A Comparison of Visual Quality and Contrast Sensitivity between Patients with Scleral-Fixated and In-Bag Intraocular Lenses
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Postoperative Ophthalmic Examinations
2.3. Contrast Sensitivity Test
2.4. Optical Aberrations
2.5. Surgical Technique
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindstrom, R. Thoughts on cataract surgery: 2015. Rev. Ophthalmol. 2015. Available online: http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422 (accessed on 28 May 2021).
- Jirásková, N. Optical Aberrations and Contrast Sensitivity of Spherical and Aspheric Intraocular Lenses—A Prospective Comparative Clinical Study. J. Clin. Exp. Ophthalmol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Matsuki, N.; Inoue, M.; Itoh, Y.; Nagamoto, T.; Hirakata, A. Changes in higher-order aberrations of intraocular lenses with intrascleral fixation. Br. J. Ophthalmol. 2015, 99, 1732–1738. [Google Scholar] [CrossRef]
- Malbran, E.S.; Malbran, E., Jr.; Negri, I. Lens guide suture for transport and fixation in secondary IOL implantation after intracapsular extraction. Int. Ophthalmol. 1986, 9, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.S.W.; Young, A.L.; Cheng, L.L. Long-term outcome of scleral-fixated intraocular lens implantation. Br. J. Ophthalmol. 2013, 97, 1308–1311. [Google Scholar] [CrossRef]
- Long, C.; Wei, Y.; Yuan, Z.; Zhang, Z.; Lin, X.; Liu, B. Modified technique for transscleral fixation of posterior chamber intraocular lenses. BMC Ophthalmol. 2015, 15, 127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Por, Y.M.; Lavin, M.J. Techniques of intraocular lens suspension in the absence of capsular/zonular support. Surv. Ophthalmol. 2005, 50, 429–462. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. Intraocular lens tilt and decentration, anterior chamber depth, and refractive error after trans-scleral suture fixation surgery. Ophthalmology 1999, 106, 878–882. [Google Scholar] [CrossRef]
- Kozaki, J.; Tanilhara, H.; Yasuda, A.; Nagata, M. Tilt and decentration of the implanted posterior chamber intraocular lens. J. Cataract. Refract. Surg. 1991, 17, 592–595. [Google Scholar] [CrossRef]
- Oshika, T.; Sugita, G.; Miyata, K.; Tokunaga, T.; Samejima, T.; Okamoto, C.; Ishii, Y. Influence of tilt and decentration of scleral-sutured intraocular lens on ocular higher-order wavefront aberration. Br. J. Ophthalmol. 2007, 91, 185–188. [Google Scholar] [CrossRef][Green Version]
- Taketani, F.; Matuura, T.; Yukawa, E.; Hara, Y. Influence of intraocular lens tilt and decentration on wavefront aberrations. J. Cataract Refract. Surg. 2004, 30, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.B.; Gilchrist, J.; Whitaker, D. Contrast sensitivity and glare sensitivity changes with three types of cataract morphology: Are these techniques necessary in a clinical evaluation of cataract? Ophthalmic Physiol. Opt. 1989, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Applegate, R.A.; Howland, H.C.; Sharp, R.P.; Cottingham, A.J.; Yee, R.W. Corneal aberrations and visual performance after radial keratotomy. J. Retract. Surg. 1998, 14, 397–4071. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Rahimy, E.; Gupta, O.P.; Hsu, J. Combined 27-Gauge Pars Plana Vitrectomy and Scleral Fixation of an Akreos AO60 Intraocular Lens Using Gore-Tex Suture. Retina 2016, 36, 1602–1604. [Google Scholar] [CrossRef]
- Mimura, T.; Amano, S.; Sugiura, T.; Funatsu, H.; Yamagami, S.; Araie, M.; Eguchi, S. Refractive change after transscleral fixation of posterior chamber intraocular lenses in the absence of capsular support. Acta Ophthalmol. Scand. 2004, 82, 544–546. [Google Scholar] [CrossRef]
- Mizuno, Y.; Sugimoto, Y. A comparative study of transscleral suture-fixated and scleral-fixated intraocular lens implantation. Int. Ophthalmol. 2019, 39, 839–845. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Tseng, C.-C.; Lin, C.-P. Myopic shift of sulcus suture-fixated posterior chamber intraocular lenses. Taiwan J. Ophthalmol. 2013, 3, 95–97. [Google Scholar] [CrossRef]
- Bayramlar, H.; Hepsen, I.F.; Yilmaz, H. Myopic shift from the predicted refraction after sulcus fixation of PMMA posterior chamber intraocular lenses. Can. J. Ophthalmol. 2006, 41, 78–82. [Google Scholar] [CrossRef]
- Ma, D.J.; Choi, H.J.; Kim, M.K.; Wee, W.R. Clinical comparison of ciliary sulcus and pars plana locations for posterior chamber intraocular lens transscleral fixation. J. Cataract. Refract. Surg. 2011, 37, 1439–1446. [Google Scholar] [CrossRef]
- Olsen, T. Sources of error in intraocular lens power calculation. J. Cataract. Refract. Surg. 1992, 18, 125–129. [Google Scholar] [CrossRef]
- Suto, C.; Hori, S.; Fukuyama, E.; Akura, J. Adjusting intraocular lens power for sulcus fixation. J. Cataract. Refract. Surg. 2003, 29, 1913–1917. [Google Scholar] [CrossRef]
- Torii, T.; Tamaoki, A.; Kojima, T.; Matsuda, T.; Kaga, T.; Ichikawa, K. Comparison of Clinical Outcomes Between Intracapsular Implantation and Intrascleral Fixation Using the Same Model of Intraocular Lens. Clin. Ophthalmol. 2020, 14, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.S.; Adamsons, I.A.; Stark, W.J. Comparison of acuity, contrast sensitivity, and disability glare before and after cataract surgery. Arch. Ophthalmol. 1993, 111, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.B.; Hurst, M.A. Simple clinical techniques to evaluate visual function in patients with early cataract. Optom. Vis. Sci. 1990, 67, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Miyata, K.; Samejima, T.; Hirohara, Y.; Mihashi, T.; Oshika, T. Influence of wavefront aberration and corneal subepithelial haze on low-contrast visual acuity after photorefractive keratectomy. Am. J. Ophthalmol. 2004, 138, 620–624. [Google Scholar] [CrossRef]
- Yamane, N.; Miyata, K.; Samejima, T.; Hiraoka, T.; Kiuchi, T.; Okamoto, F.; Hirohara, Y.; Mihashi, T.; Oshika, T. Ocular higher-order aberrations and contrast sensitivity after conventional laser in situ keratomileusis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3986–3990. [Google Scholar] [CrossRef]
- Gao, S.; Qin, T.; Wang, S.; Lu, Y. Sulcus Fixation of Foldable Intraocular Lenses Guided by Ultrasound Biomicroscopy. J. Ophthalmol. 2015, 2015, 520418. [Google Scholar] [CrossRef]
- Fass, O.N.; Herman, W.K. Four-point suture scleral fixation of a hydrophilic acrylic IOL in aphakic eyes with insufficient capsule support. J. Cataract. Refract. Surg. 2010, 36, 991–996. [Google Scholar] [CrossRef]
- Casprini, F.; Balestrazzi, A.; Tosi, G.M.; Miracco, F.; Martone, G.; Cevenini, G.; Caporossi, A. Glare disability and spherical aberration with five foldable intraocular lenses: A prospective randomized study. Acta Ophthalmol. Scand. 2005, 83, 20–25. [Google Scholar] [CrossRef]
- Kim, S.W.; Ahn, H.; Kim, E.K.; Kim, T.I. Comparison of higher order aberrations in eyes with aspherical or spherical intraocular lenses. Eye 2008, 22, 1493–1498. [Google Scholar] [CrossRef]
- Ang, R.T.; Martinez, G.A.; Caguioa, J.B.; Reyes, K.B. Comparison in the quality of vision and spherical aberration between spherical and aspheric intraocular lenses. Philipp. J. Ophthalmol. 2008, 33, 9. [Google Scholar]
- Arundale, K. An investigation into the variation of human contrast sensitivity with age and ocular pathology. Br. J. Ophthalmol. 1978, 62, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Laemmer, R.; Adler, W.; Juenemann, A.G.; Horn, F.K. Measuring contrast sensitivity in normal subjects with OPTEC 6500: Influence of age and glare. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P.; Muzyka-Woźniak, M. Methods for evaluating quality of life and vision in patients undergoing lens refractive surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1091–1099. [Google Scholar] [CrossRef]
| Characteristics | In-Bag (n = 74) | Scleral Fixation (n = 23) | p Value |
|---|---|---|---|
| Age in years, mean ± SD (range) | 69.76 ± 9.58 (46–96) | 58.13 ± 12.81 (36–79) | <0.001 * |
| Male sex, No. (male %) | 24 (32.43) | 10 (43.48) | 0.038 * |
| Laterality, No. | 0.509 | ||
| OD (%) | 36 (48.65) | 13 (56.52) | |
| OS (%) | 38 (51.35) | 10 (43.48) | |
| IOL type, No. | 0.003 * | ||
| Spherical (%) | 19 (25.68) | 9 (39.13) | |
| Aspheric (%) | 28 (37.84) | 14 (60.87) | |
| Toric (%) | 27 (36.48) | 0 (0) | |
| Postop in months, mean ± SD (range) | 19.88 ± 30.09 (1–132) | 16.22 ± 19.27 (1–60) | 0.585 |
| Pupil size (mm ± SD) | 4.81 ± 1.02 | 4.66 ± 0.99 | 0.526 |
| CDVA (logMAR ± SD) | 0.11 ± 0.14 | 0.20 ± 0.17 | 0.015 * |
| SE, mean ± SD | −0.23 ± 0.75 | −1.09 ± 3.32 | 0.0385 * |
| Astigmatism, mean ± SD | −0.81 ± 0.58 | −1.11 ± 0.87 | 0.0654 |
| In-Bag (n = 74) | Scleral Fixation (n = 23) | p Value | |
|---|---|---|---|
| Ocular | |||
| Aberrations a (μm ± SD) | 0.387 ± 0.194 | 0.785 ± 0.667 | <0.001 * |
| HOAs (μm ± SD) | 0.145 ± 0.070 | 0.255 ± 0.241 | 0.001 * |
| Tilt (μm ± SD) | 0.103 ± 0.064 | 0.187 ± 0.203 | 0.003 * |
| Trefoil (μm ± SD) | 0.121 ± 0.076 | 0.209 ± 0.189 | 0.002 * |
| Coma (μm ± SD) | 0.035 ± 0.024 | 0.058 ± 0.053 | 0.004 * |
| Astigmatism b (μm ± SD) | 0.434 ± 0.211 | 0.704 ± 0.553 | 0.001 * |
| Spherical aberration (μm ± SD) | 0.016 ± 0.013 | 0.022 ± 0.020 | 0.059 |
| Corneal | |||
| Aberrations a (μm ± SD) | 0.377 ± 0.183 | 0.432 ± 0.194 | 0.213 |
| HOAs (μm ± SD) | 0.112 ± 0.049 | 0.146 ± 0.084 | 0.019 * |
| Tilt (μm ± SD) | 0.133 ± 0.102 | 0.167 ± 0.148 | 0.208 |
| Trefoil (μm ± SD) | 0.074 ± 0.042 | 0.102 ± 0.066 | 0.017 * |
| Coma (μm ± SD) | 0.056 ± 0.038 | 0.065 ± 0.060 | 0.355 |
| Astigmatism b (μm ± SD) | 1.201 ± 0.828 | 1.340 ± 0.739 | 0.473 |
| Spherical aberration (μm ± SD) | 0.025 ± 0.024 | 0.035 ± 0.030 | 0.094 |
| Internal | |||
| Aberrations a (μm ± SD) | 0.404 ± 0.204 | 0.714 ± 0.683 | 0.001 * |
| HOAs (μm ± SD) | 0.130 ± 0.066 | 0.209 ± 0.234 | 0.011 * |
| Tilt (μm ± SD) | 0.138 ± 0.110 | 0.178 ± 0.177 | 0.198 |
| Trefoil (μm ± SD) | 0.086 ± 0.061 | 0.151 ± 0.173 | 0.007 * |
| Coma (μm ± SD) | 0.051 ± 0.041 | 0.053 ± 0.037 | 0.830 |
| Astigmatism b (μm ± SD) | 0.858 ± 0.685 | 1.340 ± 1.815 | 0.060 |
| Spherical aberration (μm ± SD) | 0.025 ± 0.024 | 0.035 ± 0.030 | 0.094 |
| (AULCSF ± SD) | In-Bag (n = 74) | Scleral Fixation (n = 23) | p Value |
|---|---|---|---|
| Day (100 cd/m2) | |||
| Glare off | 1.345 ± 0.395 | 1.073 ± 0.549 | 0.011 * |
| Glare on | 1.314 ± 0.333 | 1.065 ± 0.521 | 0.008 * |
| Twilight (10 cd/m2) | |||
| Glare off | 1.179 ± 0.317 | 1.046 ± 0.434 | 0.114 |
| Glare on | 1.060 ± 0.356 | 0.887 ± 0.479 | 0.065 |
| Night (5 cd/m2) | |||
| Glare off | 1.085 ± 0.316 | 0.972 ± 0.371 | 0.157 |
| Glare on | 0.783 ± 0.359 | 0.658 ± 0.443 | 0.173 |
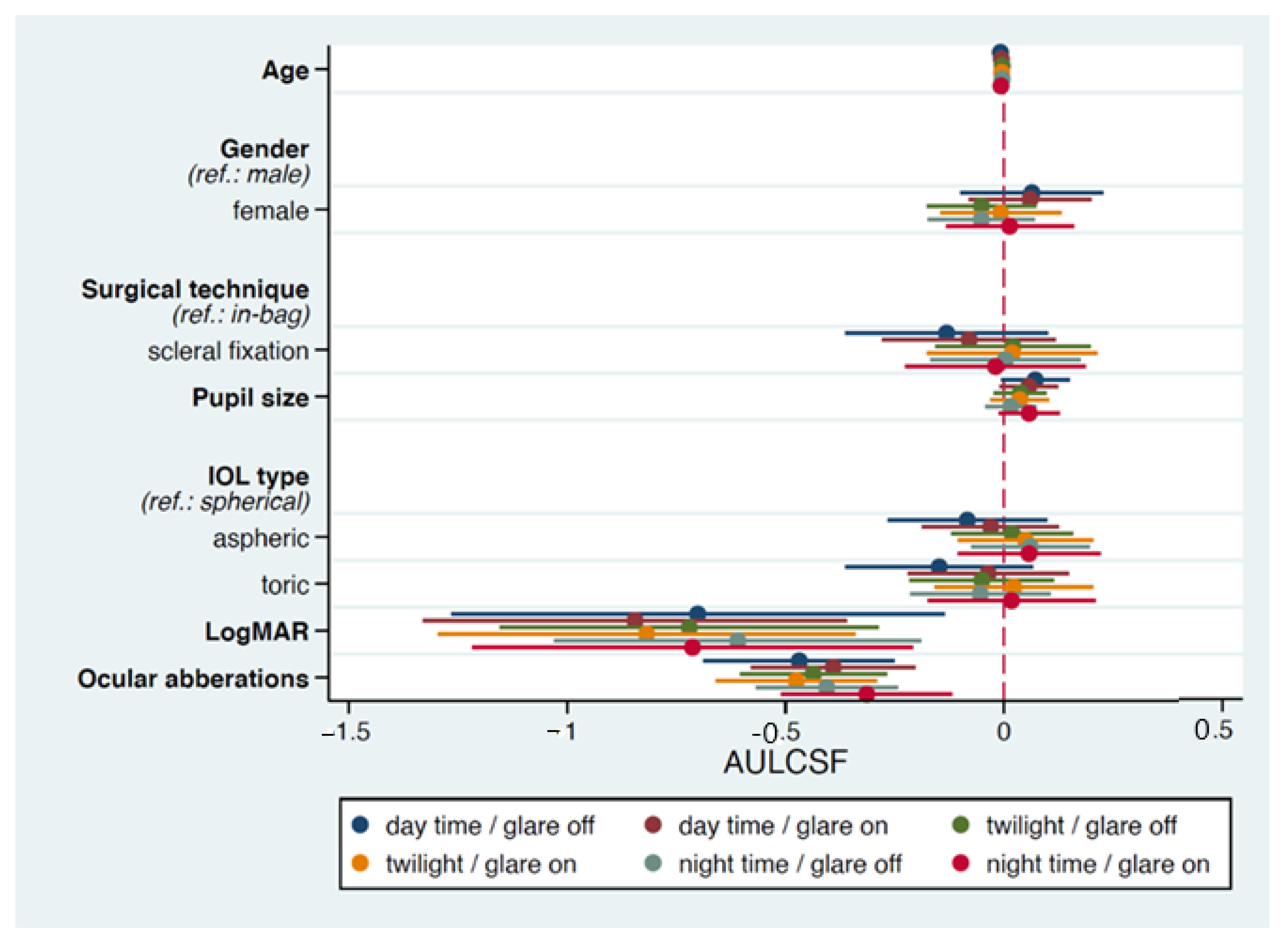
| Variables | Day Time Glare off | Day Time Glare on | Twilight Glare off | Twilight Glare on | Night Time Glare off | Night Time Glare on | |
|---|---|---|---|---|---|---|---|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | ||
| Age | −0.008 (0.004) * | −0.008 (0.004) * | −0.005 (0.003) | −0.007 (0.004) | −0.007 (0.003) * | −0.009 (0.004) * | |
| Gender | 0.080 (0.082) | 0.095 (0.076) | −0.027 (0.070) | 0.013 (0.073) | −0.027 (0.069) | 0.030 (0.077) | |
| Surgical technique | −0.127 (0.113) | −0.145 (0.097) | −0.012 (0.085) | −0.014 (0.096) | −0.039 (0.082) | −0.053 (0.102) | |
| Pupil size | 0.069 (0.039) | 0.035 (0.034) | 0.007 (0.029) | 0.014 (0.033) | −0.024 (0.028) | 0.032 (0.035) | |
| IOL type | Aspheric | −0.077 (0.091) | −0.007 (0.082) | 0.097 (0.074) | 0.077 (0.080) | 0.122 (0.072) | 0.075 (0.084) |
| Toric | −0.126 (0.107) | −0.015 (0.093) | 0.036 (0.081) | 0.059 (0.092) | 0.040 (0.078) | 0.042 (0.097) | |
| LogMAR | −0.698 (0.277) * | −0.700 (0.235) ** | −0.590 (0.200) *** | −0.776 (0.234) ** | −0.406 (0.191) ** | −0.677 (0.248) * | |
| Ocular aberrations | −0.460 (0.106) *** | −0.387 (0.090) *** | −0.417 (0.078) *** | −0.458 (0.090) *** | −0.405 (0.075) *** | −0.333 (0.095) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Pu, C.; Lin, K.-K.; Lee, J.-S.; Liu, L.; Hou, C.-H. A Comparison of Visual Quality and Contrast Sensitivity between Patients with Scleral-Fixated and In-Bag Intraocular Lenses. J. Clin. Med. 2022, 11, 2917. https://doi.org/10.3390/jcm11102917
Chen Y-L, Pu C, Lin K-K, Lee J-S, Liu L, Hou C-H. A Comparison of Visual Quality and Contrast Sensitivity between Patients with Scleral-Fixated and In-Bag Intraocular Lenses. Journal of Clinical Medicine. 2022; 11(10):2917. https://doi.org/10.3390/jcm11102917
Chicago/Turabian StyleChen, Yueh-Ling, Christy Pu, Ken-Kuo Lin, Jiahn-Shing Lee, Laura Liu, and Chiun-Ho Hou. 2022. "A Comparison of Visual Quality and Contrast Sensitivity between Patients with Scleral-Fixated and In-Bag Intraocular Lenses" Journal of Clinical Medicine 11, no. 10: 2917. https://doi.org/10.3390/jcm11102917
APA StyleChen, Y.-L., Pu, C., Lin, K.-K., Lee, J.-S., Liu, L., & Hou, C.-H. (2022). A Comparison of Visual Quality and Contrast Sensitivity between Patients with Scleral-Fixated and In-Bag Intraocular Lenses. Journal of Clinical Medicine, 11(10), 2917. https://doi.org/10.3390/jcm11102917







