Double-Blind, Randomized, Placebo-Controlled Study on hzVSF-v13, a Novel Anti-Vimentin Monoclonal Antibody Drug as Add-on Standard of Care in the Management of Patients with Moderate to Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
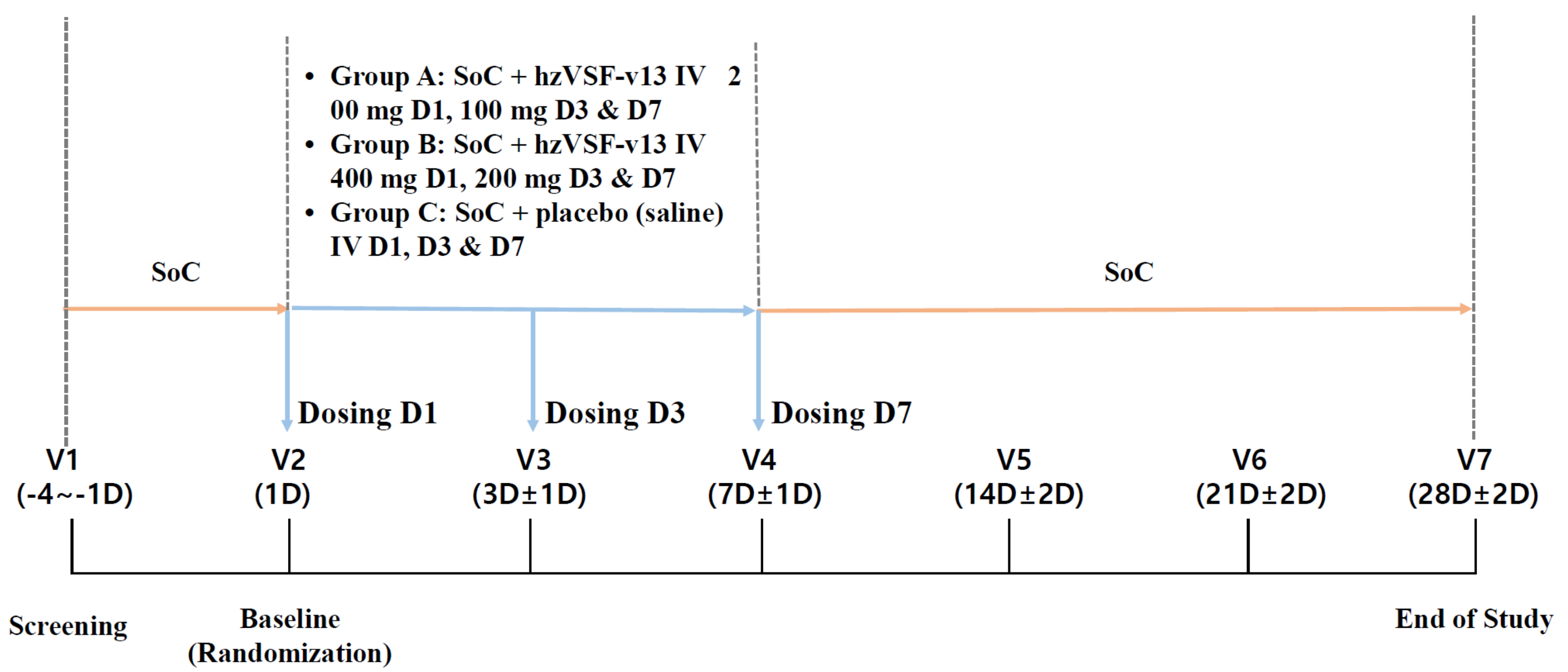
2.1. Study Design
2.2. Patients
2.3. Randomization and Masking
2.4. Interventions
- Group A: (low-dose hzVSF-v13): Standard of care (SoC) + loading dose of hzVSF-v13 200 mg at Day 1 (D1), maintenance dose of hzVSF-v13 100 mg at D3 and D7.
- Group B: (high-dose hzVSF-v13): SoC + loading dose of hzVSF-v13 400 mg at D1, maintenance dose of hzVSF-v13 200 mg at D3 and D7
- Group C: (placebo): SoC + 3 doses of the placebo (normal saline) at D1, D3 and D7.
2.5. Outcomes
2.6. Sample Size
2.7. Statistical Analysis
3. Results
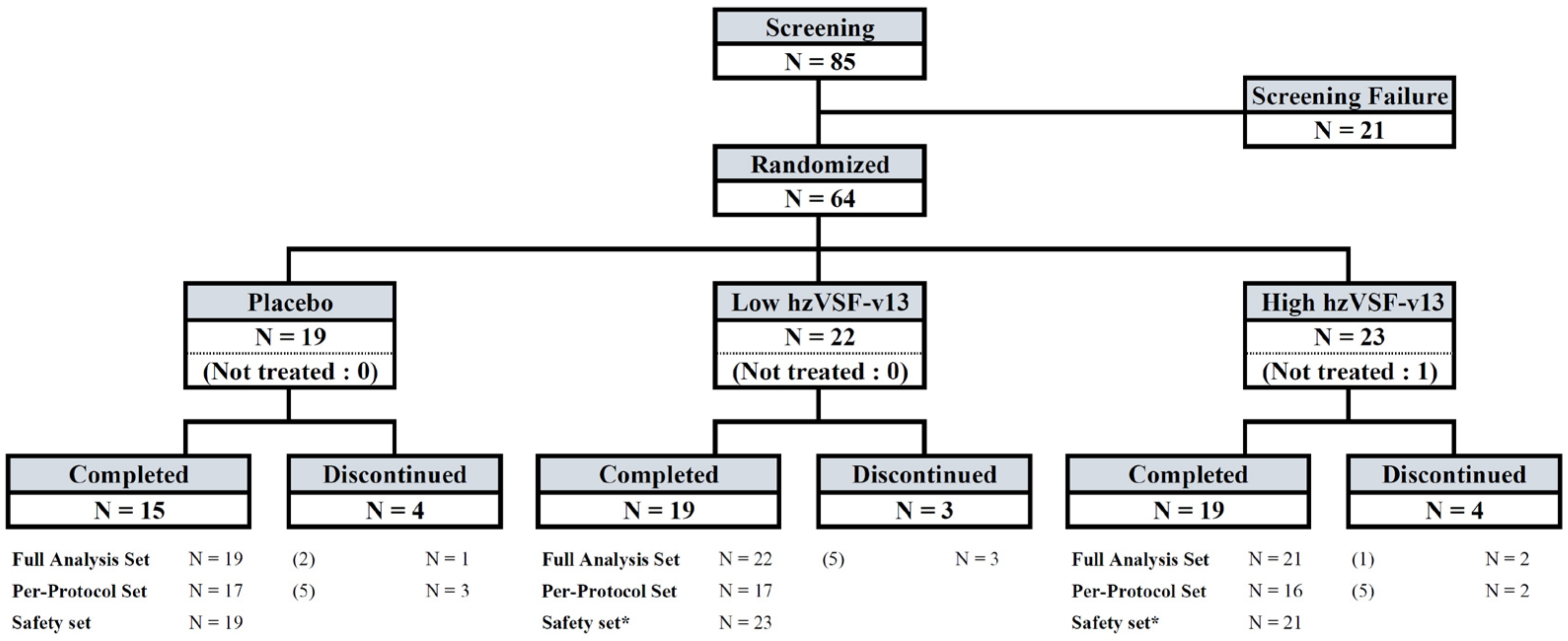
3.1. Patient Enrollment and Demographics
3.2. Primary Endpoint
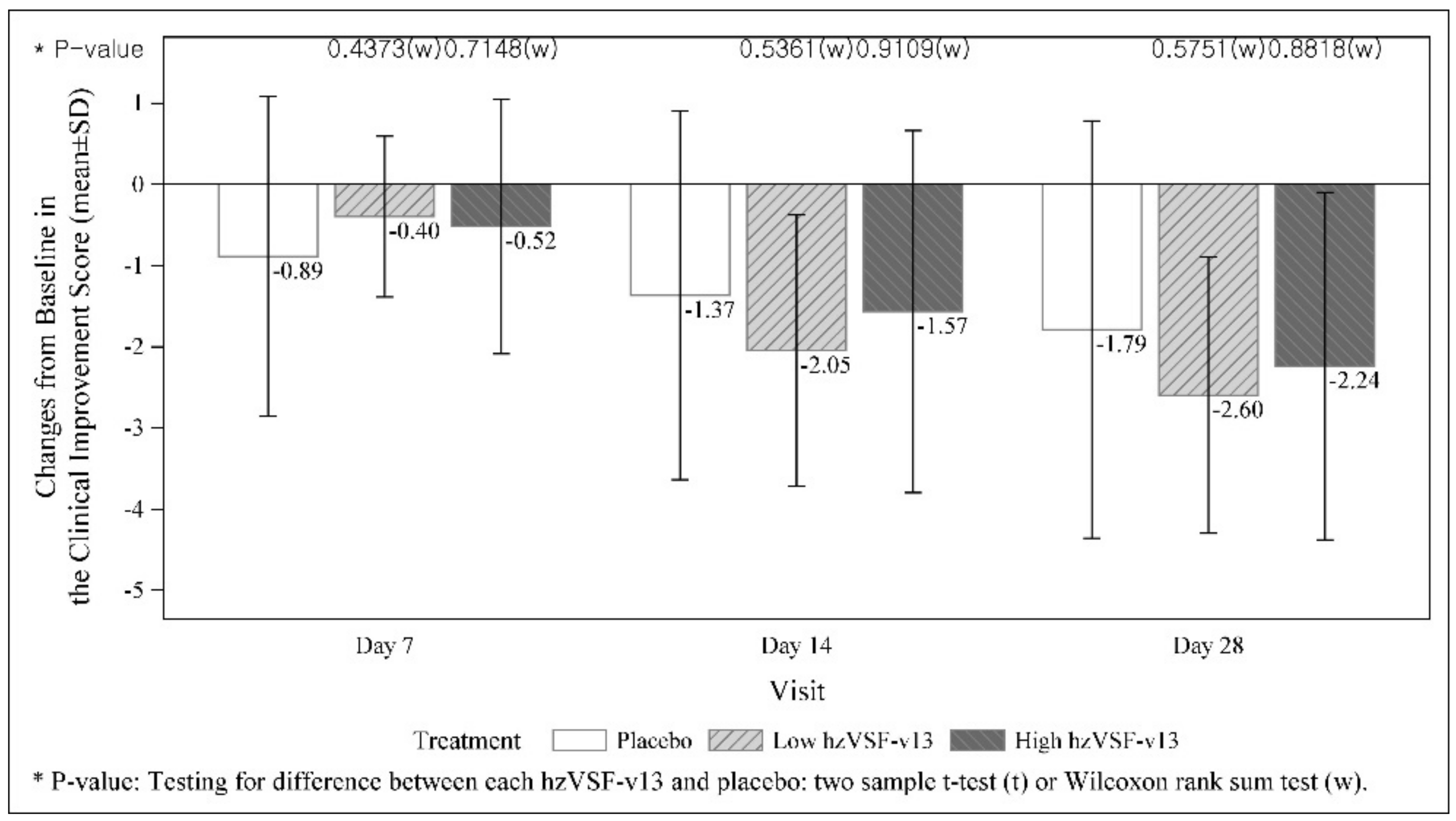
3.3. Secondary Efficacy Endpoints
3.4. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Panté, N. Vimentin plays a role in the release of the influenza A viral genome from endosomes. Virology 2016, 497, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.T.; Chien, S.C.; Chen, I.Y.; Lai, C.T.; Tsay, Y.G.; Chang, S.C.; Chang, M.F. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2021, 13, 4675. [Google Scholar] [CrossRef] [PubMed]
- Patteson, A.E.; Vahabikashi, A.; Goldman, R.D.; Janmey, P.A. Mechanical and Non-Mechanical Functions of Filamentous and Non-Filamentous Vimentin. Bioessays 2020, 42, e2000078. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Paulin, D.; Lacolley, P.; Coletti, D.; Agbulut, O. Vimentin as a target for the treatment of COVID-19. BMJ Open Respir. Res. 2020, 7, e000623. [Google Scholar] [CrossRef] [PubMed]
- Thalla, D.G.; Jung, P.; Bischoff, M.; Lautenschläger, F. Role of Extracellular Vimentin in Cancer-Cell Functionality and Its Influence on Cell Monolayer Permeability Changes Induced by SARS-CoV-2 Receptor Binding Domain. Int. J. Mol. Sci. 2021, 22, 7469. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, J.; Zhou, J.; Yuan, B.; Chen, J.; Wu, W.; Mo, L.; Qu, Z.; Zhou, F.; Dong, Y.; et al. A Vimentin-Targeting Oral Compound with Host-Directed Antiviral and Anti-Inflammatory Actions Addresses Multiple Features of COVID-19 and Related Diseases. mBio 2021, 12, e0254221. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Choe, P.G.; Park, S.; Kim, T.S.; Seong, M.W.; Kim, N.J.; Oh, M.D.; Park, W.B.; Kim, Y.W. Compassionate use of hzVSF-v13 in two patients with severe COVID-19. J. Med. Virol. 2020, 92, 2371–2373. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, Y.; Otsuka, M.; Sekiba, K.; Funato, K.; Koike, K. Humanized virus-suppressing factor inhibits hepatitis B virus infection by targeting viral cell entry. Heliyon 2021, 7, e07586. [Google Scholar] [CrossRef] [PubMed]
- Korolowicz, K.E.; Suresh, M.; Li, B.; Huang, X.; Yon, C.; Kallakury, B.V.; Lee, K.P.; Park, S.; Kim, Y.W.; Menne, S. Combination Treatment with the Vimentin-Targeting Antibody hzVSF and Tenofovir Suppresses Woodchuck Hepatitis Virus Infection in Woodchucks. Cells 2021, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.W. ImmuneMed. Seven cases of compassionate use of hzVSF-v13 in COVID-19 in Korea. Personal communication, 2021.
- Pedoman Tatalaksana COVID-19 Edisi 3, Tahun 2020 (in Indonesian). Available online: https://www.papdi.or.id/pdfs/983/Buku%20Pedoman%20Tatalaksana%20COVID-19%205OP%20Edisi%203%202020.pdf (accessed on 14 April 2022).
- FDA. COVID-19: Developing Drugs and Biological Products for Treatment or Prevention—Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- WHO R & D Blueprint. Coronavirus COVID-19 Therapeutic Trial Synopsis; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Fusina, F.; Albani, F.; Granato, E.; Meloni, A.; Rozzini, R.; Sabatini, T.; Stellini, R.; Terragnoli, P.; Rosano, A.; Abu Hilal, M.; et al. Effect of Corticosteroids on Mortality in Hospitalized COVID-19 Patients Not Receiving Invasive Mechanical Ventilation. Clin. Pharmacol. Ther. 2021, 109, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- NIH. COVID Treatment Guidelines: Anti-SARS-CoV-2 Monoclonal Antibodies; National Institutes of Health: Bethesda, MD, USA, 2022. [Google Scholar]

| Characteristic | hzVSF-v13 Low Dose (N = 22) | hzVSF-v13 High Dose (N = 21) | Placebo (N = 19) | |
|---|---|---|---|---|
| Age (years) | Mean [min~max] | 50.5 [32~70] | 50.5 [34~73] | 51.5 [28~71] |
| Age categories n (%) | <50 | 9 (40.9) | 9 (42.9) | 9 (47.4) |
| 50≤~<55 | 7 (31.8) | 7 (33.3) | 2 (10.5) | |
| 55≤~<60 | 2 (9.1) | 2 (9.5) | 4 (21.1) | |
| ≥60 | 4 (18.2) | 3 (14.3) | 4 (21.1) | |
| Gender n (%) | Male | 14 (63.6) | 11 (52.4) | 13 (68.4) |
| Female | 8 (36.4) | 10 (47.6) | 6 (31.6) | |
| Body mass index (BMI) (kg/m2) | Mean [min~max] | 27.5 [18.7, 36.7] | 26.5 [20.2, 37.1] | 26.0 [19.8, 37.5] |
| BMI categories n (%) | 18.5~24.9 | 6 (27.3) | 9 (42.9) | 9 (47.4) |
| 25.0~29.9 | 10 (45.5) | 8 (38.1) | 6 (31.6) | |
| ≥30.0 | 6 (27.3) | 4 (19.0) | 4 (21.1) | |
| N patients | Admission at enrollment | 22 (100) | 21 (100) | 19 (100) |
| COVID-19 severity n (%) | Moderate | 11 (50.0) | 12 (57.1) | 10 (52.6) |
| Severe | 11 (50.0) | 9 (42.9) | 9 (47.4) |
| Group | hzVSF-v13 Low Dose (N = 22) | hzVSF-v13 High Dose (N = 21) | Placebo (N = 19) |
|---|---|---|---|
| Patients with clinical failure at Day 28 n (%) | 2 (9.1) | 2 (9.5) | 3 (15.8) |
| Crude Odds Ratios (ORs) | 0.53 | 0.56 | |
| 85% Confidence Interval (CI) | [0.13, 2.16] | [0.14, 2.28] | |
| p-value | 0.5180 | 0.5533 | |
| Death n (%) | 2 (9.1) | 2 (9.5) | 3 (15.8) |
| Crude ORs | 0.53 | 0.56 | |
| 85% CI | [0.13, 2.16] | [0.14, 2.28] | |
| p-value | 0.5180 | 0.5533 | |
| Respiratory failure n (%) | 0 | 2 (9.5) | 3 (15.8) |
| Crude ORs | 0 | 0.56 | |
| 85% CI | [0.14, 2.28] | ||
| p-value | 0.9515 | 0.5533 | |
| Subjects with ICU Admission, n (%) | 0 | 2 (9.5) | 3 (15.8) |
| Crude ORs | 0 | 0.56 | |
| 85% CI | [0.14, 2.28] | ||
| p-value | 0.9515 | 0.5533 |
| Group | hzVSF-v13 Low Dose (N = 11) | hzVSF-v13 High Dose (N = 9) | Placebo (N = 9) |
|---|---|---|---|
| Patients with clinical failure at Day 28 n (%) | 1 (9.1) | 1 (11.1) | 3 (33.3) |
| Crude ORs | 0.20 | 0.25 | |
| 85% CI | [0.03, 1.24] | [0.04, 1.57] | |
| p-value | 0.2033 | 0.2768 |
| hzVSF-v13 Low Dose | hzVSF-v13 High Dose | Placebo | |
|---|---|---|---|
| Baseline | |||
| n | 22 | 21 | 19 |
| Mean (SD) | 4.1 (0.7) | 4.0 (0.6) | 4.1 (0.6) |
| Day 14 | |||
| n | 20 | 21 | 19 |
| Mean (SD) | 2.0 (1.9) | 2.4 (2.4) | 2.7 (2.7) |
| Change from Baseline at Day 14 | |||
| n | 20 | 21 | 19 |
| Mean (SD) | −2.1 (1.7) | −1.6 (2.2) | −1.4 (2.3) |
| p-value (Placebo vs. each hzVSF-v13) | 0.5361 | 0.9109 | |
| p-value (Low hzVSF-v13 vs. High hzVSF-v13) | 0.5936 | ||
| Improved, n (%) | 18 (90.00) | 15 (71.4) | 10 (52.6) |
| Not Improved, n (%) | 2 (10.00) | 6 (28.6) | 9 (47.4) |
| Logistic Regression with Covariate at Day 14 | |||
| Adjusted ORs | 11.53 | 2.48 | |
| 85% CI | [2.86, 46.49] | [0.84, 7.32] | |
| p-value | 0.0116 | 0.2266 | |
| Day 28 | |||
| n | 20 | 21 | 19 |
| Mean (SD) | 1.4 (1.9) | 1.8 (2.3) | 2.3 (3.0) |
| Change from Baseline at Day 28 | |||
| n | 20 | 21 | 19 |
| Mean (SD) | −2.6 (1.7) | −2.2 (2.1) | −1.8 (2.6) |
| p-value (placebo vs. each hzVSF-v13) | 0.5751 | 0.8818 | |
| p-value (low-dose hzVSF-v13 vs. high-dose hzVSF-v13) | 0.5036 | ||
| Improved, n (%) | 18 (90.0) | 18 (85.7) | 13 (68.4) |
| Not Improved, n (%) | 2 (10.0) | 3 (14.3) | 6 (31.6) |
| Logistic Regression with Covariate at Day 28 | |||
| Adjusted ORs | 4.39 | 2.78 | |
| 85% CI | [1.19, 16.21] | [0.86, 9.02] | |
| p-value | 0.1032 | 0.2108 | |
| hzVSF-v13 Low Dose | hzVSF-v13 High Dose | Placebo | |
|---|---|---|---|
| Subject with Oxygen Therapy | 11 | 9 | 9 |
| Subject who discontinued the Oxygen Therapy, n (%) | 10(90.9) | 7(77.8) | 5(55.6) |
| Time to Discontinuation of Oxygen Therapy (Days) | |||
| Median | 9 | 14 | 14 |
| 95% CI | [7.0, 10.0] | [2.0, 27.0] | [3.0, NC] |
| p-value | 0.0308 | 0.6785 | |
| Hazard Ratio | 3.33 | 1.19 | |
| 85% CI | [1.42, 7.83] | [0.50, 2.84] |
| hzVSF-v13 Low Dose | hzVSF-v13 High Dose | Placebo | |
|---|---|---|---|
| Subjects who Recovered, n (%) | 9 (81.8) | 6 (66.7) | 5 (55.6) |
| Time to Recovery (Days) | |||
| Median | 10.0 | 15.0 | 15.0 |
| 95% CI | [8.0, 11.0] | [3.0, NC] | [4.0, NC] |
| p-value | 0.0446 | 0.5550 | |
| Hazard Ratio | 3.14 | 1.33 | |
| 85% CI | [1.32, 7.51] | [0.56, 3.19] | |
| hzVSF-v13 Low Dose | hzVSF-v13 High Dose | Placebo | |
|---|---|---|---|
| Subjects with Clinical improvement n (%) | 9(81.8) | 6(66.7) | 5(55.6) |
| Time to Clinical Improvement (Days) | |||
| Median | 10 | 15 | 15 |
| 95% CI | [9.0, 16.0] | [8.0, NC] | [4.0, 22.0] |
| p-value | 0.0446 | 0.7514 | |
| Hazard Ratio | 3.18 | 1.25 | |
| 85% CI | [1.31, 7.69] | [0.52, 3.01] | |
| hzVSF-v13 Low Dose | hzVSF-v13 High Dose | Placebo | |
|---|---|---|---|
| Baseline | |||
| n | 11 | 9 | 9 |
| Mean (SD) | 4.6 (2.5) | 5.4 (0.7) | 5.1 (1.3) |
| Day 14 | |||
| n | 10 | 9 | 7 |
| Mean (SD) | 1.6 (1.5) | 2.9 (2.3) | 6.0 (4.9) |
| Change from Baseline at Day 14 | |||
| n | 10 | 9 | 7 |
| Mean (SD) | −2.9 (2.8) | −2.6 (2.7) | 1.0 (4.3) |
| p-value (Placebo vs. each hzVSF-v13) | 0.0377 (t) | 0.0612 (t) | |
| p-value (Low hzVSF-v13 vs. High hzVSF-v13) | 0.7889 (t) | ||
| ANCOVA Result at Day 14 (Placebo vs. each hzVSF-v13) | |||
| LS Mean Difference (SE) | −4.2 (1.7) | −3.7 (1.9) | |
| 95% CI for Difference | [−7.8, −0.7] | [−7.7, 0.3] | |
| p-value for Difference | 0.0235 | 0.0677 | |
| Day 21 | |||
| n | 10 | 9 | 7 |
| Mean (SD) | 1.6 (1.6) | 2.7 (2.5) | 5.4 (5.4) |
| Change from Baseline at Day 21 | |||
| n | 10 | 9 | 7 |
| Mean (SD) | −2.9 (2.9) | −2.8 (2.8) | 0.4 (4.9) |
| p-value (Placebo vs. each hzVSF-v13) | 0.0986 (t) | 0.1205 (t) | |
| p-value (Low hzVSF-v13 vs. High hzVSF-v13) | 0.9273 (t) | ||
| ANCOVA Result at Day 21 (Placebo vs. each hzVSF-v13) | |||
| LS Mean Difference (SE) | −3.7 (1.8) | −3.3 (2.1) | |
| 95% CI for Difference | [−7.6, 0.2] | [−7.7, 1.2] | |
| p-value for Difference | 0.0639 | 0.1416 | |
| Day 28/End of Treatment | |||
| n | 10 | 9 | 8 |
| Mean (SD) | 1.1 (1.1) | 2.7 (1.9) | 5.3 (4.5) |
| Change from Baseline at Day 28/End of Treatment | |||
| n | 10 | 9 | 8 |
| Mean (SD) | −3.4 (3.3) | −2.8 (2.3) | 0.4 (4.0) |
| p-value (Placebo vs. each hzVSF-v13) | 0.0441 (t) | 0.0621 (t) | |
| p-value (Low hzVSF-v13 vs. High hzVSF-v13) | 0.6429 (t) | ||
| ANCOVA Result at Day 28 (Placebo vs. each hzVSF-v13) | |||
| LS Mean Difference (SE) | −4.1 (1.5) | −3.1 (1.7) | |
| 95% CI for Difference | [−7.3, −0.9] | [−6.8, 0.5] | |
| p-value for Difference | 0.0153 | 0.0871 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasenohadi, P.; Burhan, E.; Dhunny, S.; Suharno, W.; Wabnitz, P.; Kim, Y.-W.; Petrosillo, N. Double-Blind, Randomized, Placebo-Controlled Study on hzVSF-v13, a Novel Anti-Vimentin Monoclonal Antibody Drug as Add-on Standard of Care in the Management of Patients with Moderate to Severe COVID-19. J. Clin. Med. 2022, 11, 2961. https://doi.org/10.3390/jcm11112961
Prasenohadi P, Burhan E, Dhunny S, Suharno W, Wabnitz P, Kim Y-W, Petrosillo N. Double-Blind, Randomized, Placebo-Controlled Study on hzVSF-v13, a Novel Anti-Vimentin Monoclonal Antibody Drug as Add-on Standard of Care in the Management of Patients with Moderate to Severe COVID-19. Journal of Clinical Medicine. 2022; 11(11):2961. https://doi.org/10.3390/jcm11112961
Chicago/Turabian StylePrasenohadi, Prasenohadi, Erlina Burhan, Sri Dhunny, Wahyuningsih Suharno, Paul Wabnitz, Yoon-Won Kim, and Nicola Petrosillo. 2022. "Double-Blind, Randomized, Placebo-Controlled Study on hzVSF-v13, a Novel Anti-Vimentin Monoclonal Antibody Drug as Add-on Standard of Care in the Management of Patients with Moderate to Severe COVID-19" Journal of Clinical Medicine 11, no. 11: 2961. https://doi.org/10.3390/jcm11112961
APA StylePrasenohadi, P., Burhan, E., Dhunny, S., Suharno, W., Wabnitz, P., Kim, Y.-W., & Petrosillo, N. (2022). Double-Blind, Randomized, Placebo-Controlled Study on hzVSF-v13, a Novel Anti-Vimentin Monoclonal Antibody Drug as Add-on Standard of Care in the Management of Patients with Moderate to Severe COVID-19. Journal of Clinical Medicine, 11(11), 2961. https://doi.org/10.3390/jcm11112961







