Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
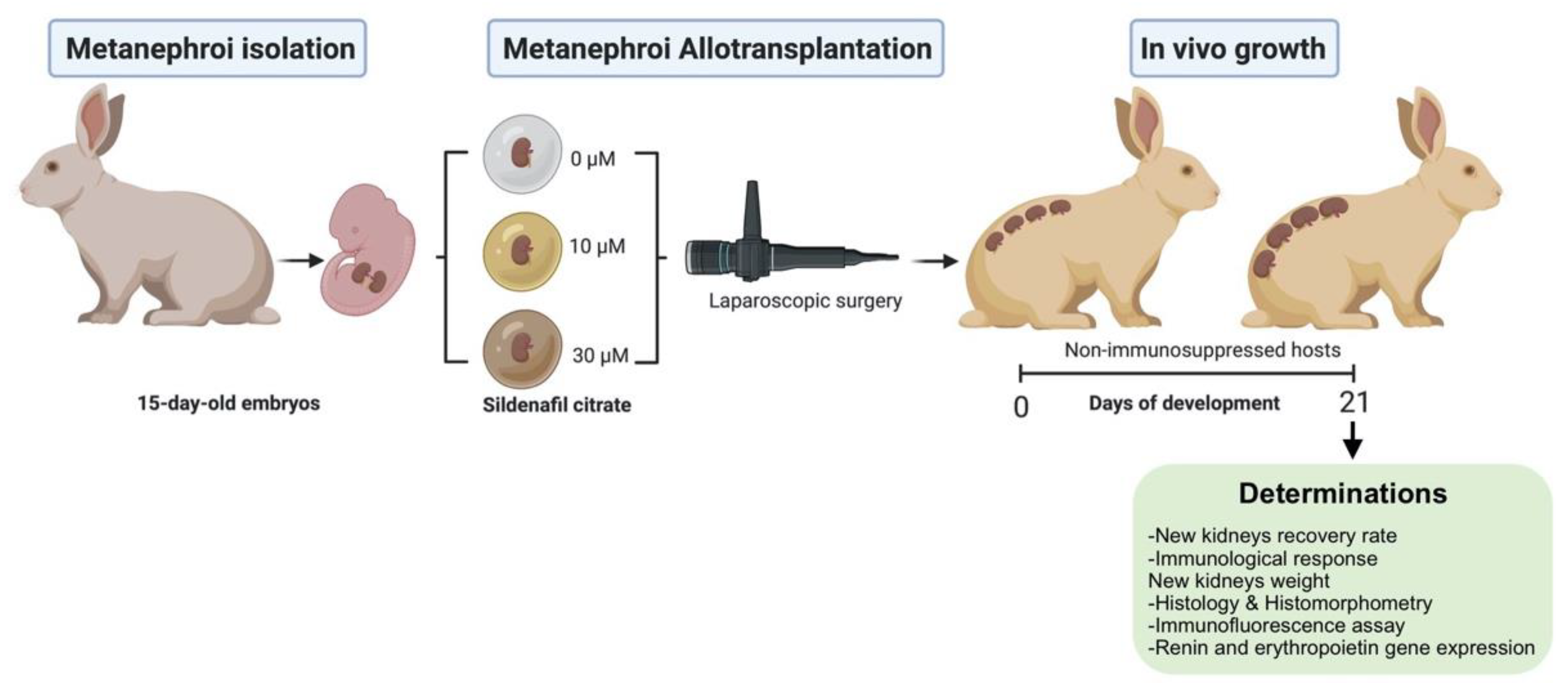
2.2. Experimental Design
2.3. Metanephroi Recovery and Transplantation
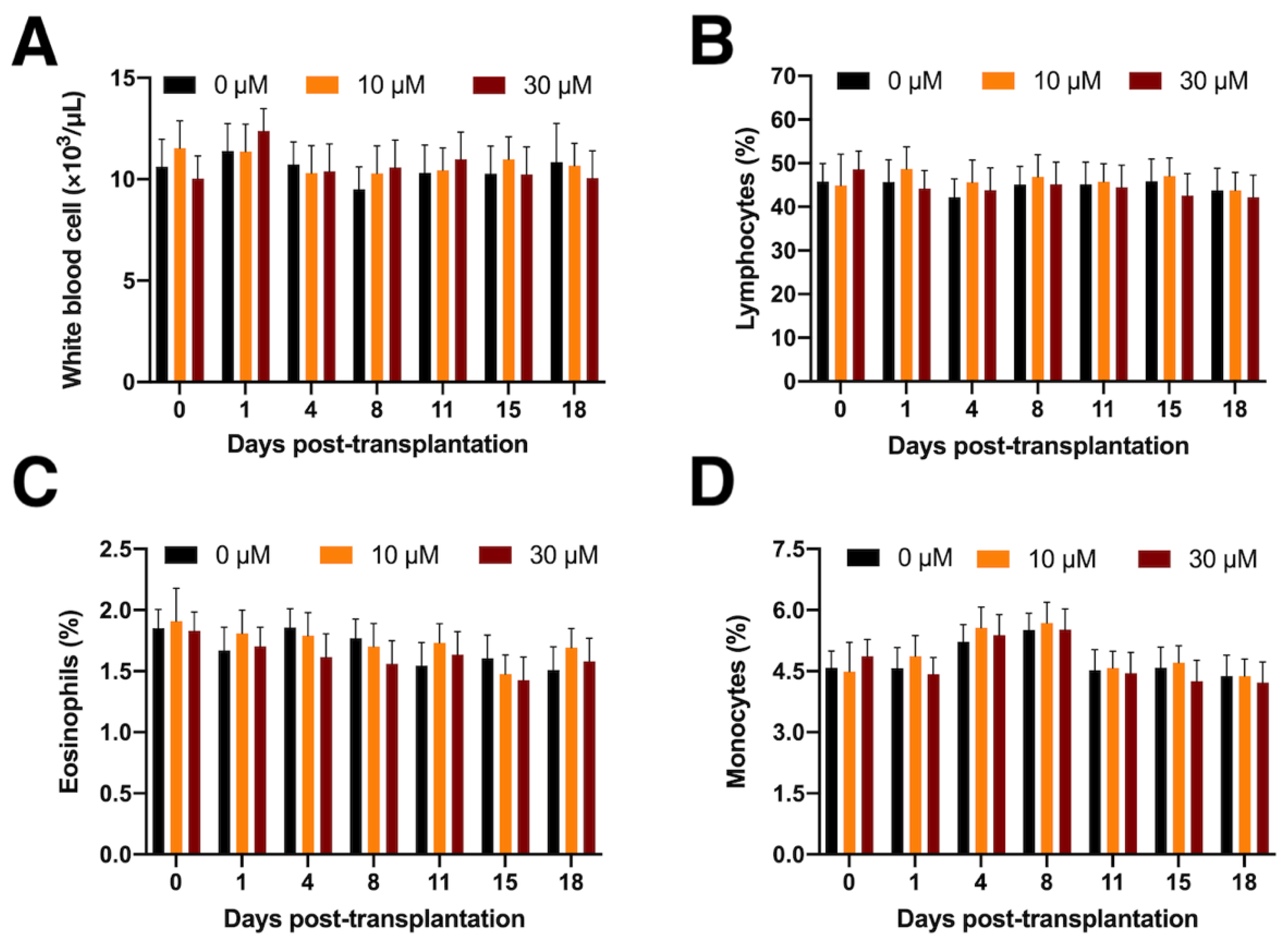
2.4. Determination of Peripheral White Blood Cells
2.5. Metanephroi Development and Histomorphometry of the Renal Corpuscle
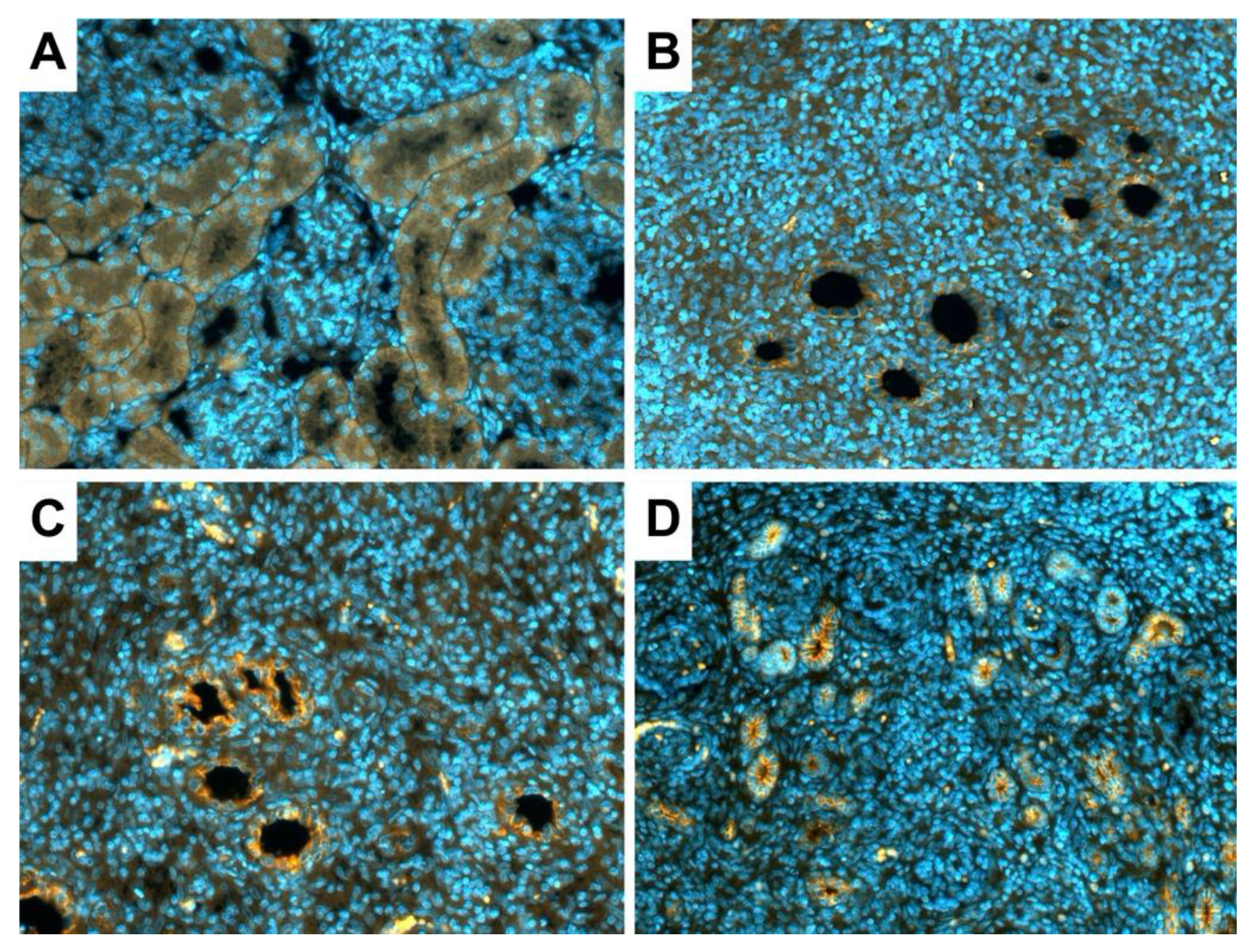
2.6. Tubule Integrity by Targeting E-Cadherin
2.7. Renin and Erythropoietin mRNA Gene Expression
2.8. Statistical Analyses
3. Results
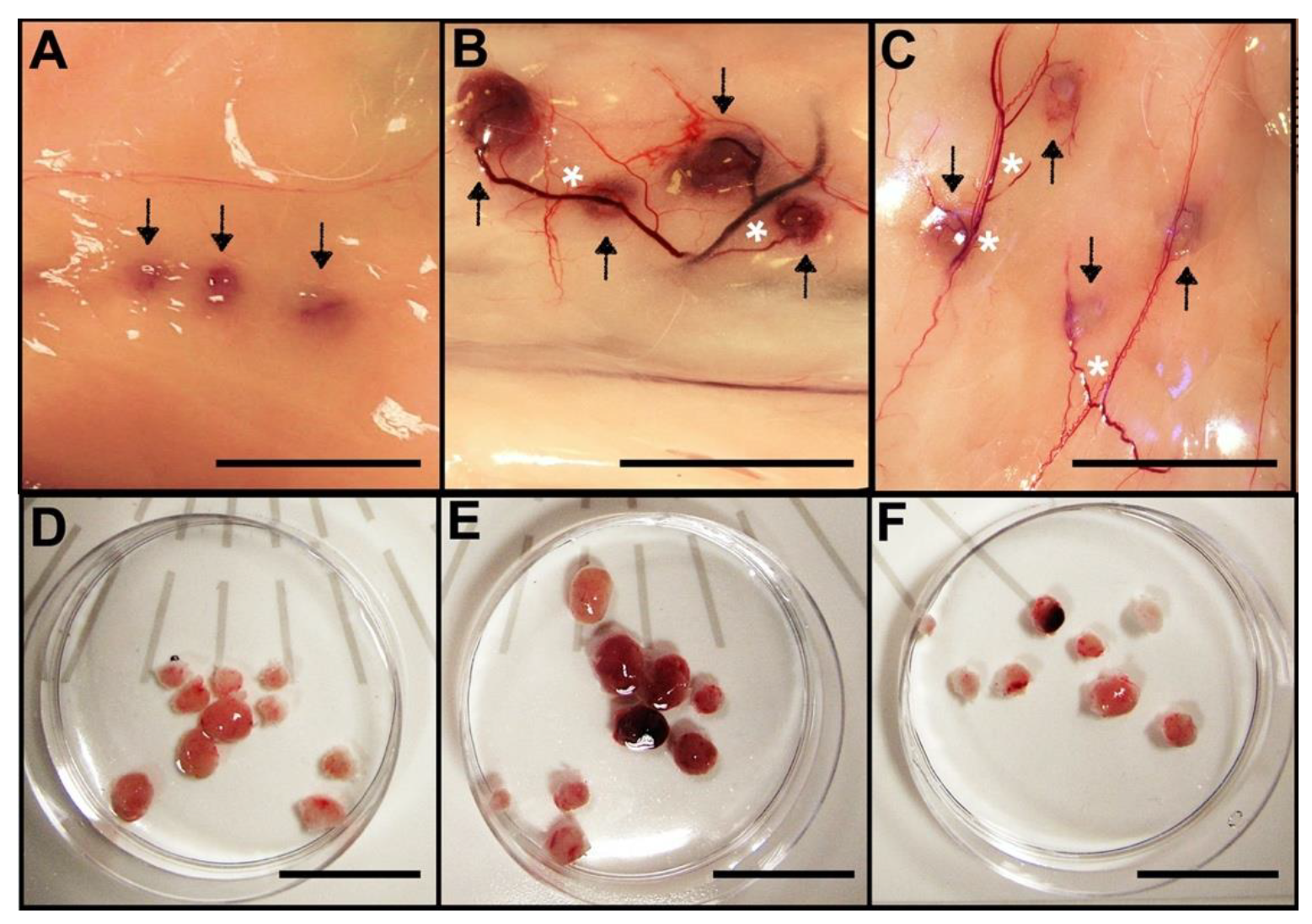
3.1. Allotransplanted Metanephroi Form Adult Organs
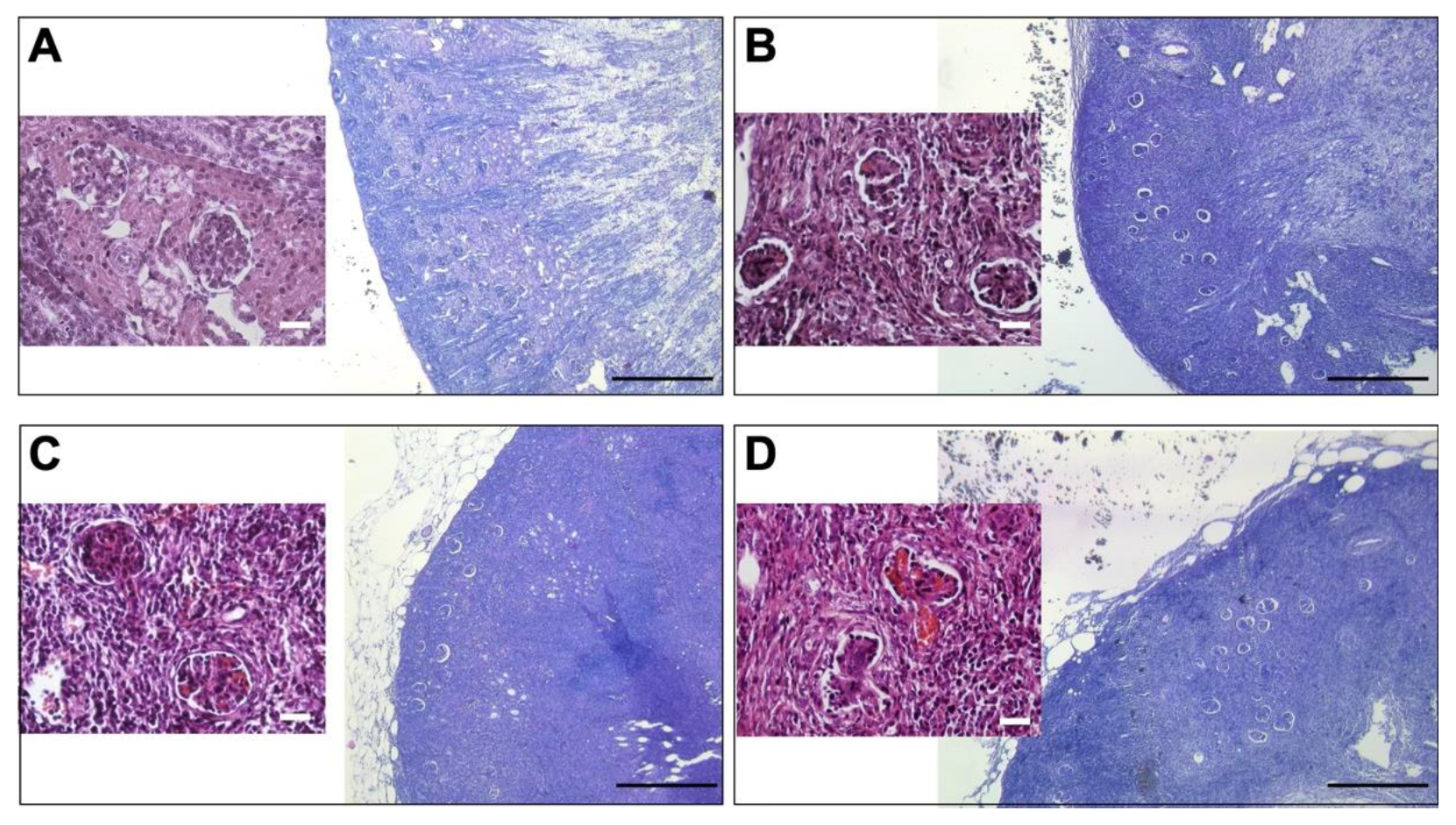
3.2. Comparative Renal Weight and Histomorphometry Study
3.3. Tubule Integrity in the New Kidneys
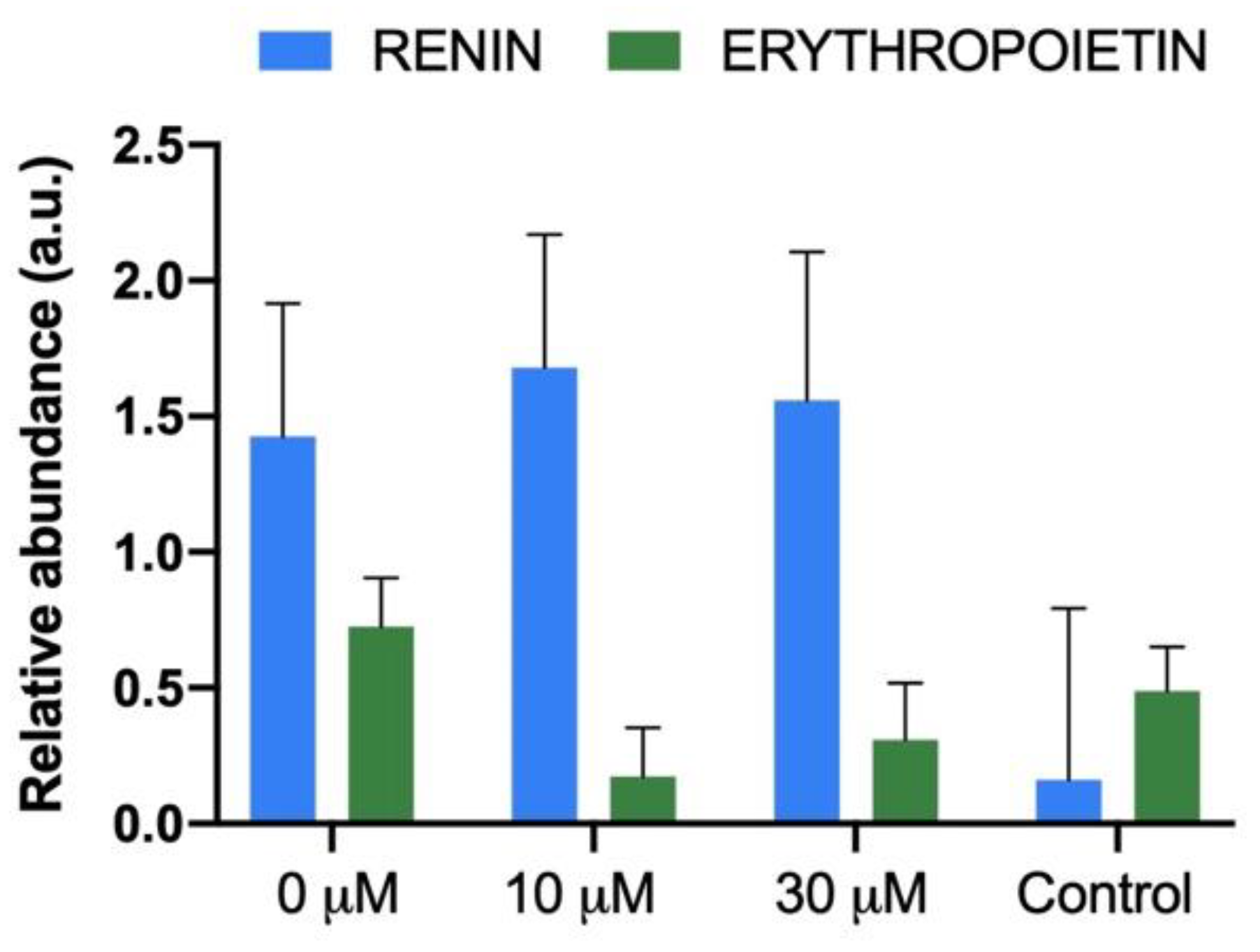
3.4. Glomerular Renin and Erythropoietin Production in the New Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chambers, J.M.; McKee, R.A.; Drummond, B.E.; Wingert, R.A. Evolving technology: Creating kidney organoids from stem cells. AIMS Bioeng. 2016, 3, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, M.; Peloso, A.; Katari, R.; Orlando, G. Regeneration and Bioengineering of the Kidney: Current Status and Future Challenges. Curr. Urol. Rep. 2014, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Organización Nacional de Trasplantes (ONT). Nota de Prensa. Available online: http://www.ont.es/prensa/NotasDePrensa/07.09.2020NPONTRegistroMundial.pdf (accessed on 14 October 2020).
- Organización Nacional de Trasplantes (ONT). Memoria Actividad Donación Y Trasplante Renal. España. 2019. Available online: http://www.ont.es/infesp/Memorias/Actividad_de_Donación_y_Trasplante_Renal_2019.pdf (accessed on 13 October 2020).
- Organ Procurement and Transplantation Network (OPTN) Web Site. Available online: https://optn.transplant.hrsa.gov/ (accessed on 10 October 2020).
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Foutz, J.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am. J. Transplant. 2020, 20, 20–130. [Google Scholar] [CrossRef] [PubMed]
- Heilman, R.L.; Smith, M.L.; Smith, B.H.; Kumar, A.; Srinivasan, A.; Huskey, J.L.; Khamash, H.A.; Jadlowiec, C.C.; Mathur, A.K.; Moss, A.A.; et al. Long-term Outcomes Following Kidney Transplantation From Donors With Acute Kidney Injury. Transplantation 2019, 103, e263–e272. [Google Scholar] [CrossRef]
- García-Domínguez, X.; Vicente, J.S.; Vera-Donoso, C.D.; Marco-Jimenez, F. Current Bioengineering and Regenerative Strategies for the Generation of Kidney Grafts on Demand. Curr. Urol. Rep. 2017, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Peired, A.J.; Mazzinghi, B.; De Chiara, L.; Guzzi, F.; Lasagni, L.; Romagnani, P.; Lazzeri, E. Bioengineering strategies for nephrologists: Kidney was not built in a day. Expert Opin. Biol. Ther. 2020, 20, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Montserrat, N.; Garreta, E.; Belmonte, J.C.I. Regenerative strategies for kidney engineering. FEBS J. 2016, 283, 3303–3324. [Google Scholar] [CrossRef] [Green Version]
- Hammerman, M.R. Transplantation of renal primordia: Renal organogenesis. Pediatr. Nephrol. 2007, 22, 1991–1998. [Google Scholar] [CrossRef]
- Dekel, B.; Burakova, T.; Arditti, F.D.; Reich-Zeliger, S.; Milstein, O.; Aviel-Ronen, S.; Rechavi, G.; Friedman, N.; Kaminski, N.; Passwell, J.H.; et al. Human and porcine early kidney precursors as a new source for transplantation. Nat. Med. 2003, 9, 53–60. [Google Scholar] [CrossRef]
- Takeda, S.-I.; Rogers, S.A.; Hammerman, M.R. Differential origin for endothelial and mesangial cells after transplantation of pig fetal renal primordia into rats. Transpl. Immunol. 2006, 15, 211–215. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yokoo, T.; Yokote, S.; Utsunomiya, Y.; Ohashi, T.; Hosoya, T. Functional development of a transplanted embryonic kidney: Effect of transplantation site. J. Nephrol. 2012, 25, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yokote, S.; Yokoo, T.; Matsumoto, K.; Utsunomiya, Y.; Kawamura, T.; Hosoya, T. The effect of metanephros transplantation on blood pressure in anephric rats with induced acute hypotension. Nephrol. Dial. Transplant. 2012, 27, 3449–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokote, S.; Yokoo, T.; Matsumoto, K.; Ohkido, I.; Utsunomiya, Y.; Kawamura, T.; Hosoya, T. Metanephros Transplantation Inhibits the Progression of Vascular Calcification in Rats with Adenine-Induced Renal Failure. Nephron Exp. Nephrol. 2012, 120, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yokoo, T.; Matsunari, H.; Iwai, S.; Yokote, S.; Teratani, T.; Gheisari, Y.; Tsuji, O.; Okano, H.; Utsunomiya, Y.; et al. Xenotransplanted Embryonic Kidney Provides a Niche for Endogenous Mesenchymal Stem Cell Differentiation into Erythropoietin-Producing Tissue. Stem Cells 2012, 30, 1228–1235. [Google Scholar] [CrossRef]
- Yokote, S.; Matsunari, H.; Iwai, S.; Yamanaka, S.; Uchikura, A.; Fujimoto, E.; Matsumoto, K.; Nagashima, H.; Kobayashi, E.; Yokoo, T. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc. Natl. Acad. Sci. USA 2015, 112, 12980–12985. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.A.; Lowell, J.A.; Hammerman, N.A.; Hammerman, M.R. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998, 54, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.A.; Talcott, M.; Hammerman, M.R. Transplantation of Pig Metanephroi. ASAIO J. 2003, 49, 48–52. [Google Scholar] [CrossRef]
- Woolf, A.S.; Palmer, S.J.; Snow, M.L.; Fine, L.G. Creation of a functioning chimeric mammalian kidney. Kidney Int. 1990, 38, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Abrahamson, D.R.; St. John, P.L.; Pillion, D.J.; Tucker, D.C. Glomerular development in intraocular and intrarenal grafts of fetal kidneys. Lab. Investig. 1991, 64, 629–639. [Google Scholar]
- Rogers, S.A.; Hammerman, M.R. Transplantation of rat metanephroi into mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1865–R1869. [Google Scholar] [CrossRef]
- Hammerman, M.R. Xenotransplantation of developing kidneys. Am. J. Physiol. Ren. Physiol. 2002, 283, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Noordergraaf, J.; Schucker, A.; Martin, M.; Schuurman, H.-J.; Ordway, B.; Cooley, K.; Sheffler, M.; Theis, K.; Armstrong, C.; Klein, L.; et al. Pathogen elimination and prevention within a regulated, Designated Pathogen Free, closed pig herd for long-term breeding and production of xenotransplantation materials. Xenotransplantation 2018, 25, e12428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.A.; Liapis, H.; Hammerman, M.R. Transplantation of metanephroi across the major histocompatibility complex in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, 132–136. [Google Scholar] [CrossRef]
- Hammerman, M.R. Transplantation of renal precursor cells: A new therapeutic approach. Pediatr. Nephrol. 2000, 14, 513–517. [Google Scholar] [CrossRef]
- Rogers, S.A.; Powell-Braxton, L.; Hammerman, M.R. Insulin-like growth factor I regulates renal development in rodents. Dev. Genet. 1999, 24, 293–298. [Google Scholar] [CrossRef]
- Rogers, S.A.; Hammerman, M.R. Prolongation of Life in Anephric Rats following de novo Renal Organogenesis. Organogenesis 2004, 1, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Hammerman, M.R. Organogenesis of kidneys following transplantation of renal progenitor cells. Transpl. Immunol. 2004, 12, 229–239. [Google Scholar] [CrossRef]
- Zahran, M.H.; Barakat, N.; Khater, S.; Awadalla, A.; Mosbah, A.; Nabeeh, A.; Hussein, A.M.; Shokeir, A.A. Renoprotective effect of local sildenafil administration in renal ischaemia–reperfusion injury: A randomised controlled canine study. Arab J. Urol. 2019, 17, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, G.; Zisis, I.-E.; Docea, A.O.; Tsarouhas, K.; Fragkiadoulaki, I.; Mavridis, C.; Karavitakis, M.; Stratakis, S.; Stylianou, K.; Tsitsimpikou, C.; et al. Current Concepts on the Reno-Protective Effects of Phosphodiesterase 5 Inhibitors in Acute Kidney Injury: Systematic Search and Review. J. Clin. Med. 2020, 9, 1284. [Google Scholar] [CrossRef]
- Koneru, S.; Penumathsa, S.V.; Thirunavukkarasu, M.; Vidavalur, R.; Zhan, L.; Singal, P.K.; Engelman, R.M.; Das, D.K.; Maulik, N. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: Role of VEGF/angiopoietin-1. J. Cell. Mol. Med. 2008, 12, 2651–2664. [Google Scholar] [CrossRef] [Green Version]
- Kemaloğlu, C.A.; Tekin, Y. The effect of sildenafil on fascia-wrapped diced cartilage grafts. Laryngoscope 2015, 125, E168–E172. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.H.; Kim, I.J.; Joo, S.Y.; Kim, E.Y.; Kim, C.S.; Choi, J.S.; Ma, S.K.; Kim, S.H.; Lee, J.U.; Kima, S.W. Renoprotective Effects of Sildenafil in DOCA-Salt Hypertensive Rats. Kidney Blood Press. Res. 2012, 36, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lledó-García, E.; Subirá-Ríos, D.; Rodríguez–Martínez, D.; Dulín, E.; Alvarez-Fernández, E.; Hernández-Fernández, C.; Del Cañizo-López, J.F. Sildenafil as a Protecting Drug for Warm Ischemic Kidney Transplants: Experimental Results. J. Urol. 2009, 182, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Lledo-Garcia, E.; Rodriguez-Martinez, D.; Cabello-Benavente, R.; Moncada-Iribarren, I.; Tejedor-Jorge, A.; Dulin, E.; Hernandez-Fernandez, C.; Del Canizo-Lopez, J.F. Sildenafil Improves Immediate Posttransplant Parameters in Warm-Ischemic Kidney Transplants: Experimental Study. Transplant. Proc. 2007, 39, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Vera-Donoso, C.D.; García-Dominguez, X.; Jiménez-Trigos, E.; García-Valero, L.; Vicente, J.S.; Marco-Jiménez, F. Laparoscopic transplantation of metanephros: A first step to kidney xenotransplantation. Actas Urológicas Españolas (Engl. Ed.) 2015, 39, 527–534. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Marco-Jimenez, F.; Viudes-De-Castro, M.P.; Vicente, J.S. Minimally Invasive Embryo Transfer and Embryo Vitrification at the Optimal Embryo Stage in Rabbit Model. J. Vis. Exp. 2019, 147, e58055. [Google Scholar] [CrossRef] [Green Version]
- Weltzien, F.-A.; Pasqualini, C.; Vernier, P.; Dufour, S. A quantitative real-time RT-PCR assay for European eel tyrosine hydroxylase. Gen. Comp. Endocrinol. 2005, 142, 134–142. [Google Scholar] [CrossRef]
- Llobat, L.; Marco-Jiménez, F.; Peñaranda, D.S.; Saenz-de-Juano, M.D.; Vicente, J.S. Effect of Embryonic Genotype on Reference Gene Selection for RT-qPCR Normalization. Reprod. Domest. Anim. 2012, 47, 629–634. [Google Scholar] [CrossRef]
- Imberti, B.; Corna, D.; Rizzo, P.; Xinaris, C.; Abbate, M.; Longaretti, L.; Cassis, P.; Benedetti, V.; Benigni, A.; Zoja, C.; et al. Renal Primordia Activate Kidney Regenerative Events in a Rat Model of Progressive Renal Disease. PLoS ONE 2015, 10, e0120235. [Google Scholar] [CrossRef] [Green Version]
- Organ Donation and Transplantation Activities 2018 Report. Global Observatory on Donation and Transplantation (GODT). Available online: http://www.transplant-observatory.org/wp-content/uploads/2020/10/glorep2018-2.pdf (accessed on 10 October 2020).
- Yamanaka, S.; Yokoo, T. Current Bioengineering Methods for Whole Kidney Regeneration. Stem Cells Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Rostaing, L.; Tran-Van, T.; Ader, J.-L. Increased Glomerular Filtration Rate in Kidney-Transplant Recipients Who Take Sildenafil. N. Engl. J. Med. 2000, 342, 1679–1680. [Google Scholar] [CrossRef] [PubMed]
- Pyriochou, A.; Zhou, Z.; Koika, V.; Petrou, C.; Cordopatis, P.; Sessa, W.C.; Papapetropoulos, A. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J. Cell. Physiol. 2007, 211, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Vidavalur, R.; Penumathsa, S.V.; Zhan, L.; Thirunavukkarasu, M.; Maulik, N. Sildenafil induces angiogenic response in human coronary arteriolar endothelial cells through the expression of thioredoxin, hemeoxygenase and vascular endothelial growth factor. Vasc. Pharmacol. 2006, 45, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, X.; Dai, Y.; Zheng, F.; Wang, D.; Huang, Y.; Bian, J.; Deng, C. Chronic Administration of Sildenafil Modified the Impaired VEGF System and Improved the Erectile Function in Rats with Diabetic Erectile Dysfunction. J. Sex. Med. 2010, 7, 3868–3878. [Google Scholar] [CrossRef]
- Yardimci, S.; Bostanci, E.B.; Ozer, I.; Dalgic, T.; Surmelioglu, A.; Aydog, G.; Akoglu, M. Sildenafil Accelerates Liver Regeneration after Partial Hepatectomy in Rats. Transplant. Proc. 2012, 44, 1747–1750. [Google Scholar] [CrossRef]
- Gao, L.; Liu, M.-M.; Zang, H.-M.; Ma, Q.-Y.; Yang, Q.; Jiang, L.; Ren, G.-L.; Li, H.-D.; Wu, W.-F.; Wang, J.-N.; et al. Restoration of E-cadherin by PPBICA protects against cisplatin-induced acute kidney injury by attenuating inflammation and programmed cell death. Lab. Investig. 2018, 98, 911–923. [Google Scholar] [CrossRef] [Green Version]
- Hammerman, M.R. Windows of opportunity for organogenesis. Transpl. Immunol. 2005, 15, 1–8. [Google Scholar] [CrossRef]
- Marshall, D.; Dilworth, M.R.; Clancy, M.; Bravery, C.A.; Ashton, N. Increasing renal mass improves survival in anephric rats following metanephros transplantation. Exp. Physiol. 2007, 92, 263–271. [Google Scholar] [CrossRef]
- Andersen, O.S.; Jonasson, O.; Merkel, F.K. En Bloc Transplantation of Pediatric Kidneys Into Adult Patients. Arch. Surg. 1974, 108, 35–37. [Google Scholar] [CrossRef]
- Damji, S.; Callaghan, C.J.; Loukopoulos, I.; Kessaris, N.; Stojanovic, J.; Marks, S.D.; Mamode, N. Utilisation of small paediatric donor kidneys for transplantation. Pediatr. Nephrol. 2019, 34, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- García-Domínguez, X.; Vera-Donoso, C.D.; García-Valero, L.; Vicente, J.S.; Marco-Jimenez, F. Embryonic Organ Transplantation: The New Era of Xenotransplantation. In Frontiers in Transplantology; Abdeldayem, H., El-Kased, A.F., El-Shaarawy, A., Eds.; InTech: London, UK, 2016; pp. 25–46. [Google Scholar]
- Marco-Jiménez, F.; García-Domínguez, X.; Jiménez-Trigos, E.; Vera-Donoso, C.D.; Vicente, J.S. Vitrification of kidney precursors as a new source for organ transplantation. Cryobiology 2015, 70, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Dominguez, X.; Vicente, J.S.; Vera-Donoso, C.D.; Marco-Jimenez, F. Successful development of vitrified embryonic kidney after laparoscopy transplantation into non-immunosuppressed hosts. Trends Transplant. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Vera-Donoso, C.D.; Jimenez-Trigos, E.; Vicente, J.S.; Marco-Jimenez, F. First steps towards organ banks: Vitrification of renal primordial. Cryo Lett. 2016, 37, 47–52. [Google Scholar]

| Genes | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| REN | Forward: 5′-GGGACTCCTGCTGGTACTCT-3′ | 100 |
| Reverse: 5′-CTGAGGGCATTTTCTTGAGG-3′ | ||
| EPO | Forward: 5′-ACGTGGACAAGGCTGTCAGT-3′ | 162 |
| Reverse: 5′-TGGAGTAGATGCGGAAAAGC-3′ | ||
| GAPDH | Forward: 5′-GCCGCTTCTTCTCGTGCAG-3′ | 144 |
| Reverse: 5′-ATGGATCATTGATGGCGACAACAT-3′ |
| Sildenafil Citrate | n | Renal Corpuscle | Glomerulus | |||
|---|---|---|---|---|---|---|
| Area (μm2) | Perimeter (μm) | Area (μm2) | Perimeter (μm) | Cell Number | ||
| 0 µM SC | 10 | 3034.6± 176.44 b | 201.1 ± 6.06 b | 2132.5 ± 142.56 b | 170.9 ± 5.70 b | 41.0 ± 2.22 b |
| 10 µM SC | 9 | 3639.7 ± 179.94 a | 218.5 ± 6.18 a | 2749.5 ± 145.39 a | 192.0 ± 5.81 a | 49.9 ± 2.26 a |
| 30 µM SC | 8 | 3582.44 ± 187.59 a | 218.3 ± 6.45 a | 2655.7 ± 151.85 a | 190.2 ± 6.06 a | 48.3 ± 2.36 a |
| Control | 6 | 2633.4 ± 92.31 c | 184.2 ± 3.17 c | 2104.7 ± 74.58 b | 165.3 ± 2.98 b | 52.7 ± 1.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Dominguez, X.; Vera-Donoso, C.D.; Lopez-Moncholi, E.; Moreno-Manzano, V.; Vicente, J.S.; Marco-Jiménez, F. Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts. J. Clin. Med. 2022, 11, 3068. https://doi.org/10.3390/jcm11113068
Garcia-Dominguez X, Vera-Donoso CD, Lopez-Moncholi E, Moreno-Manzano V, Vicente JS, Marco-Jiménez F. Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts. Journal of Clinical Medicine. 2022; 11(11):3068. https://doi.org/10.3390/jcm11113068
Chicago/Turabian StyleGarcia-Dominguez, Ximo, César D. Vera-Donoso, Eric Lopez-Moncholi, Victoria Moreno-Manzano, José S. Vicente, and Francisco Marco-Jiménez. 2022. "Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts" Journal of Clinical Medicine 11, no. 11: 3068. https://doi.org/10.3390/jcm11113068
APA StyleGarcia-Dominguez, X., Vera-Donoso, C. D., Lopez-Moncholi, E., Moreno-Manzano, V., Vicente, J. S., & Marco-Jiménez, F. (2022). Sildenafil Citrate Enhances Renal Organogenesis Following Metanephroi Allotransplantation into Non-Immunosuppressed Hosts. Journal of Clinical Medicine, 11(11), 3068. https://doi.org/10.3390/jcm11113068






