Dapagliflozin Impact on the Exercise Capacity of Non-Diabetic Heart Failure with Reduced Ejection Fraction Patients
Abstract
:1. Background
2. Methods
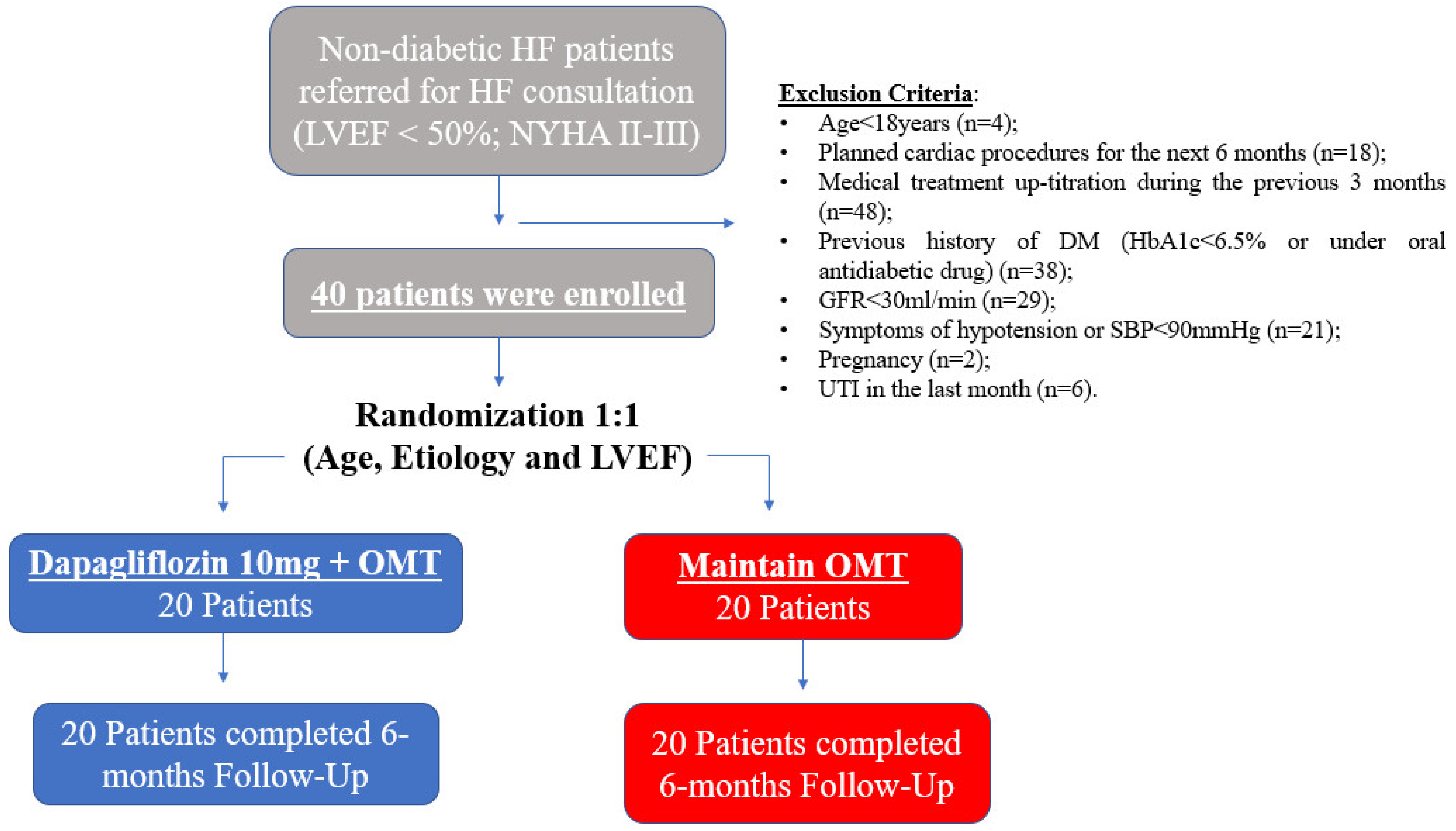
2.1. Patient Population and Study Design
2.2. Baseline and Follow-Up Evaluation
- Anthropometric and physical examination data: age, gender, weight, height, body mass index, heart rate and blood pressure.
- Clinical data: etiology of HF, assessment of control of the patient’s comorbidities and record of the patient’s medication; NYHA functional class and risk assessment through risk scores validated in HF (Heart Failure Survival Score, Seattle Heart Failure Model and Heart Failure Risk Calculator).
- Functional capacity data: peak oxygen consumption (pVO2), ventilation/CO2 production slope (VE/VCO2 slope), time to anaerobic threshold and heart rate recovery after the first minute of effort.
- Echocardiography parameters: left ventricular dimension, left ventricular ejection fraction, left ventricular strain, right ventricular function, RV–AD gradient, valvular function, and other summary examination parameters.
- Blood tests, including hemoglobin, creatinine, high-sensitivity troponin I and NTproBNP levels, glycated hemoglobin and lipid profile.
- Record of concomitant therapy with a potential impact on the evolution of HF, such as the use of intravenous iron therapy, implantation of CIED, changes of HF, surgical or percutaneous valve procedures, coronary procedures, and arrhythmia ablation procedures.
- The occurrence of major cardiovascular events was also recorded, including death (all causes, cardiovascular and due to HF), hospitalizations (from cardiovascular causes and due to HF) and need for advanced HF therapy (cardiac transplantation and left ventricular assist device implantation).
Cardiopulmonary Exercise Testing
2.3. Follow-Up and Endpoint
2.4. Ethics
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPET | Cardiopulmonary exercise test |
| HF | Heart failure |
| HFrEF | Heart failure with reduced ejection fraction |
| LV | Left ventricle |
| LVEF | Left-ventricular ejection fraction |
| MACE | Major adverse cardiovascular events |
| NT-proBNP | N-terminal pro-hormone BNP |
| pVO2 | Peak oxygen uptake |
| SGLT2i | Sodium–glucose co-transporter 2 inhibitors |
References
- Seferović, P.M.; Coats, A.J.S.; Ponikowski, P.; Filippatos, G.; Huelsmann, M.; Jhund, P.S.; Polovina, M.M.; Komajda, M.; Seferović, J.; Sari, I.; et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur. J. Heart Fail. 2019, 22, 196–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Cardoso, R.; Graffunder, F.P.; Ternes, C.M.P.; Fernandes, A.; Rocha, A.V.; Fernandes, G.; Bhatt, D.L. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100933. [Google Scholar] [CrossRef]
- ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar]
- Tromp, J.; Ouwerkerk, W.; van Veldhuisen, D.J.; Hillege, H.L.; Richards, A.M.; van der Meer, P.; Anand, I.S.; Lam, C.S.P.; Voors, A.A. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2022, 10, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.M.; Gandy, S.; McCrimmon, R.; Houston, J.G.; Struthers, A.D.; Lang, C.C. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: The DAPA-LVH trial. Eur. Heart J. 2020, 41, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. EMPA-TROPISM (ATRU-4) Investigators. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef]
- Tanaka, H.; Soga, F.; Tatsumi, K.; Mochizuki, Y.; Sano, H.; Toki, H.; Matsumoto, K.; Shite, J.; Takaoka, H.; Doi, T.; et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc. Diabetol. 2020, 19, 6. [Google Scholar] [CrossRef]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Singh, J.S.S.; Mordi, I.R.; Vickneson, K.; Fathi, A.; Donnan, P.T.; Mohan, M.; Choy, A.M.J.; Gandy, S.; George, J.; Khan, F.; et al. Dapagliflozin Versus Placebo on Left Ventricular Remodeling in Patients With Diabetes and Heart Failure: The REFORM Trial. Diabetes Care 2020, 43, 1356–1359. [Google Scholar] [CrossRef] [Green Version]
- Petrie, M.C.; Lee, M.M.; Lang, N.N. EMPEROR-REDUCED reigns while EMPERIAL whimpers. Eur. Heart J. 2021, 42, 711–714. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Kane, S.P. ClincCalc Sample Size Calculator. 2022. Available online: https://clincalc.com/stats/samplesize.aspx# (accessed on 1 November 2019).
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef]
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail 2011, 4, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Shim, C.Y.; Seo, J.; Cho, I.; Lee, C.J.; Cho, I.J.; Lhagvasuren, P.; Kang, S.M.; Ha, J.W.; Han, G.; Jang, Y.; et al. Randomized, Controlled Trial to Evaluate the Effect of Dapagliflozin on Left Ventricular Diastolic Function in Patients With Type 2 Diabetes Mellitus: The IDDIA Trial. Circulation 2021, 143, 510–512. [Google Scholar] [CrossRef]

| Total Population (n = 40) | Dapagliflozin (n = 20) | Control (n = 20) | p Value | |
|---|---|---|---|---|
| Age (years) | 60.9 ± 13.0 | 60.3 ± 11.6 | 61.7 ± 14.8 | 0.740 |
| Male gender | 33 (82.5%) | 17 (85.0%) | 16 (80.0%) | 0.787 |
| Ischemic etiology | 28 (78.0%) | 16 (80.0%) | 12 (60.0%) | 0.369 |
| Previous MI | 28 (78.0%) | 16 (80.0%) | 12 (60.0%) | 0.369 |
| Previous PCI | 28 (78.0%) | 16 (80.0%) | 12 (60.0%) | 0.369 |
| Previous CABG | 3 (7.5%) | 3 (15.0%) | 0 (0%) | 0.087 |
| Previous valvular heart surgery | 5 (12.5%) | 3 (15.0%) | 2 (10.0%) | 0.720 |
| HF hospitalizations in the previous 12 months | 11 (27.5%) | 8 (40.0%) | 3 (15.0%) | 0.115 |
| Heart failure medication | ||||
| beta-blocker | 39 (97.5%) | 20 (100%) | 19 (95.0%) | 0.675 |
| ACE-i/ARB/ARNI | 40 (100%) | 20 (100%) | 20 (100%) | 1.000 |
| MRA | 36 (90.0%) | 19 (95.0%) | 17 (85.0%) | 0.510 |
| Ivabradine | 7 (17.5%) | 3 (15.0%) | 4 (20.0%) | 0.675 |
| Digitalis | 2 (5.0%) | 0 (0%) | 2 (10.0%) | 0.227 |
| DAPT | 6 (15.0%) | 2 (10.0%) | 4 (20.0%) | 0.308 |
| Oral anticoagulation | 19 (47.5%) | 8 (40.0%) | 11 (55.0%) | 0.210 |
| Furosemide dose (mg) | 30.5 ± 31.0 | 25.7 ± 29.8 | 33.7 ± 32.6 | 0.421 |
| Ferric carboxymaltose | 17 (42.5%) | 13 (65.0%) | 4 (20.0%) | 0.012 |
| Cardiac rehabilitation | 21 (52.5%) | 12 (60.0%) | 9 (45.0%) | 0.563 |
| Hypertension | 25 (62.5%) | 14 (70.0%) | 11 (55.0%) | 0.567 |
| Dyslipidemia | 26 (65.0%) | 16 (80.0%) | 10 (50.0%) | 0.119 |
| Current or former smoker | 28 (70.0%) | 17 (85.0%) | 11 (55.0%) | 0.334 |
| Atrial fibrillation | 12 (30.0%) | 6 (30.0%) | 6 (30.0%) | 1.000 |
| ICD | 25 (62.5%) | 14 (70.0%) | 11 (55.0%) | 0.567 |
| CRT device | 6 (15.0%) | 3 (15.0%) | 3 (15.0%) | 1.000 |
| Chronic kidney disease | 8 (20.0%) | 5 (25.0%) | 3 (15.0%) | 0.698 |
| Peripheral artery disease | 9 (22.5%) | 4 (20.0%) | 5 (25.0%) | 0.712 |
| COPD | 11 (27.5%) | 5 (25.0%) | 6 (30.0%) | 0.583 |
| NYHA Class III–IV | 8 (20.0%) | 6 (30.0%) | 2 (10.0%) | 0.241 |
| HFSS | 8.6 ± 0.7 | 8.7 ± 0.6 | 8.6 ± 0.7 | 0.871 |
| SHFM | 88.9 ± 5.9 | 89.5 ± 6.7 | 88.3 ± 4.9 | 0.517 |
| MAGGIC score | 17.9 ± 5.4 | 18.1 ± 5.4 | 17.8 ± 5.6 | 0.860 |
| Hemoglobin (g/dL) | 13.9 ± 1.4 | 14.0 ± 1.6 | 13.8 ± 1.2 | 0.707 |
| Creatinine (mg/dL) | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.495 |
| GFR (mL/min) | 70.4 ± 20.6 | 68.7 ± 23.8 | 72.5 ± 17.1 | 0.596 |
| hs-cTnI (ng/mL) | 15.2 ± 31.6 | 9.2 ± 6.7 | 21.9 ± 45.1 | 0.041 |
| NT-proBNP (pg/mL) | 781.0 (350.7–1599.1) | 890.5 (426.5–1652.0) | 747.4 (287.7–1490.2) | 0.881 |
| HbA1c (%) | 5.8 ± 0.4 | 5.8 ± 0.4 | 5.7 ± 0.5 | 0.352 |
| LDL (mg/dL) | 90.3 ± 35.0 | 85.2 ± 33.2 | 95.8 ± 36.9 | 0.150 |
| LVEDD (mm) | 66.1 ± 7.2 | 65.1 ± 6.0 | 67.2 ± 8.4 | 0.373 |
| LVESD (mm) | 48.3 ± 11.9 | 49.4 ± 9.6 | 46.9 ± 14.3 | 0.522 |
| LVEF (%) | 34.1 ± 8.3 | 34.5 ± 8.9 | 33.5 ± 7.8 | 0.708 |
| GLS (%) | 8.2 ± 3.0 | 8.2 ± 3.2 | 8.4 ± 2.8 | 0.866 |
| TAPSE (mm) | 18.8 ± 4.9 | 19.0 ± 5.7 | 18.6 ± 4.2 | 0.831 |
| LA indexed volume (mL/m2) | 30.7 ± 14.4 | 43.4 ± 12.2 | 41.9 ± 17.5 | 0.740 |
| PASP (mmHg) | 33.0 ± 9.8 | 36.6 ± 7.8 | 29.8 ± 10.4 | 0.040 |
| E/e’ | 12.5 ± 4.7 | 13.3 ± 4.5 | 11.8 ± 3.1 | 0.372 |
| CPET duration (min) | 10.4 ± 3.6 | 10.0 ± 4.0 | 10.9 ± 3.2 | 0.451 |
| Peak RER | 1.10 ± 0.11 | 1.11 ± 0.12 | 1.09 ± 0.12 | 0.661 |
| pVO2 (mL/kg/min) | 16.5 ± 4.5 | 16.4 ± 3.9 | 16.5 ± 5.1 | 0.921 |
| VE/VCO2 | 34.3 ± 8.3 | 34.4 ± 7.9 | 34.1 ± 8.9 | 0.919 |
| Number of Events | |
|---|---|
| Dapagliflozin + OMT | |
| 3 2 0 0 |
| Control Group (maintain OMT) | |
| 2 2 0 0 |
| p-Value (Partial η2) | |||||||
|---|---|---|---|---|---|---|---|
| Dapagliflozin (n = 20) | Control (n = 20) | Time | Group | Interaction | |||
| Baseline | 6 m Fup | Baseline | 6 m Fup | ||||
| NYHA Class III (No vs. Yes) (a) | 6 (28.6%) | 1 (4.8%) | 2 (10.5%) | 5 (26.3%) | 0.031 (8.00) | 0.087 (7.14) | 0.005 (0.041) |
| Furosemide Dose (mg) | 25.7 ± 29.8 | 20.9 ± 29.9 | 33.7 ± 32.6 | 38.9 ± 32.9 | 0.931 (0.00) | 0.182 (0.05) | 0.061 (0.09) |
| HFSS | 8.7 ± 0.6 | 8.9 ± 0.8 | 8.6 ± 0.7 | 8.7 ± 0.9 | 0.087 (0.11) | 0.512 (0.02) | 0.639 (0.10) |
| SHFM | 89.5 ± 6.7 | 92.5 ± 4.4 | 88.3 ± 4.9 | 87.6 ± 6.1 | 0.137 (0.06) | 0.080 (0.09) | 0.014 (0.17) |
| MAGGIC Score | 18.1 ± 5.4 | 17.0 ± 5.0 | 17.8 ± 5.6 | 19.2 ± 7.4 | 0.992 (0.00) | 0.550 (0.01) | 0.073 (0.09) |
| Hemoglobin (g/dL) | 14.0 ± 1.6 | 14.5 ± 1.6 | 13.8 ± 1.2 | 13.9 ± 1.6 | 0.091 (0.08) | 0.422 (0.02) | 0.238 (0.04) |
| Creatinine (mg/dL) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.033 (0.12) | 0.757 (0.00) | 0.183 (0.05) |
| GFR (mL/min) | 68.7 ± 23.8 | 66.8 ± 22.2 | 72.5 ± 17.1 | 65.9 ± 16.5 | 0.010 (0.17) | 0.834 (0.00) | 0.144 (0.06) |
| hs-cTnI (ng/mL) | 9.2 ± 6.7 | 8.4 ± 5.2 | 21.9 ± 45.1 | 27.7 ± 43.4 | 0.935 (0.00) | 0.161 (0.06) | 0.200 (0.00) |
| NT-proBNP (pg/mL) | 1201.5 ± 920.9 | 983.9 ± 823.5 | 1132.1 ± 1774.4 | 1782.4 ± 2513.7 | 0.162 (0.05) | 0.485 (0.01) | 0.007 (0.19) |
| HbA1c (%) | 5.8 ± 0.4 | 5.6 ± 0.2 | 5.7 ± 0.5 | 6.1 ± 0.4 | 0.404 (0.02) | 0.854 (0.00) | <0.001 (0.28) |
| LDL (mg/dL) | 85.2 ± 33.2 | 86.9 ± 48.9 | 95.8 ± 36.9 | 88.8 ± 52.3 | 0.478 (0.01) | 0.610 (0.01) | 0.281 (0.03) |
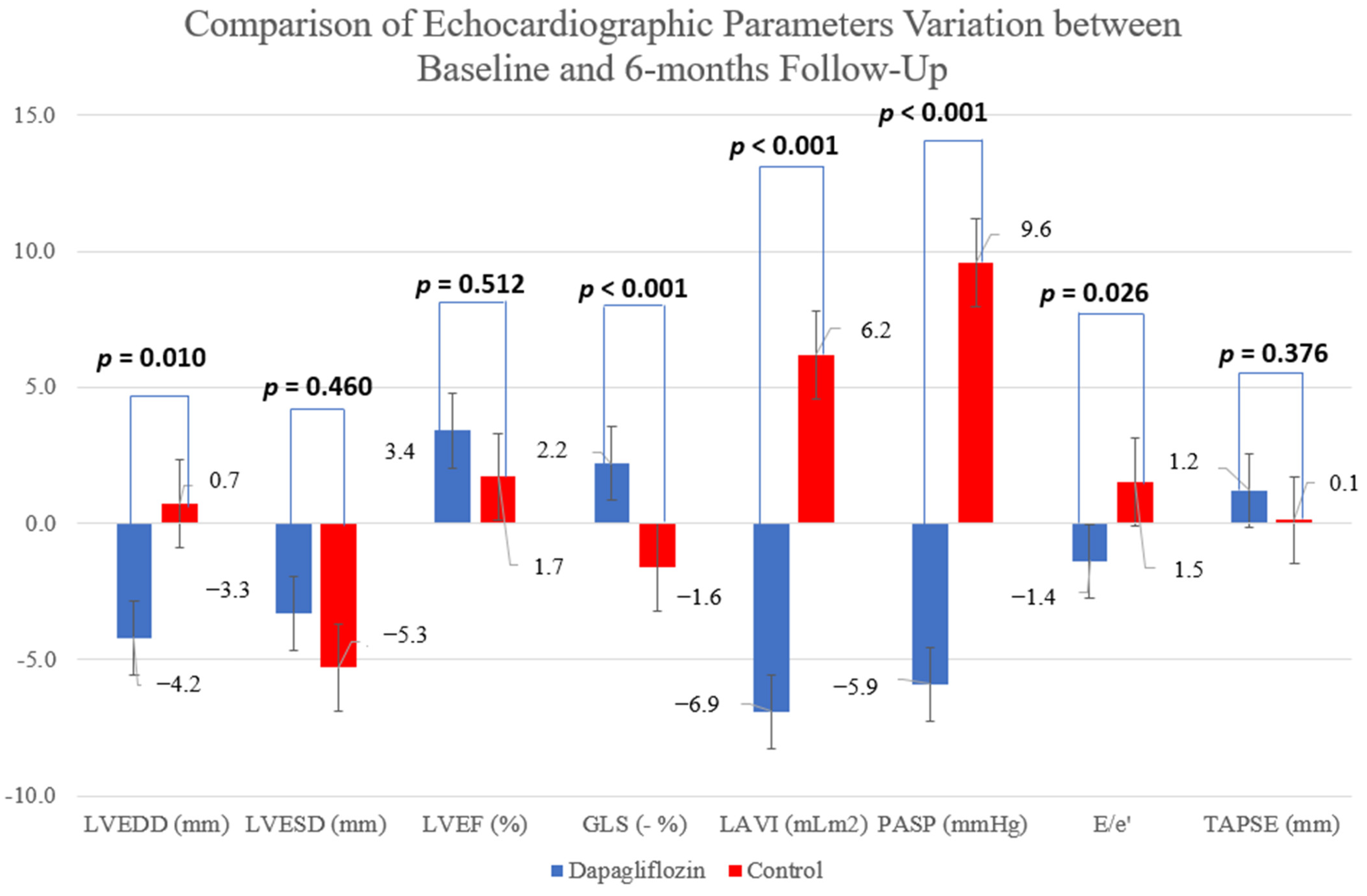
| LVEDD (mm) | 67.2 ± 8.4 | 63.0 ± 9.7 | 65.1 ± 6.0 | 65.8 ± 8.6 | 0.055 (0.10) | 0.618 (0.01) | 0.010 (0.17) |
| LVESD (mm) | 49.4 ± 9.6 | 46.1 ± 13.4 | 46.9 ± 14.3 | 41.6 ± 10.8 | 0.004 (0.23) | 0.760 (0.00) | 0.460 (0.02) |
| LVEF (%) | 33.5 ± 7.8 | 36.9 ± 8.5 | 34.5 ± 8.9 | 36.2 ± 8.1 | 0.057 (0.10) | 0.759 (0.00) | 0.512 (0.01) |
| GLS (%) | −8.2 ± 3.2 | −10.4 ± 2.3 | −8.4 ± 2.8 | −6.8 ± 2.8 | 0.549 (0.02) | 0.092 (0.11) | <0.001 (0.39) |
| TAPSE (mm) | 19.0 ± 5.7 | 20.2 ± 5.7 | 18.6 ± 4.2 | 18.7 ± 4.2 | 0.376 (0.00) | 0.883 (0.00) | 0.376 (0.00) |
| LA Indexed Volume (ml/m2) | 43.4 ± 12.2 | 36.5 ± 10.1 | 41.9 ± 17.5 | 48.1 ± 11.2 | 0.760 (0.00) | 0.296 (0.03) | <0.001 (0.08) |
| PASP (mmHg) | 36.6 ± 7.8 | 30.7 ± 8.6 | 29.8 ± 11.4 | 39.4 ± 10.4 | 0.560 (0.00) | 0.344 (0.04) | <0.001 (0.15) |
| E/e’ | 13.3 ± 4.5 | 11.9 ± 3.8 | 11.8 ± 4.9 | 13.3 ± 7.5 | 0.938 (0.00) | 0.783 (0.00) | 0.026 (0.16) |
| CPET Duration (minutes) | 10.0 ± 4.0 | 11.9 ± 4.6 | 10.9 ± 3.2 | 11.5 ± 4.4 | 0.025 (0.17) | 0.755 (0.00) | 0.225 (0.05) |
| Peak RER | 1.10 ± 011 | 1.11 ± 0.12 | 1.09 ± 0.12 | 0.99 ± 0.09 | 0.003 (0.27) | 0.185 (0.06) | 0.020 (0.18) |
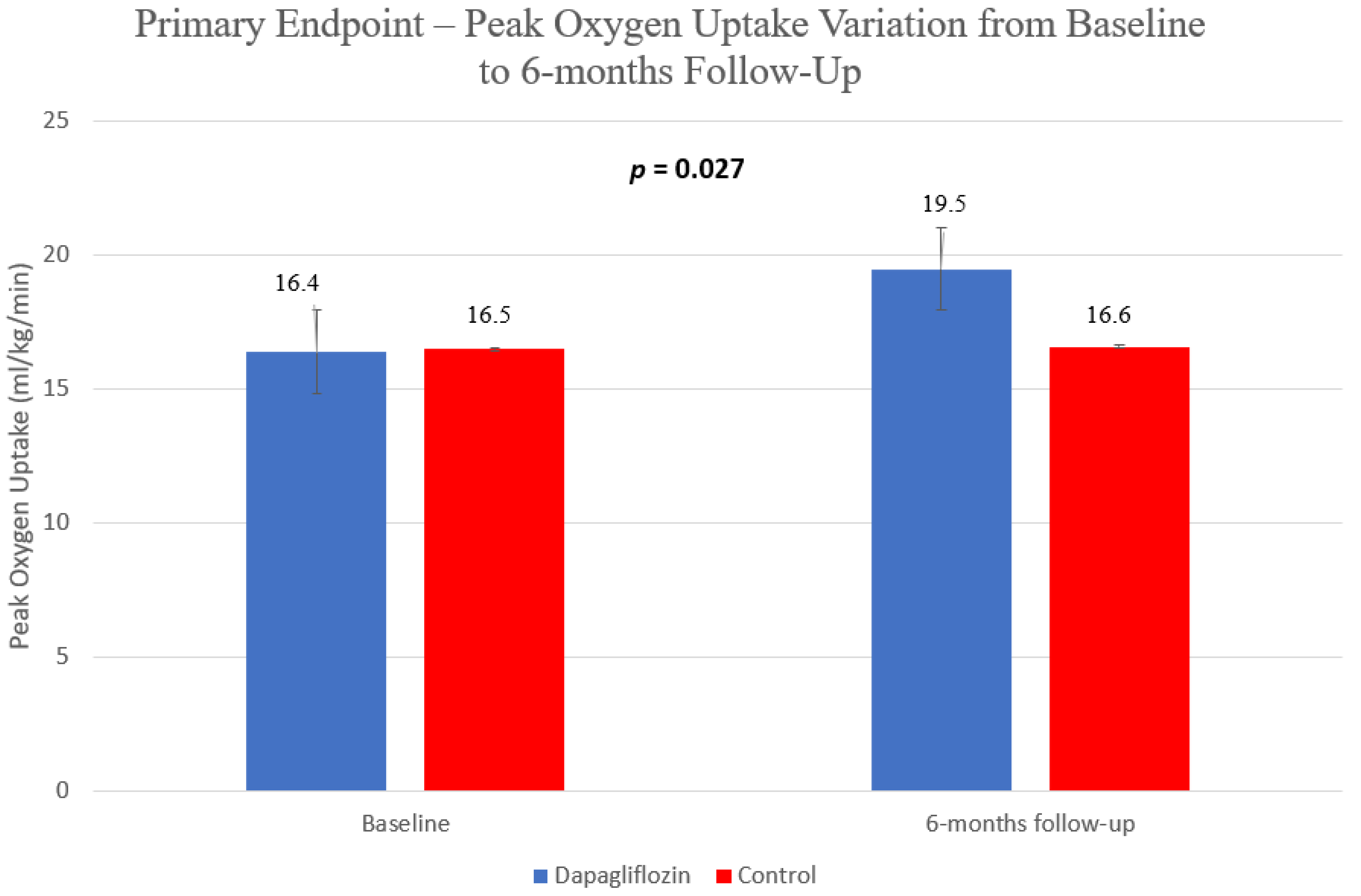
| pVO2 (ml/kg/min) | 16.4 ± 3.9 | 19.5 ± 6.0 | 16.5 ± 5.1 | 16.6 ± 5.2 | 0.018 (0.18) | 0.537 (0.01) | 0.027 (0.16) |
| VE/VCO2 | 34.4 ± 7.9 | 33.6 ± 6.7 | 34.1 ± 8.9 | 37.4 ± 9.4 | 0.169 (0.06) | 0.789 (0.00) | 0.027 (0.15) |
| pVO2 at LANA | 11.8 ± 2.8 | 12.4 ± 3.6 | 11.7 ± 3.4 | 12.9 ± 3.2 | 0.081 (0.11) | 0.857 (0.00) | 0.556 (0.01) |
| Cardiorrespiratory Optimal Point | 30.7 ± 5.6 | 28.8 ± 4.5 | 28.0 ± 6.6 | 30.5 ± 8.2 | 0.719 (0.01) | 0.476 (0.02) | 0.018 (0.21) |
| HR at LANA | 87.9 ± 14.8 | 91.0 ± 18.0 | 97.2 ± 21.6 | 94.3 ± 17.3 | 0.786 (0.00) | 0.403 (0.03) | 0.233 (0.05) |
| HRR1 | 21.2 ± 12.6 | 17.5 ± 13.2 | 21.0 ± 12.0 | 20.7 ± 10.8 | 0.369 (0.03) | 0.943 (0.00) | 0.583 (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, J.; Teixeira, A.R.; Gonçalves, A.V.; Moreira, R.I.; Silva, T.P.; Timóteo, A.T.; Ferreira, R.C. Dapagliflozin Impact on the Exercise Capacity of Non-Diabetic Heart Failure with Reduced Ejection Fraction Patients. J. Clin. Med. 2022, 11, 2935. https://doi.org/10.3390/jcm11102935
Reis J, Teixeira AR, Gonçalves AV, Moreira RI, Silva TP, Timóteo AT, Ferreira RC. Dapagliflozin Impact on the Exercise Capacity of Non-Diabetic Heart Failure with Reduced Ejection Fraction Patients. Journal of Clinical Medicine. 2022; 11(10):2935. https://doi.org/10.3390/jcm11102935
Chicago/Turabian StyleReis, João, Ana Rita Teixeira, António Valentim Gonçalves, Rita Ilhão Moreira, Tiago Pereira Silva, Ana Teresa Timóteo, and Rui Cruz Ferreira. 2022. "Dapagliflozin Impact on the Exercise Capacity of Non-Diabetic Heart Failure with Reduced Ejection Fraction Patients" Journal of Clinical Medicine 11, no. 10: 2935. https://doi.org/10.3390/jcm11102935
APA StyleReis, J., Teixeira, A. R., Gonçalves, A. V., Moreira, R. I., Silva, T. P., Timóteo, A. T., & Ferreira, R. C. (2022). Dapagliflozin Impact on the Exercise Capacity of Non-Diabetic Heart Failure with Reduced Ejection Fraction Patients. Journal of Clinical Medicine, 11(10), 2935. https://doi.org/10.3390/jcm11102935







