) | |
| Follow-up time (median [IQR]) | 27.00 [5.00, 91.00] | 37.00 [6.00, 93.75] | 0.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Kong, J.; Luo, M.; Shen, Z.; Fang, S.; Hu, J.; Xu, Z.; Dong, W.; Huang, J.; Lin, T. Development and Validation of Survival Nomograms in Patients with Primary Bladder Lymphoma. J. Clin. Med. 2022, 11, 3188. https://doi.org/10.3390/jcm11113188
Lin J, Kong J, Luo M, Shen Z, Fang S, Hu J, Xu Z, Dong W, Huang J, Lin T. Development and Validation of Survival Nomograms in Patients with Primary Bladder Lymphoma. Journal of Clinical Medicine. 2022; 11(11):3188. https://doi.org/10.3390/jcm11113188
Chicago/Turabian StyleLin, Junyi, Jianbin Kong, Mingli Luo, Zefeng Shen, Shuogui Fang, Jintao Hu, Zixin Xu, Wen Dong, Jian Huang, and Tianxin Lin. 2022. "Development and Validation of Survival Nomograms in Patients with Primary Bladder Lymphoma" Journal of Clinical Medicine 11, no. 11: 3188. https://doi.org/10.3390/jcm11113188
APA StyleLin, J., Kong, J., Luo, M., Shen, Z., Fang, S., Hu, J., Xu, Z., Dong, W., Huang, J., & Lin, T. (2022). Development and Validation of Survival Nomograms in Patients with Primary Bladder Lymphoma. Journal of Clinical Medicine, 11(11), 3188. https://doi.org/10.3390/jcm11113188





