Clinical Implications of Determining Individualized Positive End-Expiratory Pressure Using Electrical Impedance Tomography in Post-Cardiac Surgery Patients: A Prospective, Non-Randomized Interventional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
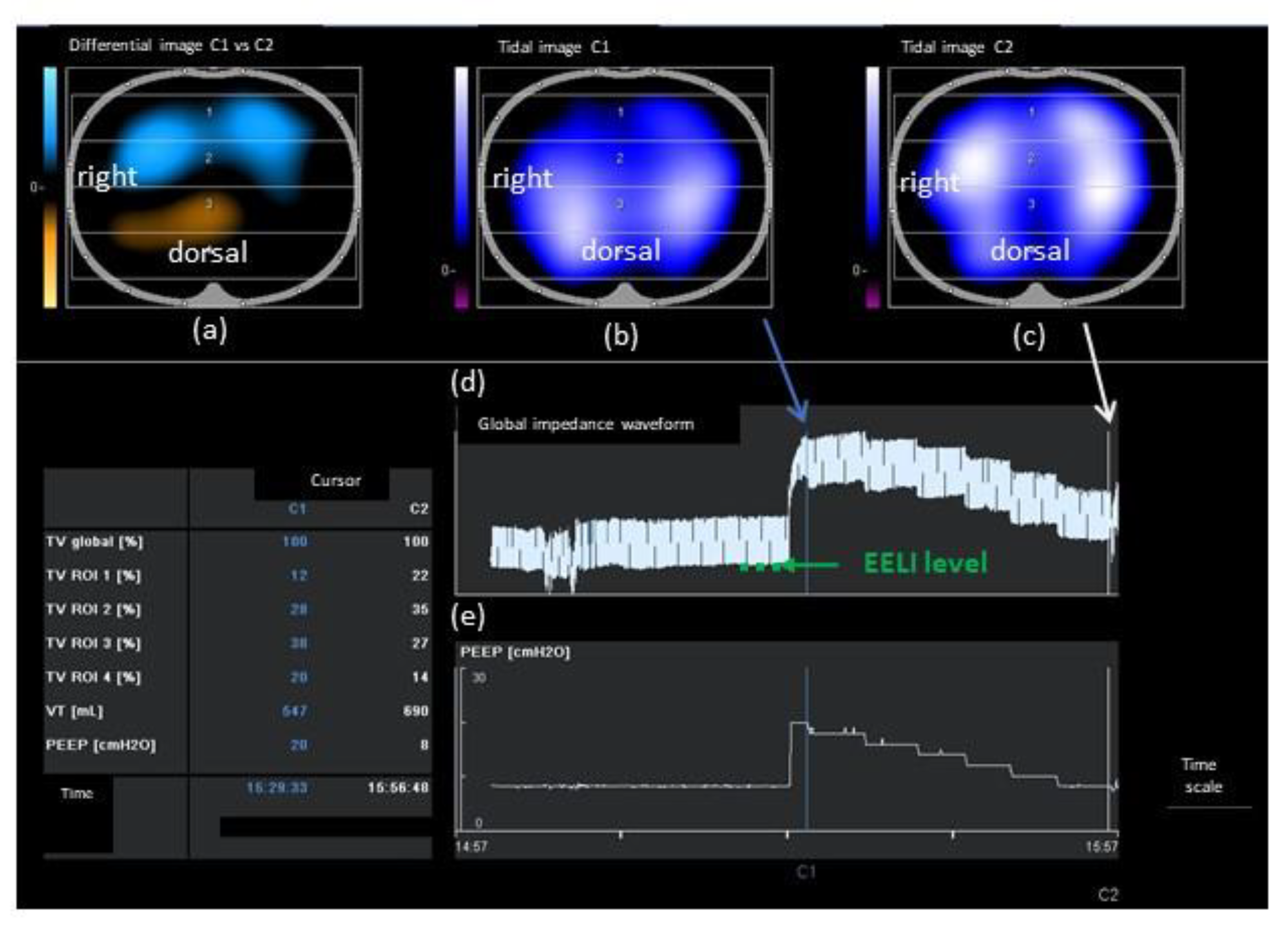
2.3. Individualized PEEP Setting by EIT (PEEPONLINE)
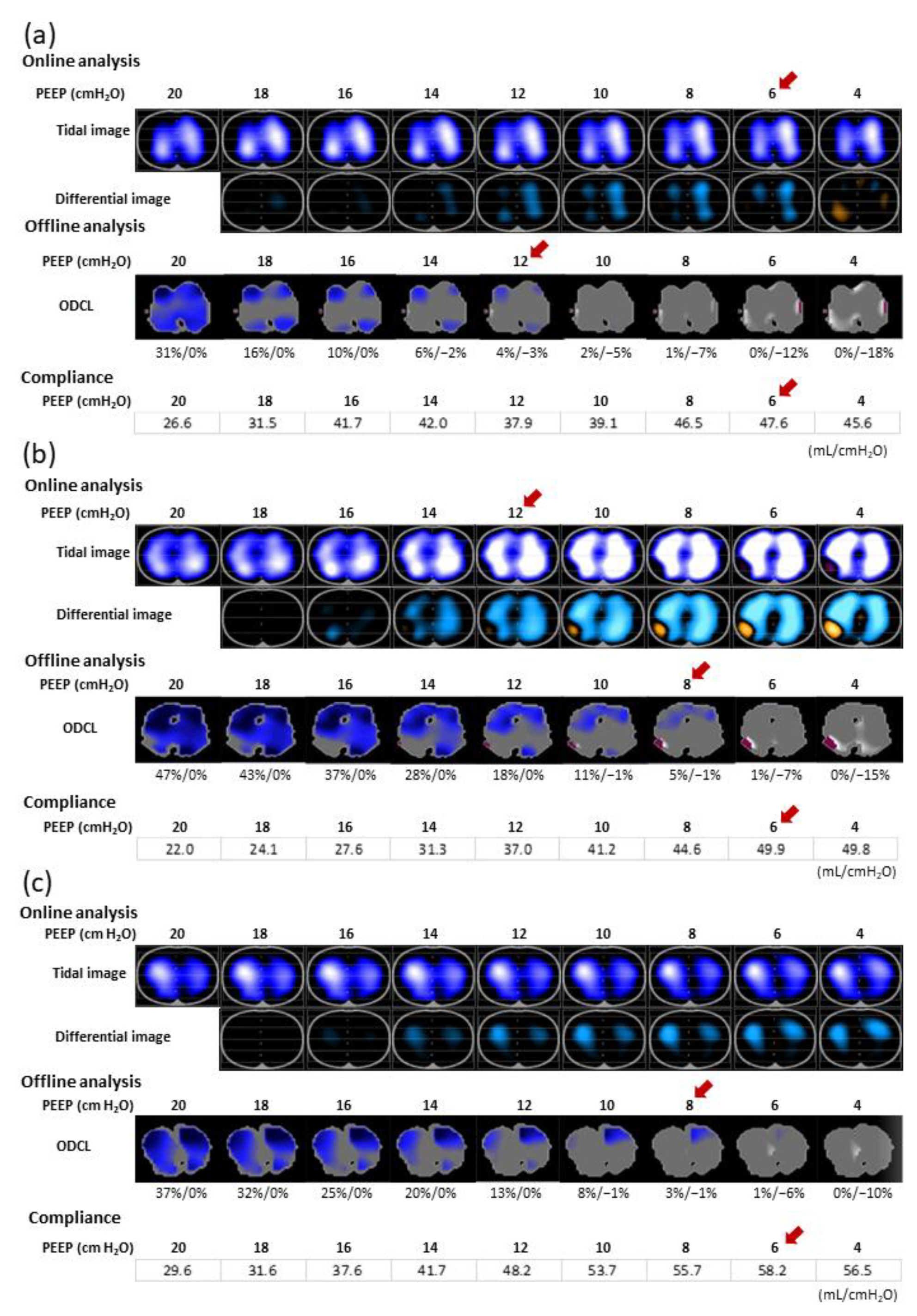
2.4. Offline EIT Data Analysis
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Ventilation Distribution during the PEEP Trial
3.3. Effects of PEEPONLINE
3.4. Comparison between PEEPONLINE and PEEPODCL
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neto, A.S.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Hollmann, M.W.; Jaber, S.; Kozian, A.; Licker, M.; et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis. Lancet Respir. Med. 2014, 2, 1007–1015. [Google Scholar] [CrossRef]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative mechanical ventilation, and postoperative pulmonary complications after cardiac surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Tenling, A.; Hachenberg, T.; Tydén, H.; Wegenius, G.; Hedenstierna, G. Atelectasis and gas exchange after cardiac surgery. Anesthesiology 1998, 89, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachmann, B. Open up the lung and keep the lung open. Intensiv. Care Med. 1992, 18, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Costa Leme, A.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; De Santis Santiago, R.R.; Osawa, E.A.; Pinheiro de Almeida, J.; Gerent, A.M.; Franco, R.A.; Zanetti Feltrim, M.I.; et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: A randomized clinical trial. JAMA 2017, 317, 1422–1432. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Chen, M.; Cheng, L.; Chen, Q.; Jiang, H.; Sun, Z. An open lung strategy in the management of acute respiratory distress syndrome: A systematic review and meta-analysis. Shock 2017, 48, 43–53. [Google Scholar] [CrossRef]
- Goligher, E.C.; Hodgson, C.L.; Adhikari, N.K.J.; Meade, M.O.; Wunsch, H.; Uleryk, E.; Gajic, O.; Amato, M.P.B.; Ferguson, N.D.; Rubenfeld, G.D.; et al. Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann. Am. Thorac. Soc. 2017, 14, S304–S311. [Google Scholar] [CrossRef]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Dudykevych, T.; Quintel, M.; Hellige, G. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J. Appl. Physiol. 2002, 93, 660–666. [Google Scholar] [CrossRef]
- Brabant, O.; Crivellari, B.; Hosgood, G.; Raisis, A.; Waldmann, A.D.; Auer, U.; Adler, A.; Smart, L.; Laurence, M.; Mosing, M. Effects of PEEP on the relationship between tidal volume and total impedance change measured via electrical impedance tomography (EIT). J. Clin. Monit. Comput. 2022, 36, 325–334. [Google Scholar] [CrossRef]
- Blankman, P.; Hasan, D.; Erik, G.; Gommers, D. Detection of “best” positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit. Care 2014, 18, R95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, G.K.; Gómez-Laberge, C.; Rettig, J.S.; Vargas, S.O.; Smallwood, C.D.; Prabhu, S.P.; Vitali, S.H.; Zurakowski, D.; Arnold, J.H. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit. Care Med. 2013, 41, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Amato, M.B.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use, and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sella, N.; Pettenuzzo, T.; Zarantonello, F.; Andreatta, G.; De Cassai, A.; Schiavolin, C.; Simoni, C.; Pasin, L.; Boscolo, A.; Navalesi, P. Electrical impedance tomography: A compass for the safe route to optimal PEEP. Respir. Med. 2021, 187, 106555. [Google Scholar] [CrossRef]
- Eronia, N.; Mauri, T.; Maffezzini, E.; Gatti, S.; Bronco, A.; Alban, L.; Binda, F.; Sasso, T.; Marenghi, C.; Grasselli, G.; et al. Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: A feasibility study. Ann. Intensiv. Care 2017, 7, 76. [Google Scholar] [CrossRef]
- Zhao, Z.; Steinmann, D.; Frerichs, I.; Guttmann, J.; Möller, K. PEEP titration guided by ventilation homogeneity: A feasibility study using electrical impedance tomography. Crit. Care 2010, 14, R8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Chang, M.Y.; Chang, M.Y.; Gow, C.H.; Zhang, J.H.; Hsu, Y.L.; Frerichs, I.; Chang, H.T.; Möller, K. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann. Intensiv. Care 2019, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.M.; Tucci, M.R.; Morais, C.C.A.; Simões, C.M.; Tonelotto, B.F.F.; Pompeo, M.S.; Kay, F.U.; Pelosi, P.; Vieira, J.E.; Amato, M.B.P.; et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 2018, 129, 1070–1081. [Google Scholar] [CrossRef]
- Costa, E.L.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensiv. Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef]
- Mauri, T.; Eronia, N.; Turrini, C.; Battistini, M.; Grasselli, G.; Rona, R.; Volta, C.A.; Bellani, G.; Pesenti, A. Bedside assessment of the effects of positive end-expiratory pressure on lung inflation and recruitment by the helium dilution technique and electrical impedance tomography. Intensiv. Care Med. 2016, 42, 1576–1587. [Google Scholar] [CrossRef]
- Karsten, J.; Grusnick, C.; Paarmann, H.; Heringlake, M.; Heinze, H. Positive end-expiratory pressure titration at bedside using electrical impedance tomography in post-operative cardiac surgery patients. Acta Anaesthesiol. Scand. 2015, 59, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, Z.; Chen, J.; Liu, Y.; Tang, Y.; Han, Q.; Lu, F.; Tang, H.; Xu, Z. Risk factors for noninvasive ventilation failure in patients with post-extubation acute respiratory failure after cardiac surgery. J. Thorac. Dis. 2018, 10, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Bartz, R.R.; Ferreira, R.G.; Schroder, J.N.; Davies, J.; Liu, W.W.; Camara, A.; Welsby, I.J. Prolonged pulmonary support after cardiac surgery: Incidence, risk factors and outcomes: A retrospective cohort study. J. Crit. Care 2015, 30, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Franchineau, G.; Bréchot, N.; Lebreton, G.; Hekimian, G.; Nieszkowska, A.; Trouillet, J.L.; Leprince, P.; Chastre, J.; Luyt, C.E.; Combes, A.; et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.; Luepschen, H.; Karsten, J.; Leibecke, T.; Grossherr, M.; Gehring, H.; Leonhardt, S. Assessment of regional lung recruitment and derecruitment during a PEEP trial based on electrical impedance tomography. Intensiv. Care Med. 2008, 34, 543–550. [Google Scholar] [CrossRef]
- Suarez-Sipmann, F.; Böhm, S.H.; Tusman, G.; Pesch, T.; Thamm, O.; Reissmann, H.; Reske, A.; Magnusson, A.; Hedenstierna, G. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit. Care Med. 2007, 35, 214–221. [Google Scholar] [CrossRef] [Green Version]
- El-Dash, S.A.; Borges, J.B.; Costa, E.L.; Tucci, M.R.; Ranzani, O.T.; Caramez, M.P.; Carvalho, C.R.; Amato, M.B. There is no cephalocaudal gradient of computed tomography densities or lung behavior in supine patients with acute respiratory distress syndrome. Acta Anaesthesiol. Scand. 2016, 60, 767–779. [Google Scholar] [CrossRef]
- Bikker, I.G.; Preis, C.; Egal, M.; Bakker, J.; Gommers, D. Electrical impedance tomography measured at two thoracic levels can visualize the ventilation distribution changes at the bedside during a decremental positive end-expiratory lung pressure trial. Crit. Care 2011, 15, R193. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.M.; Jung, H.; Koh, Y.; Lee, J.S.; Shim, T.S.; Lee, S.D.; Kim, W.S.; Kim, D.S.; Kim, W.D. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit. Care Med. 2003, 31, 411–418. [Google Scholar] [CrossRef]
- Oczenski, W.; Hörmann, C.; Keller, C.; Lorenzl, N.; Kepka, A.; Schwarz, S.; Fitzgerald, R.D. Recruitment maneuvers after a positive end-expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology 2004, 101, 620–625. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Number of patients | 35 |
| Age (years) | 71.3 ± 10.0 |
| Sex (female/male) | 18/17 |
| Height (cm) | 155.2 ± 10.0 |
| Weight (kg) | 58.3 ± 13.2 |
| Body mass index (kg m−2) | 24.1 ± 4.5 |
| Duration of CPB (min) | 95 (65–127) |
| Duration of surgery (min) | 224.3 ± 47.5 |
| Comorbidity | |
| Hypertension | 22 |
| Diabetes | 7 |
| Chronic kidney disease | 10 |
| EuroSCORE II | 2.0 (1.4–3.5) |
| Type of surgery | |
| Aortic valve | 17 |
| Mitral valve | 2 |
| Combination | 13 |
| Others | 3 |
| Parameter | Before PEEP Trial | 5 min after PEEPONLINE Setting | 1 h after PEEPONLINE Setting | p-Value |
|---|---|---|---|---|
| TIV in the dependent region (%) | 41.3 ± 8.5 | 49.1 ± 9.3 * | 48.7 ± 9.4 * | <0.001 |
| ΔEELI (%) | 0.0 | 142.7 ± 109.4 * | 125.5 ± 119.9 * | <0.001 |
| PaO2/FiO2 ratio | 310.3 ± 93.4 | 428.7 ± 119.1 * | 429.9 ± 115.9 * | <0.001 |
| Respiratory compliance (mL cmH2O−1) | 41.3 (32.8–53.0) | 46.8 (39.8–63.8) * | 47.0 (35.8–61.1) *,☨ | <0.001 |
| Parameter | PEEPONLINE ≥ PEEPODCL (n = 19) | PEEPONLINE < PEEPODCL (n = 16) | p-Value |
|---|---|---|---|
| PEEPONLINE (cmH2O) | 10 (9–12) | 7.5 (6–10) | 0.008 |
| PEEPODCL (cmH2O) | 10 (8–10) | 10.5 (8.5–12) # | 0.047 |
| PEEPCOMPLIANCE (cmH2O) | 6 (6–8) # | 8 (6–9.5) | 0.106 |
| TIV in the dependent region (%) | |||
| before PEEP trial | 41.8 ± 9.1 | 40.8 ± 7.8 | 0.753 |
| 5 min after PEEPONLINE setting | 50.9 ± 9.9 * | 47 ± 8.4 * | 0.229 |
| 1 h after PEEPONLINE setting | 50.6 ± 10.1 * | 46.4 ± 8.3 * | 0.189 |
| PaO2/FiO2 ratio | |||
| before PEEP trial | 322.1 ± 86.1 | 296.3 ± 102.5 | 0.424 |
| 5 min after PEEPONLINE setting | 456.4 ± 116.5 * | 395.8 ± 117.2 * | 0.136 |
| 1 h after PEEPONLINE setting | 456.5 ± 111.4 * | 398.5 ± 110.4 * | 0.143 |
| Respiratory compliance (mL cmH2O−1) | |||
| before PEEP trial | 41.3 (35.9–55.4) | 42.5 (31.4–51.1) | 0.417 |
| 5 min after PEEPONLINE setting | 52.6 (40.6–78.1) * | 44.7 (39–59.4) * | 0.267 |
| 1 h after PEEPONLINE setting | 48.1 (37.3–71.3) * | 42.5 (34.3–58.9) | 0.202 |
| ΔEELV after PEEPONLINE setting (mL) | −27.2 ± 87.7 | −122.8 ± 98.6 | 0.005 |
| Lowest PaO2/FiO2 ratio within 12 h after extubation (mmHg) | 214.5 ± 62.7 | 165.0 ± 76.1 | 0.034 |
| Patients who required rescue oxygen therapy (n) | 1 | 6 | 0.018 |
| Patients with pneumonia (n) | 1 | 1 | 0.900 |
| Characteristic | PEEPONLINE ≥ PEEPODCL (n = 19) | PEEPONLINE < PEEPODCL (n = 16) | p-Value |
|---|---|---|---|
| Age (years) | 71 ± 8.7 | 71.6 ± 11.7 | 0.872 |
| Sex (female/male) | 9/10 | 8/8 | 0.877 |
| Height (cm) | 155.9 ± 9.0 | 154.3 ± 11.4 | 0.632 |
| Weight (kg) | 58.5 ± 10.4 | 58.0 ± 16.3 | 0.908 |
| BMI (kg m−2) | 24.1 ± 4.2 | 24.1 ± 5.0 | 0.989 |
| EuroSCORE II | 1.9 (1.0–3.8) | 2.4 (1.7–3.5) | 0.275 |
| Type of surgery | 0.302 | ||
| Aortic valve | 11 | 6 | |
| Mitral valve | 0 | 2 | |
| Combination | 6 | 7 | |
| Others | 2 | 1 | |
| Duration of CPB (min) | 92.5 ± 43.7 | 110.6 ± 31.5 | 0.178 |
| Duration of surgery (min) | 211.6 ± 43.6 | 239.3 ± 48.8 | 0.085 |
| Time of extubation after ICU admission (h) | 16.3 (8.7–19.2) | 16.3 (9.1–19.7) | 0.778 |
| Patients who underwent blood transfusion (n) | 10 | 11 | 0.332 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bito, K.; Shono, A.; Kimura, S.; Maruta, K.; Omoto, T.; Aoki, A.; Oe, K.; Kotani, T. Clinical Implications of Determining Individualized Positive End-Expiratory Pressure Using Electrical Impedance Tomography in Post-Cardiac Surgery Patients: A Prospective, Non-Randomized Interventional Study. J. Clin. Med. 2022, 11, 3022. https://doi.org/10.3390/jcm11113022
Bito K, Shono A, Kimura S, Maruta K, Omoto T, Aoki A, Oe K, Kotani T. Clinical Implications of Determining Individualized Positive End-Expiratory Pressure Using Electrical Impedance Tomography in Post-Cardiac Surgery Patients: A Prospective, Non-Randomized Interventional Study. Journal of Clinical Medicine. 2022; 11(11):3022. https://doi.org/10.3390/jcm11113022
Chicago/Turabian StyleBito, Kiyoko, Atsuko Shono, Shinya Kimura, Kazuto Maruta, Tadashi Omoto, Atsushi Aoki, Katsunori Oe, and Toru Kotani. 2022. "Clinical Implications of Determining Individualized Positive End-Expiratory Pressure Using Electrical Impedance Tomography in Post-Cardiac Surgery Patients: A Prospective, Non-Randomized Interventional Study" Journal of Clinical Medicine 11, no. 11: 3022. https://doi.org/10.3390/jcm11113022
APA StyleBito, K., Shono, A., Kimura, S., Maruta, K., Omoto, T., Aoki, A., Oe, K., & Kotani, T. (2022). Clinical Implications of Determining Individualized Positive End-Expiratory Pressure Using Electrical Impedance Tomography in Post-Cardiac Surgery Patients: A Prospective, Non-Randomized Interventional Study. Journal of Clinical Medicine, 11(11), 3022. https://doi.org/10.3390/jcm11113022







