Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind?
Abstract
:1. Introduction
2. Current Mastectomy Techniques and Their Implications for Implant-Based Breast Reconstruction
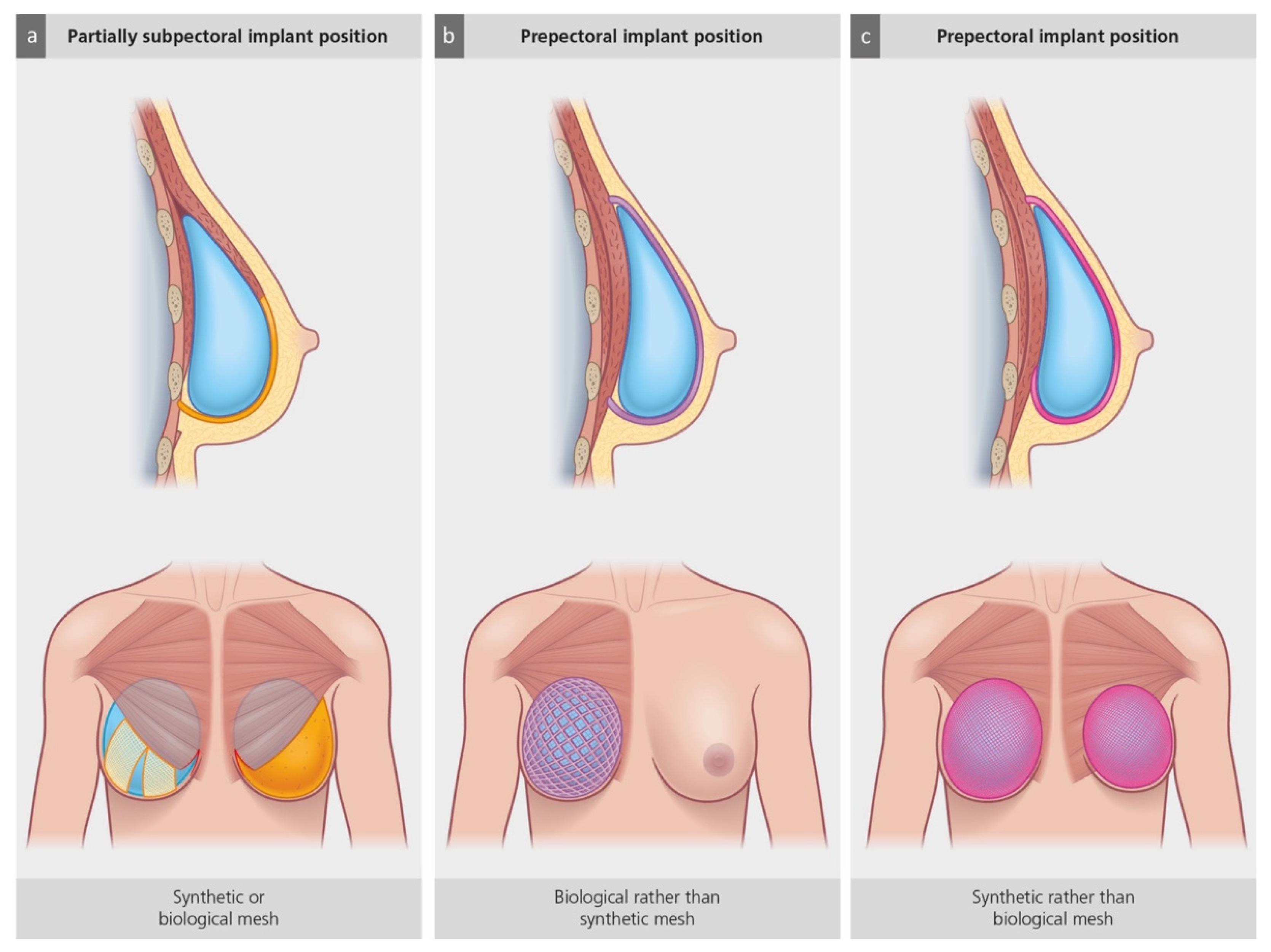
3. The Use of Biologic and Synthetic Meshes in Prepectoral IBBR
4. Autologous Fat Grafting and Hybrid Prepectoral Reconstruction
5. Radiation Therapy and Prepectoral IBBR
6. Further Technical Developments
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breasted, J.H. The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes. JAMA J. Am. Med. Assoc. 1931, 96, 1534. [Google Scholar] [CrossRef]
- Freeman, M.D.; Gopman, J.M.; Salzberg, C.A. The Evolution of Mastectomy Surgical Technique: From Mutilation to Medicine. Gland. Surg. 2018, 7, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Izuo, M. Medical History: Seishu Hanaoka and His Success in Breast Cancer Surgery under General Anesthesia Two Hundred Years Ago. Breast Cancer 2004, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Halsted, W.S. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann. Surg. 1894, 20, 497–555. [Google Scholar] [CrossRef] [PubMed]
- Halsted, W.S. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann. Surg. 1907, 46, 1–19. [Google Scholar] [CrossRef]
- Madden, J.L. Modified Radical Mastectomy. Surg. Gynecol. Obstet. 1965, 121, 1221–1230. [Google Scholar] [CrossRef]
- Freeman, B.S. Subcutaneous Mastectomy for Benign Breast Lesions with Immediate or Delayed Prosthetic Replacement. Plast. Reconstr. Surg. 1962, 30, 676–682. [Google Scholar] [CrossRef]
- Toth, B.A.; Lappert, P. Modified Skin Incisions for Mastectomy: The Need for Plastic Surgical Input in Preoperative Planning. Plast. Reconstr. Surg. 1991, 87, 1048–1053. [Google Scholar] [CrossRef]
- Murthy, V.; Chamberlain, R.S. Defining a Place for Nipple Sparing Mastectomy in Modern Breast Care: An Evidence Based Review. Breast J. 2013, 19, 571–581. [Google Scholar] [CrossRef]
- Czerny, V. Drei Plastische Operationen. III. Plastischer Ersatz Der Brustdruse Durch Ein Lipom. Arch. F Klin. Chir. 1895, 50, 216–217. [Google Scholar]
- Young, V.L.; Watson, M.E. Breast Implant Research: Where We Have Been, Where We Are, Where We Need to Go. Clin. Plast. Surg. 2001, 28, 451–483. [Google Scholar] [CrossRef]
- Snyderman, R.K.; Guthrie, R.H. Reconstruction of the Female Breast Following Radical Mastectomy. Plast. Reconstr. Surg. 1971, 47, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.D.; Brauer, R.O. Augmentation Mammaplasty. Surg. Clin. N. Am. 1971, 51, 441–452. [Google Scholar] [CrossRef]
- Sbitany, H. Pre-Pectoral Breast Reconstruction: A Less Invasive Option. Gland. Surg. 2019, 8, 1–2. [Google Scholar] [CrossRef]
- Rigo, M.H.; Piccinini, P.S.; Sartori, L.D.P.; de Carvalho, L.A.R.; Uebel, C.O. SMS—Split Muscle Support: A Reproducible Approach for Breast Implant Stabilization. Aesthetic Plast. Surg. 2020, 44, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kraenzlin, F.; Chopra, K.; Kokosis, G.; Venturi, M.L.; Mesbahi, A.; Nahabedian, M.Y. Revision Breast Reconstruction with Prepectoral Pocket Conversion of Submuscular Breast Implants. Plast. Reconstr. Surg. 2021, 147, 743e–748e. [Google Scholar] [CrossRef] [PubMed]
- Leonardis, J.M.; Lyons, D.A.; Giladi, A.M.; Momoh, A.O.; Lipps, D.B. Functional Integrity of the Shoulder Joint and Pectoralis Major Following Subpectoral Implant Breast Reconstruction. J. Orthop. Res. 2019, 37, 1610–1619. [Google Scholar] [CrossRef]
- Mennie, J.C.; Mohanna, P.N.; O’Donoghue, J.M.; Rainsbury, R.; Cromwell, D.A. National Trends in Immediate and Delayed Post-Mastectomy Reconstruction Procedures in England: A Seven-Year Population-Based Cohort Study. Eur. J. Surg. Oncol. 2017, 43, 52–61. [Google Scholar] [CrossRef]
- Cemal, Y.; Albornoz, C.R.; Disa, J.J.; Mccarthy, C.M.; Mehrara, B.J.; Pusic, A.L.; Cordeiro, P.G.; Matros, E. A Paradigm Shift in U.S. Breast Reconstruction: Part 2. the Influence of Changing Mastectomy Patterns on Reconstructive Rate and Method. Plast. Reconstr. Surg. 2013, 131, 320e–326e. [Google Scholar] [CrossRef]
- de Felice, F.; Marchetti, C.; Musella, A.; Palaia, I.; Perniola, G.; Musio, D.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Bilateral Risk-Reduction Mastectomy in BRCA1 and BRCA2 Mutation Carriers: A Meta-Analysis. Ann. Surg. Oncol. 2015, 22, 2876–2880. [Google Scholar] [CrossRef]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk Reduction and Survival Benefit of Prophylactic Surgery in BRCA Mutation Carriers, a Systematic Review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Chen, K.; Li, Y.; Lai, J.; Fang, Y.; Shen, S.; Liu, Y.; Su, F.; Yu, F. Factors Associated with the Increasing Trend of Contralateral Prophylactic Mastectomy among Patients with Ductal Carcinoma in Situ: Analysis of Surveillance, Epidemiology, and End Results Data. Breast 2018, 40, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.; Goodman, M.; Lin, C.C.; Freedman, R.A.; Dominici, L.S.; Ward, K.; Jemal, A. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004–2012. JAMA Surg. 2017, 152, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, P.; Zhang, M.; Wang, M.; Bai, F.; Wu, K. Growing Trends of Contralateral Prophylactic Mastectomy and Reconstruction in Young Breast Cancer. J. Surg. Res. 2019, 239, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Coopey, S.B.; Gadd, M.A.; Hughes, K.S.; Chang, D.C.; Oseni, T.O. Trends in Unilateral and Contralateral Prophylactic Mastectomy Use in Ductal Carcinoma In Situ of the Breast: Patterns and Predictors. Ann. Surg. Oncol. 2019, 26, 3863–3873. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, T.M.; Habermann, E.B.; Grund, E.H.; Morris, T.J.; Virnig, B.A. Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend toward More Aggressive Surgical Treatment. J. Clin. Oncol. 2007, 25, 5203. [Google Scholar] [CrossRef]
- Gutnik, L.; Fayanju, O.M. Controversies in Breast Cancer Surgery. Surg. Clin. N. Am. 2021, 101, 1033–1044. [Google Scholar] [CrossRef]
- Maruccia, M.; Elia, R.; Tedeschi, P.; Gurrado, A.; Moschetta, M.; Testini, M.; Giudice, G. Prepectoral Breast Reconstruction: An Ideal Approach to Bilateral Risk-Reducing Mastectomy. Gland Surg. 2021, 10, 2997–3006. [Google Scholar] [CrossRef]
- Rezai, M.; Strauß, S.; Kimmig, R.; Kern, P. Risk-Reducing, Conservative Mastectomy-Analysis of Surgical Outcome and Quality of Life in 272 Implant-Based Reconstructions Using TiLoop® Bra versus Autologous Corial Flaps. Gland Surg. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Veronesi, U.; Stafyla, V.; Luini, A.; Veronesi, P. Breast Cancer: From “Maximum Tolerable” to “Minimum Effective” Treatment. Front. Oncol. 2012, 2, 125. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-Sparing and Skin-Sparing Mastectomy: Review of Aims, Oncological Safety and Contraindications. Breast 2017, 34, S82–S84. [Google Scholar] [CrossRef] [PubMed]
- de La Cruz, L.; Moody, A.M.; Tappy, E.E.; Blankenship, S.A.; Hecht, E.M. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple–Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann. Surg. Oncol. 2015, 22, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.G.; Muhsen, S.; Moo, T.A.; Zabor, E.C.; Stempel, M.; Pusic, A.; Gemignani, M.L.; Morrow, M.; Sacchini, V.S. Increase in Utilization of Nipple-Sparing Mastectomy for Breast Cancer: Indications, Complications, and Oncologic Outcomes. Ann. Surg. Oncol. 2020, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Vicini, E.; de Lorenzi, F.; Invento, A.; Corso, G.; Radice, D.; Bozzo, S.; Fontana, S.K.R.; Caldarella, P.; Veronesi, P.; Galimberti, V. Is Nipple-Sparing Mastectomy Indicated after Previous Breast Surgery? A Series of 387 Institutional Cases. Plast. Reconstr. Surg. 2021, 148, 21–30. [Google Scholar] [CrossRef]
- Balci, F.L.; Kara, H.; Dulgeroglu, O.; Uras, C. Oncologic Safety of Nipple-Sparing Mastectomy in Patients with Short Tumor-Nipple Distance. Breast J. 2019, 25, 612–618. [Google Scholar] [CrossRef]
- Akdeniz Dogan, Z.; Farhadi, J.; Farhadi, J. Evaluation of Sensation on Mastectomy Skin Flaps Following Immediate Breast Reconstruction. J. Reconstr. Microsurg. 2020, 36, 420–425. [Google Scholar] [CrossRef]
- Didier, F.; Arnaboldi, P.; Gandini, S.; Maldifassi, A.; Goldhirsch, A.; Radice, D.; Minotti, I.; Ballardini, B.; Luini, A.; Santillo, B.; et al. Why Do Women Accept to Undergo a Nipple Sparing Mastectomy or to Reconstruct the Nipple Areola Complex When Nipple Sparing Mastectomy Is Not Possible? Breast Cancer Res. Treat. 2012, 132, 1177–1184. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Cil, T.D.; Semple, J.L.; Li, L.D.X.; Bagher, S.; Zhong, T.; Virani, S.; Narod, S.; Pal, T. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann. Surg. Oncol. 2015, 22, 3324–3330. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Knight, S.; Lambaudie, E.; Cohen, M. Robotic Breast and Reconstructive Surgery: 100 Procedures in 2-Years for 80 Patients. Surg. Oncol. 2019, 31, 38–45. [Google Scholar] [CrossRef]
- Lai, H.W.; Wang, C.C.; Lai, Y.C.; Chen, C.J.; Lin, S.L.; Chen, S.T.; Lin, Y.J.; Chen, D.R.; Kuo, S.J. The Learning Curve of Robotic Nipple Sparing Mastectomy for Breast Cancer: An Analysis of Consecutive 39 Procedures with Cumulative Sum Plot. Eur. J. Surg. Oncol. 2019, 45, 125–133. [Google Scholar] [CrossRef]
- Sarfati, B.; Struk, S.; Leymarie, N.; Honart, J.F.; Alkhashnam, H.; Tran de Fremicourt, K.; Conversano, A.; Rimareix, F.; Simon, M.; Michiels, S.; et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2018, 25, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.D.; de Bock, E.; Postma, E.L.; Bastian, O.W.; Schellekens, P.P.A.; Vriens, M.R.; Witkamp, A.J.; Richir, M.C. Robotic Nipple-Sparing Mastectomy Complication Rate Compared to Traditional Nipple-Sparing Mastectomy: A Systematic Review and Meta-Analysis. J. Robot. Surg. 2021, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.K. Reconstruction of the Ptotic Breast Using Wise Pattern Skin Deepithelialization. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e1077. [Google Scholar] [CrossRef] [PubMed]
- Rammos, C.K.; Mammolito, D.; King, V.A.; Yoo, A. Two-Stage Reconstruction of the Large and Ptotic Breasts: Skin Reduction Mastectomy with Prepectoral Device Placement. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1853. [Google Scholar] [CrossRef]
- Maruccia, M.; Elia, R.; Gurrado, A.; Moschetta, M.; Nacchiero, E.; Bolletta, A.; Testini, M.; Giudice, G. Skin-Reducing Mastectomy and Pre-Pectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast. Surg. 2020, 44, 664–672. [Google Scholar] [CrossRef]
- Safran, T.; Al-Halabi, B.; Viezel-Mathieu, A.; Boileau, J.F.; Dionisopoulos, T. Skin-Reducing Mastectomy with Immediate Prepectoral Reconstruction: Surgical, Aesthetic, and Patient-Reported Outcomes with and without Dermal Matrices. Plast. Reconstr. Surg. 2021, 147, 1046–1057. [Google Scholar] [CrossRef]
- Movassaghi, K.; Stewart, C.N. The “Smile Mastopexy”: A Novel Technique to Aesthetically Address the Excess Skin Envelope in Large, Ptotic Breasts While Preserving Nipple Areolar Complex during Prosthetic Breast Reconstruction. Aesthetic Surg. J. 2022, 42, NP393–NP403. [Google Scholar] [CrossRef]
- Albright, W.B.; Hawkes, P.J. The Bell Pattern: A Novel Breast Incision Approach to Skin-Reducing Mastectomies. Aesthetic Surg. J. Open Forum 2020, 2, ojz031. [Google Scholar] [CrossRef] [Green Version]
- Momeni, A.; Kanchwala, S.; Sbitany, H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast. Reconstr. Surg. 2020, 145, 914–920. [Google Scholar] [CrossRef]
- Gunnarsson, G.L.; Bille, C.; Reitsma, L.C.; Wamberg, P.; Thomsen, J.B. Prophylactic Nipple-Sparing Mastectomy and Direct-to-Implant Reconstruction of the Large and Ptotic Breast: Is Preshaping of the Challenging Breast a Key to Success? Plast. Reconstr. Surg. 2017, 140, 449–454. [Google Scholar] [CrossRef]
- Gunnarsson, G.L.; Thomsen, J.B. Reply: How to Preshape the Breast for a Successful Nipple-Sparing Mastectomy and Direct-to-Implant Breast Reconstruction in the Challenging Breast. Plast. Reconstr. Surg. 2018, 141, 610e–611e. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y. Current Approaches to Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. 2018, 142, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Breuing, K.H.; Warren, S.M. Immediate Bilateral Breast Reconstruction with Implants and Inferolateral AlloDerm Slings. Ann. Plast. Surg. 2005, 55, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Rolph, R.; Farhadi, J. The Use of Meshes and Matrices in Breast Reconstruction. Br. J. Hosp. Med. 2018, 79, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y. Prosthetic Breast Reconstruction and Red Breast Syndrome: Demystification and a Review of the Literature. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2108. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.F.; Perez Colman, M.C.; Stoppani, I. Red Breast Syndrome (RBS) Associated to the Use of Polyglycolic Mesh in Breast Reconstruction: A Case Report. Acta Chir. Plast. 2020, 62, 50–52. [Google Scholar]
- Cabalag, M.S.; Rostek, M.; Miller, G.S.; Chae, M.P.; Quinn, T.; Rozen, W.M.; Hunter-Smith, D.J. Alloplastic Adjuncts in Breast Reconstruction. Gland. Surg. 2016, 5, 158–173. [Google Scholar]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef]
- Haynes, D.F.; Kreithen, J.C. Vicryl Mesh in Expander/Implant Breast Reconstruction: Long-Term Follow-Up in 38 Patients. Plast. Reconstr. Surg. 2014, 134, 892–899. [Google Scholar] [CrossRef]
- Moyer, H.R.; Pinell-White, X.; Losken, A. The Effect of Radiation on Acellular Dermal Matrix and Capsule Formation in Breast Reconstruction: Clinical Outcomes and Histologic Analysis. Plast. Reconstr. Surg. 2014, 133, 214–221. [Google Scholar] [CrossRef]
- Seth, A.K.; Hirsch, E.M.; Fine, N.A.; Kim, J.Y.S. Utility of Acellular Dermis-Assisted Breast Reconstruction in the Setting of Radiation: A Comparative Analysis. Plast. Reconstr. Surg. 2012, 130, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.; Buckingham, B.; Matthews, J.; Sabino, J.; Tadisina, K.K.; Silverman, R.P.; Goldberg, N.H.; Slezak, S.; Singh, D.P. Acellular Dermal Matrix Reduces Capsule Formation in Two-Stage Breast Reconstruction. Int. Wound J. 2017, 14, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, J.; Li, Z.; Wang, B.; Sun, J. Efficacy of Acellular Dermal Matrix in Capsular Contracture of Implant-Based Breast Reconstruction: A Single-Arm Meta-Analysis. Aesthetic Plast. Surg. 2020, 44, 735–742. [Google Scholar] [CrossRef]
- Lardi, A.M.; Ho-Asjoe, M.; Junge, K.; Farhadi, J. Capsular Contracture in Implant Based Breast Reconstruction-the Effect of Porcine Acellular Dermal Matrix. Gland Surg. 2017, 6, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessler, O.; Reish, R.G.; Maman, D.Y.; Smith, B.L.; Austen, W.G. Beyond Biologics: Absorbable Mesh as a Low-Cost, Low-Complication Sling for Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2014, 133, 90e–99e. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Paepke, S.; Zwiefel, K.; Dieterich, H.; Blohmer, J.; Faridi, A.; Klein, E.; Gerber, B.; Nestle-Kraemling, C. Implant-Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLOOP Bra): A Multicenter Study of 231 Cases. Plast. Reconstr. Surg. 2013, 132, 8e–19e. [Google Scholar] [CrossRef]
- Rose, J.F.; Zafar, S.N.; Ellsworth, W.A., IV. Does Acellular Dermal Matrix Thickness Affect Complication Rate in Tissue Expander Based Breast Reconstruction? Plast. Surg. Int. 2016, 2016, 2867097. [Google Scholar] [CrossRef] [Green Version]
- Garcia, O.; Scott, J.R. Analysis of Acellular Dermal Matrix Integration and Revascularization Following Tissue Expander Breast Reconstruction in a Clinically Relevant Large-Animal Model. Plast. Reconstr. Surg. 2013, 131, 741e–751e. [Google Scholar] [CrossRef]
- Sorrentino, L.; Regolo, L.; Scoccia, E.; Petrolo, G.; Bossi, D.; Albasini, S.; Caruso, A.; Vanna, R.; Morasso, C.; Mazzucchelli, S.; et al. Autologous Fat Transfer after Breast Cancer Surgery: An Exact-Matching Study on the Long-Term Oncological Safety. Eur. J. Surg. Oncol. 2019, 45, 1827–1834. [Google Scholar] [CrossRef]
- Gale, K.L.; Rakha, E.A.; Ball, G.; Tan, V.K.; McCulley, S.J.; Macmillan, R.D. A Case-Controlled Study of the Oncologic Safety of Fat Grafting. Plast. Reconstr. Surg. 2015, 135, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- Cogliandro, A.; Barone, M.; Tenna, S.; Morelli Coppola, M.; Persichetti, P. The Role of Lipofilling After Breast Reconstruction: Evaluation of Outcomes and Patient Satisfaction with BREAST-Q. Aesthetic Plast. Surg. 2017, 41, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.W.; Kabir, M.; Sherman, K.A.; Meybodi, F.; French, J.R.; Elder, E.B. Patient Reported Outcomes of Autologous Fat Grafting after Breast Cancer Surgery. Breast 2017, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Panettiere, P.; Marchetti, L.; Accorsi, D. The Serial Free Fat Transfer in Irradiated Prosthetic Breast Reconstructions. Aesthetic Plast. Surg. 2009, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Gronovich, Y.; Winder, G.; Maisel-Lotan, A.; Lysy, I.; Sela, E.; Spiegel, G.; Carmon, M.; Hadar, T.; Elami, A.; Eizenman, N.; et al. Hybrid Prepectoral Direct-to-Implant and Autologous Fat Graft Simultaneously in Immediate Breast Reconstruction: A Single Surgeon’s Experience with 25 Breasts in 15 Consecutive Cases. Plast. Reconstr. Surg. 2022, 149, 386e–391e. [Google Scholar] [CrossRef] [PubMed]
- Stillaert, F.B.J.L.; Lannau, B.; Van Landuyt, K.; Blondeel, P.N. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2966. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Manyam, B.V.; Shah, C.; Woody, N.M.; Reddy, C.A.; Weller, M.A.; Juloori, A.; Naik, M.; Valente, S.; Grobmyer, S.; Durand, P.; et al. Long-Term Complications and Reconstruction Failures in Previously Radiated Breast Cancer Patients Receiving Salvage Mastectomy with Autologous Reconstruction or Tissue Expander/Implant-Based Reconstruction. Breast J. 2019, 25, 1071–1078. [Google Scholar] [CrossRef]
- Lesniak, D.M.; Sarfati, I.; Meredith, I.; Millochau, J.; Wang, K.C.; Nos, C.; Clough, K.B. Fat Grafting before Delayed Prophylactic Mastectomy and Immediate Implant Reconstruction for Patients at High Risk of Complications. Plast. Reconstr. Surg. 2022, 149, 52–56. [Google Scholar] [CrossRef]
- Ribuffo, D.; Atzeni, M.; Guerra, M.; Bucher, S.; Politi, C.; Deidda, M.; Atzori, F.; Dessi, M.; Madeddu, C.; Lay, G. Treatment of Irradiated Expanders: Protective Lipofilling Allows Immediate Prosthetic Breast Reconstruction in the Setting of Postoperative Radiotherapy. Aesthetic Plast. Surg. 2013, 37, 1146–1152. [Google Scholar] [CrossRef]
- Gentilucci, M.; Mazzocchi, M.; Alfano, C. Effects of Prophylactic Lipofilling after Radiotherapy Compared to Non-Fat Injected Breasts: A Randomized, Objective Study. Aesthetic Surg. J. 2020, 40, NP597–NP607. [Google Scholar] [CrossRef]
- Nahabedian, M.Y. What Are the Long-Term Aesthetic Issues in Prepectoral Breast Reconstruction? Aesthetic Surg. J. 2020, 40, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, I.; Ihrai, T.; Duvernay, A.; Nos, C.; Clough, K. Autologous Fat Grafting to the Postmastectomy Irradiated Chest Wall Prior to Breast Implant Reconstruction: A Series of 68 Patients. Ann. Chir. Plast. Esthet. 2013, 58, 35–40. [Google Scholar] [CrossRef]
- Lee, B.T.; Adesiyun, T.A.; Colakoglu, S.; Curtis, M.S.; Yueh, J.H.; Anderson, K.E.; Tobias, A.M.; Recht, A. Postmastectomy Radiation Therapy and Breast Reconstruction: An Analysis of Complications and Patient Satisfaction. Ann. Plast. Surg. 2010, 64, 679–683. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.H.; Ryu, J.Y.; Park, S.H.; Park, J.Y.; Han, M.H.; Lee, J.; Park, H.Y.; Yang, J.D. Influence of Irradiation on Capsules of Silicone Implants Covered with Acellular Dermal Matrix in Mice. Aesthetic Plast. Surg. 2021, 46, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, C.J.; Persing, S.M.; Pronovost, M.; Hodyl, C.; McConnell, D.; Ott Young, A. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann. Surg. Oncol. 2018, 25, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Neoh, D. Neoadjuvant Radiotherapy: Changing the Treatment Sequence to Allow Immediate Free Autologous Breast Reconstruction. J. Reconstr. Microsurg. 2018, 34, 624–631. [Google Scholar] [CrossRef]
- Tansley, P.; Ramsey, K.; Wong, S.; Guerrieri, M.; Pitcher, M.; Grinsell, D. New Treatment Sequence Protocol to Reconstruct Locally Advanced Breast Cancer. ANZ J. Surg. 2013, 83, 630–635. [Google Scholar] [CrossRef]
- Monrigal, E.; Dauplat, J.; Gimbergues, P.; le Bouedec, G.; Peyronie, M.; Achard, J.L.; Chollet, P.; Mouret-Reynier, M.A.; Nabholtz, J.M.; Pomel, C. Mastectomy with Immediate Breast Reconstruction after Neoadjuvant Chemotherapy and Radiation Therapy. A New Option for Patients with Operable Invasive Breast Cancer. Results of a 20 Years Single Institution Study. Eur. J. Surg. Oncol. 2011, 37, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Paillocher, N.; Florczak, A.S.; Richard, M.; Classe, J.M.; Oger, A.S.; Raro, P.; Wernert, R.; Lorimier, G. Evaluation of Mastectomy with Immediate Autologous Latissimus Dorsi Breast Reconstruction Following Neoadjuvant Chemotherapy and Radiation Therapy: A Single Institution Study of 111 Cases of Invasive Breast Carcinoma. Eur. J. Surg. Oncol. 2016, 42, 949–955. [Google Scholar] [CrossRef]
- Kung, T.A.; Kidwell, K.M.; Speth, K.A.; Pang, J.C.; Jagsi, R.; Newman, L.A.; Wilkins, E.G.; Momoh, A.O. Radiation-Induced Skin Changes after Postmastectomy Radiation Therapy: A Pilot Study on Indicators for Timing of Delayed Breast Reconstruction. J. Reconstr. Microsurg. 2019, 35, 209–215. [Google Scholar] [CrossRef]
- Deng, Y.; Li, H.; Zheng, Y.; Zhai, Z.; Wang, M.; Lin, S.; Li, Y.; Wei, B.; Xu, P.; Wu, Y.; et al. Impact of Preoperative vs Postoperative Radiotherapy on Overall Survival of Locally Advanced Breast Cancer Patients. Front. Oncol. 2021, 11, 779185. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Cro, S.C.; Coomber, B.; Rolph, R.; Cornelius, V.; Farhadi, J. A Randomised Controlled Feasibility Trial to Evaluate Local Heat Preconditioning on Wound Healing after Reconstructive Breast Surgery: The PreHEAT Trial. Pilot Feasibility Stud. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.; Rolph, R.; Cornelius, V.; Harder, Y.; Farhadi, J. Local Heat Preconditioning in Skin Sparing Mastectomy: A Pilot Study. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Fredman, R.; Wiser, I.; Friedman, T.; Heller, L.; Karni, T. Skin-Sparing Mastectomy Flap Ischemia Salvage Using Urgent Hyperbaric Chamber Oxygen Therapy: A Case Report. Undersea Hyperb. Med. 2014, 41, 145–147. [Google Scholar] [PubMed]
- Lauritzen, E.; Damsgaard, T.E. Use of Indocyanine Green Angiography Decreases the Risk of Complications in Autologous- and Implant-Based Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1703–1717. [Google Scholar] [CrossRef]
- Pruimboom, T.; Schols, R.M.; van Kuijk, S.M.J.; van der Hulst, R.R.W.J.; Qiu, S.S. Indocyanine Green Angiography for Preventing Postoperative Mastectomy Skin Flap Necrosis in Immediate Breast Reconstruction. Cochrane Database Syst. Rev. 2020, 4, CD013280. [Google Scholar] [CrossRef]
- Johnson, A.C.; Colakoglu, S.; Chong, T.W.; Mathes, D.W. Indocyanine Green Angiography in Breast Reconstruction: Utility, Limitations, and Search for Standardization. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2694. [Google Scholar] [CrossRef]
- Groth, A.K.; Graf, R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthetic Plast. Surg. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Govrin-Yehudain, J.; Dvir, H.; Preise, D.; Govrin-Yehudain, O.; Govreen-Segal, D. Lightweight Breast Implants: A Novel Solution for Breast Augmentation and Reconstruction Mammaplasty. Aesthetic Surg. J. 2015, 35, 965–971. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinzierl, A.; Schmauss, D.; Brucato, D.; Harder, Y. Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind? J. Clin. Med. 2022, 11, 3079. https://doi.org/10.3390/jcm11113079
Weinzierl A, Schmauss D, Brucato D, Harder Y. Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind? Journal of Clinical Medicine. 2022; 11(11):3079. https://doi.org/10.3390/jcm11113079
Chicago/Turabian StyleWeinzierl, Andrea, Daniel Schmauss, Davide Brucato, and Yves Harder. 2022. "Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind?" Journal of Clinical Medicine 11, no. 11: 3079. https://doi.org/10.3390/jcm11113079
APA StyleWeinzierl, A., Schmauss, D., Brucato, D., & Harder, Y. (2022). Implant-Based Breast Reconstruction after Mastectomy, from the Subpectoral to the Prepectoral Approach: An Evidence-Based Change of Mind? Journal of Clinical Medicine, 11(11), 3079. https://doi.org/10.3390/jcm11113079







