Galactose-Deficient IgA1 as a Candidate Urinary Marker of IgA Nephropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Pathological Parameters
2.3. Measurement of Gd-IgA1
2.4. Statistical Analyses
3. Results
3.1. Demographic, Clinical, and Laboratory Findings
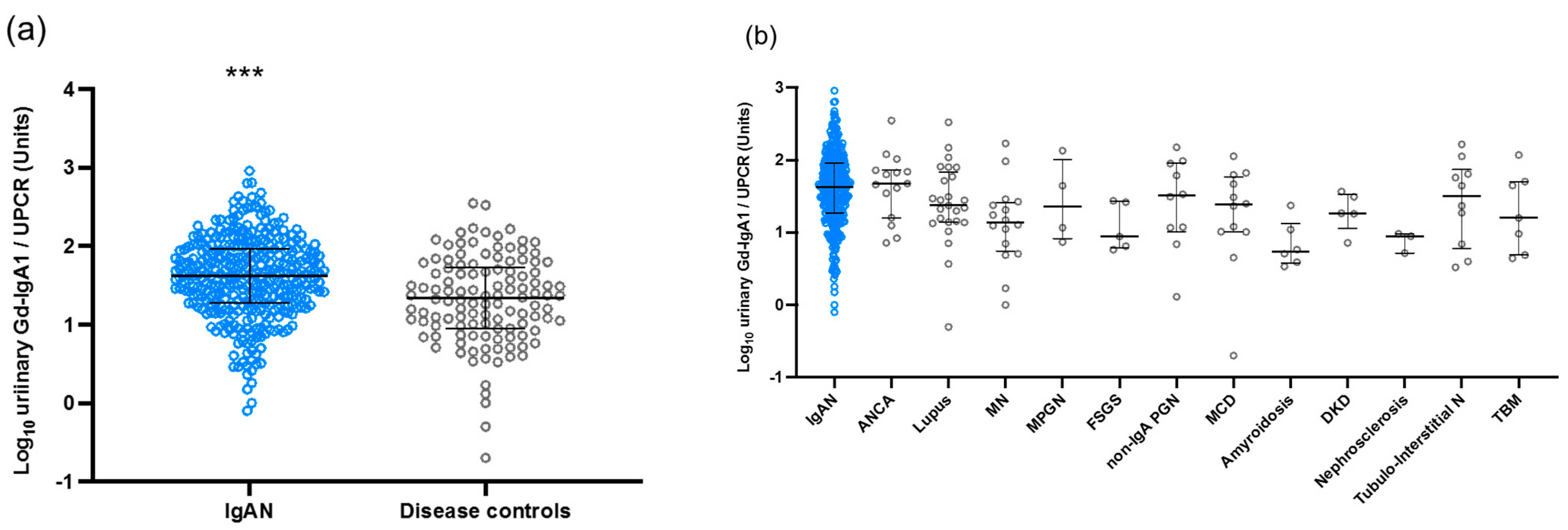
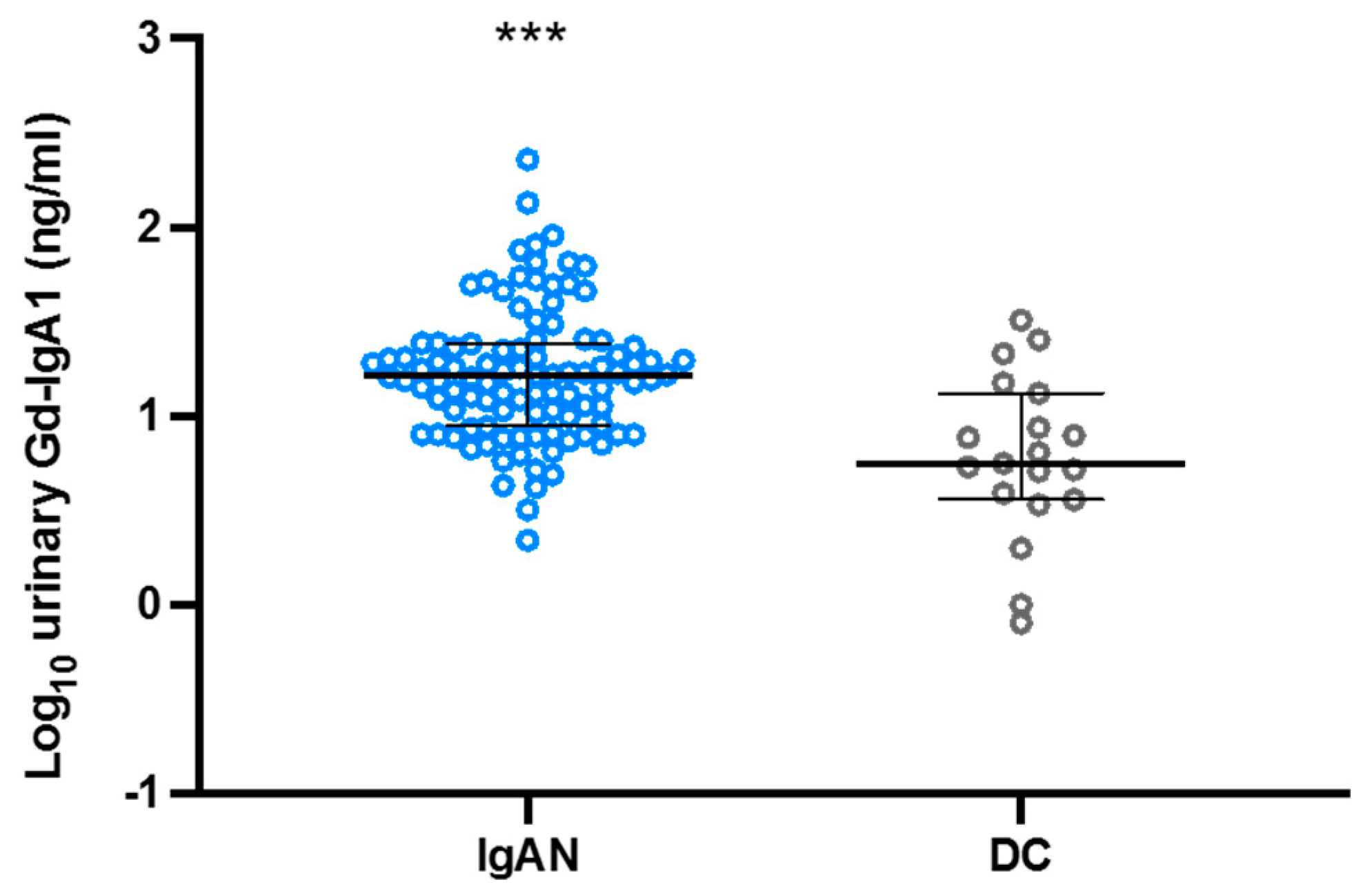
3.2. Urinary Levels of Gd-IgA1 in the Japanese Cohort
3.3. Correlation between Urinary Gd-IgA1 and Laboratory and Pathological Findings in IgAN Patients from the Japanese Cohort
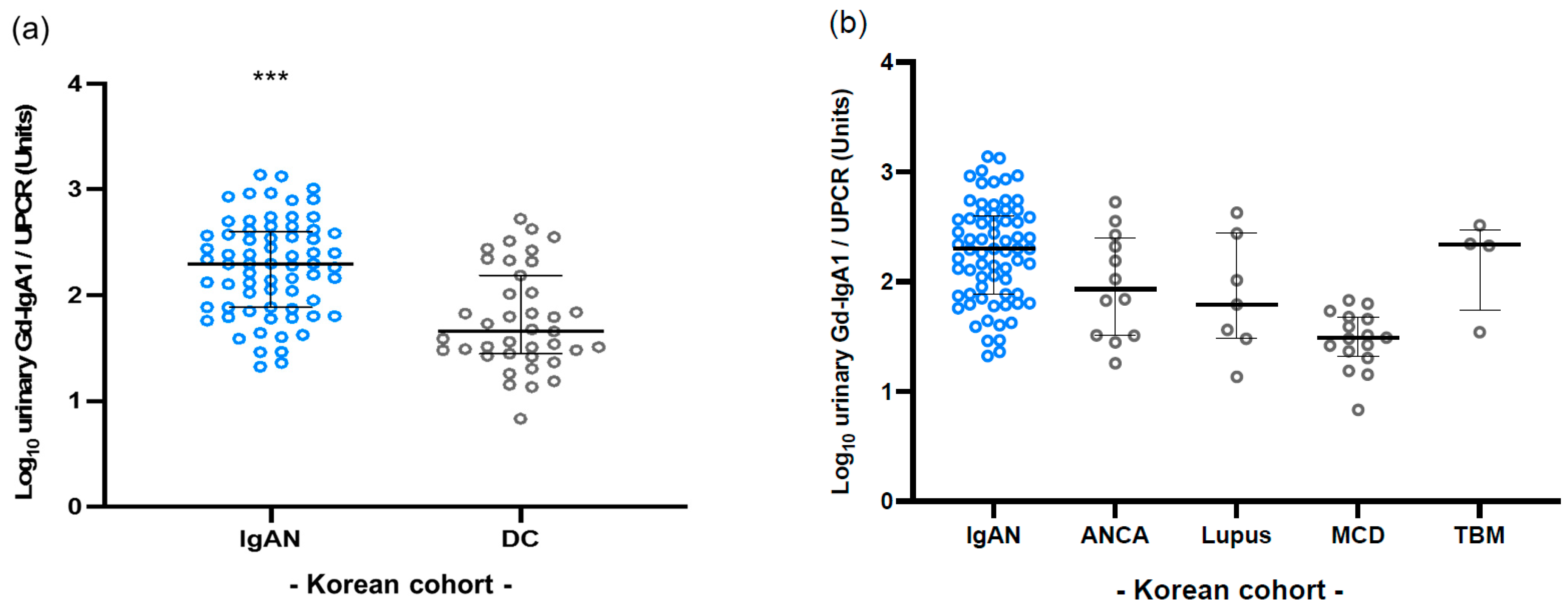
3.4. Validation in Korean Cohort
3.5. Difference in Clinical Features at the Time of Renal Biopsy in Patients with IgAN
3.6. Urinary Gd-IgA1 Excretion with Trace Proteinuria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, M.; Berger, J. Worldwide perspective of IgA nephropathy. Am. J. Kidney Dis. 1988, 12, 340–347. [Google Scholar] [CrossRef]
- McGrogan, A.; Franssen, C.F.; de Vries, C.S. The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol. Dial. Transpl. 2011, 26, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, G. The commonest glomerulonephritis in the world: IgA nephropathy. Q. J. Med. 1987, 64, 709–727. [Google Scholar] [PubMed]
- Brandtzaeg, P.; Johansen, F.E. Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 2005, 206, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Wyatt, R.J.; Lee, J.Y.; Tomana, M.; Julian, B.A.; Mestecky, J.; Huang, W.Q.; Anreddy, S.R.; Hall, S.; Hastings, M.C.; et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007, 71, 1148–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Maixnerova, D.; Ling, C.; Hall, S.; Reily, C.; Brown, R.; Neprasova, M.; Suchanek, M.; Honsova, E.; Zima, T.; Novak, J.; et al. Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS ONE 2019, 14, e0212254. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Hou, P.; Lv, J.; Moldoveanu, Z.; Li, Y.; Kiryluk, K.; Gharavi, A.G.; Novak, J.; Zhang, H. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012, 82, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Gharavi, A.G.; Moldoveanu, Z.; Wyatt, R.J.; Barker, C.V.; Woodford, S.Y.; Lifton, R.P.; Mestecky, J.; Novak, J.; Julian, B.A. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J. Am. Soc. Nephrol. 2008, 19, 1008–1014. [Google Scholar] [CrossRef]
- Suzuki, H.; Yasutake, J.; Makita, Y.; Tanbo, Y.; Yamasaki, K.; Sofue, T.; Kano, T.; Suzuki, Y. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018, 93, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Yasutake, J.; Suzuki, Y.; Suzuki, H.; Hiura, N.; Yanagawa, H.; Makita, Y.; Kaneko, E.; Tomino, Y. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol. Dial. Transpl. 2015, 30, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, K.; Suzuki, Y.; Suzuki, H.; Satake, K.; Horikoshi, S.; Novak, J.; Tomino, Y. The kinetics of glomerular deposition of nephritogenic IgA. PLoS ONE 2014, 9, e113005. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ruotsalainen, V.; Tryggvason, K.; Shaw, A.S.; Miner, J.H. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am. J. Physiol. Renal. Physiol. 2000, 279, F785–F792. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Allegri, L.; Suzuki, Y.; Hall, S.; Moldoveanu, Z.; Wyatt, R.J.; Novak, J.; Julian, B.A. Galactose-Deficient IgA1 as a Candidate Urinary Polypeptide Marker of IgA Nephropathy? Dis. Markers 2016, 2016, 7806438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosik, L.P.; Lajoie, G.; Sugar, L.; Cattran, D.C. Predicting progression in IgA nephropathy. Am. J. Kidney Dis. 2001, 38, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Donadio, J.V.; Bergstralh, E.J.; Grande, J.P.; Rademcher, D.M. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol. Dial. Transpl. 2002, 17, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Matsuzaki, K.; Suzuki, H.; Sakamoto, N.; Joh, K.; Kawamura, T.; Tomino, Y.; Matsuo, S. Proposal of remission criteria for IgA nephropathy. Clin. Exp. Nephrol. 2014, 18, 481–486. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Ieiri, N.; Yamazaki, S.; Hayashino, Y.; Gillespie, B.; Miyazaki, M.; Taguma, Y.; Fukuhara, S.; Hotta, O. Clinical effectiveness of steroid pulse therapy combined with tonsillectomy in patients with immunoglobulin A nephropathy presenting glomerular haematuria and minimal proteinuria. Nephrology 2010, 15, 116–123. [Google Scholar] [CrossRef]
- Komatsu, H.; Sato, Y.; Miyamoto, T.; Tamura, M.; Nakata, T.; Tomo, T.; Nishino, T.; Miyazaki, M.; Fujimoto, S. Significance of tonsillectomy combined with steroid pulse therapy for IgA nephropathy with mild proteinuria. Clin. Exp. Nephrol. 2016, 20, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Kaga, T.; Abe, Y.; Endo, M.; Wakai, S.; Tsuchiya, K.; Nitta, K. Renal biopsy findings and clinical indicators of patients with hematuria without overt proteinuria. Clin. Exp. Nephrol. 2015, 19, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Kawamura, T.; Joh, K.; Utsunomiya, Y.; Okonogi, H.; Miyazaki, Y.; Koike, K.; Yokoo, T.; Matsushima, M.; Yoshimura, M.; et al. Clinical guides for immunoglobulin A (IgA) nephropathy in Japan, third version. Jpn. J. Nephrol. 2011, 53, 123–135. [Google Scholar]
- Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef] [Green Version]
- Yap, H.K.; Quek, C.M.; Shen, Q.; Joshi, V.; Chia, K.S. Role of urinary screening programmes in children in the prevention of chronic kidney disease. Ann. Acad. Med. Singap. 2005, 34, 3–7. [Google Scholar]
- Donadio, J.V.; Grande, J.P. IgA nephropathy. N. Engl. J. Med. 2002, 347, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Nakanishi, I.; Suzuki, A.; Hayashi, T.; Togawa, M.; Okada, N.; Imai, E.; Hori, M.; Tsubakihara, Y. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am. J. Kidney Dis. 2000, 35, 194–201. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Z.; Zhang, H.; Liu, X. Aberrant IgA1 Glycosylation in IgA Nephropathy: A Systematic Review. PLoS ONE 2016, 11, e0166700. [Google Scholar] [CrossRef] [Green Version]
- Kaihan, A.B.; Yasuda, Y.; Katsuno, T.; Kato, S.; Imaizumi, T.; Ozeki, T.; Hishida, M.; Nagata, T.; Ando, M.; Tsuboi, N.; et al. The Japanese Histologic Classification and T-score in the Oxford Classification system could predict renal outcome in Japanese IgA nephropathy patients. Clin. Exp. Nephrol. 2017, 21, 986–994. [Google Scholar] [CrossRef]
- Haaskjold, Y.L.; Bjørneklett, R.; Bostad, L.; Bostad, L.S.; Lura, N.G.; Knoop, T. Utilizing the MEST score for prognostic staging in IgA nephropathy. BMC Nephrol. 2022, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Espino-Hernandez, G.; Reich, H.N.; Coppo, R.; Roberts, I.S.; Feehally, J.; Herzenberg, A.M.; Cattran, D.C.; Bavbek, N.; Cook, T.; et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016, 89, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hwang, J.H.; Paik, J.H.; Ryu, H.J.; Kim, D.K.; Chin, H.J.; Oh, Y.K.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; et al. Long-term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol. 2014, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patients (n) | Age (year) | Sex | sCr (mg/dL) | eGFR (mL/min/1.73 m2) | UPCR (g/gCr) | |
|---|---|---|---|---|---|---|
| Japanese | data | |||||
| IgAN | 338 | 38.1 | M177/F161 | 0.8 | 83.1 | 0.9 |
| DC | 120 | 49.2 | M53/F67 | 1.0 | 79.9 | 2.4 |
| Korean | ||||||
| IgAN | 69 | 40.5 | M28/F41 | 1.3 | 76.5 | 1.5 |
| DC | 39 | 44.4 | M16/F20 | 1.9 | 69.4 | 4.6 |
| Disease Controls | Patients (n) | sCr (mg/dL) | eGFR (mL/min/1.73 m2) | UPCR (g/gCr) |
|---|---|---|---|---|
| Japanese cohort | ||||
| ANCA-associated glomerulonephritis | 15 | 1.7 | 37.1 | 2.0 |
| Lupus nephritis | 26 | 0.7 | 95.7 | 1.7 |
| Minimal change disease | 12 | 0.8 | 87.1 | 5.6 |
| Membranous nephropathy | 16 | 0.7 | 90.0 | 3.8 |
| Membranoproliferative glomerulonephritis | 4 | 0.9 | 103.9 | 1.6 |
| Non-IgA mesangial proliferative glomerulonephritis | 10 | 0.7 | 101.7 | 0.3 |
| Focal segmental glomerulosclerosis | 5 | 0.9 | 62.3 | 3.2 |
| Tubulointerstitial nephritis | 10 | 2.0 | 37.2 | 1.7 |
| Renal amyloidosis | 6 | 1.0 | 72.1 | 5.1 |
| Diabetic kidney disease | 5 | 1.3 | 56.1 | 1.8 |
| Nephrosclerosis | 4 | 0.7 | 77.4 | 0.3 |
| Thin basement membrane disease | 7 | 0.6 | 119.0 | 0.9 |
| Korean cohort | ||||
| ANCA-associated glomerulonephritis | 12 | 3.9 | 24.9 | 2.3 |
| Lupus nephritis | 7 | 0.9 | 85.8 | 4.2 |
| Minimal change disease | 16 | 1.2 | 85.7 | 7.6 |
| Thin basement membrane disease | 4 | 0.6 | 108.8 | 0.5 |
| Patients (n) | Age (year) | Sex | sCr (mg/dL) | eGFR (mL/min/1.73 m2) | UPCR (g/gCr) | |
|---|---|---|---|---|---|---|
| Japanese | data | |||||
| IgAN | 338 | 38.1 | M177/F161 | 0.8 | 83.1 | 0.9 |
| Korean | ||||||
| IgAN | 69 | 40.5 | M28/F41 | 1.3 | 76.5 | 1.5 |
| Taiwanese | ||||||
| IgAN | 35 | 36.6 | M21/F14 | 1.9 | 51.9 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukao, Y.; Suzuki, H.; Kim, J.S.; Jeong, K.H.; Makita, Y.; Kano, T.; Nihei, Y.; Nakayama, M.; Lee, M.; Kato, R.; et al. Galactose-Deficient IgA1 as a Candidate Urinary Marker of IgA Nephropathy. J. Clin. Med. 2022, 11, 3173. https://doi.org/10.3390/jcm11113173
Fukao Y, Suzuki H, Kim JS, Jeong KH, Makita Y, Kano T, Nihei Y, Nakayama M, Lee M, Kato R, et al. Galactose-Deficient IgA1 as a Candidate Urinary Marker of IgA Nephropathy. Journal of Clinical Medicine. 2022; 11(11):3173. https://doi.org/10.3390/jcm11113173
Chicago/Turabian StyleFukao, Yusuke, Hitoshi Suzuki, Jin Sug Kim, Kyung Hwan Jeong, Yuko Makita, Toshiki Kano, Yoshihito Nihei, Maiko Nakayama, Mingfeng Lee, Rina Kato, and et al. 2022. "Galactose-Deficient IgA1 as a Candidate Urinary Marker of IgA Nephropathy" Journal of Clinical Medicine 11, no. 11: 3173. https://doi.org/10.3390/jcm11113173
APA StyleFukao, Y., Suzuki, H., Kim, J. S., Jeong, K. H., Makita, Y., Kano, T., Nihei, Y., Nakayama, M., Lee, M., Kato, R., Chang, J.-M., Lee, S. H., & Suzuki, Y. (2022). Galactose-Deficient IgA1 as a Candidate Urinary Marker of IgA Nephropathy. Journal of Clinical Medicine, 11(11), 3173. https://doi.org/10.3390/jcm11113173






