High Resting Heart Rates Are Associated with Early Posthospitalization Mortality in Low Ejection Fraction Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
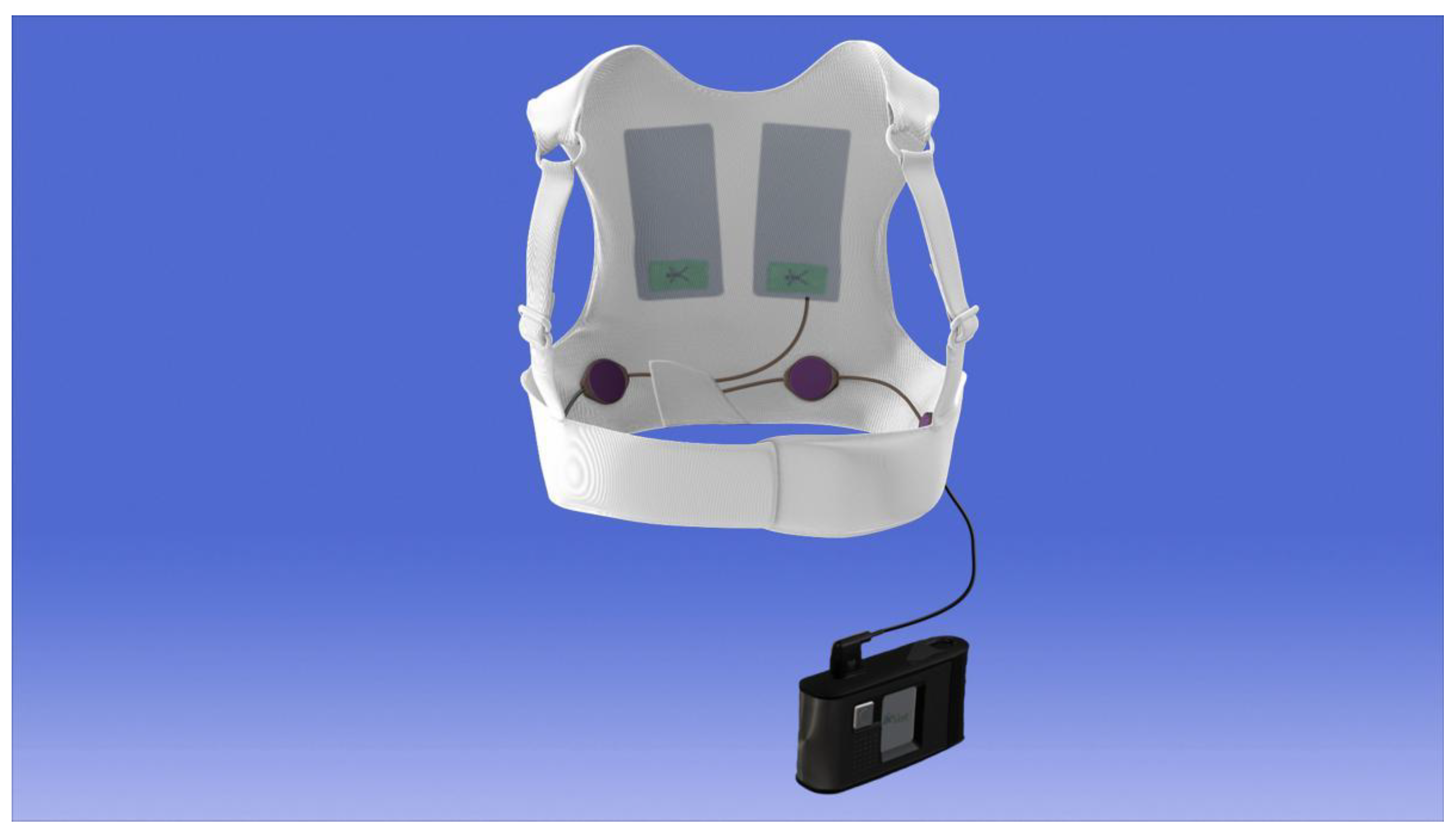
2.2. Device Description
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Tavazzi, L. Rationale and design of a randomized, double-blind, placebo-controlled outcome trial of ivabradine in chronic heart failure: The Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT). Eur. J. Heart Fail. 2010, 12, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jouven, X.; Empana, J.P.; Schwartz, P.J.; Desnos, M.; Dominique, C.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death [2] (multiple letters). N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Lechat, P.; Hulot, J.-S.; Escolano, S.; Mallet, A.; Leizorovicz, A.; Werhlen-Grandjean, M.; Pochmalicki, G.; Dargie, H.; on behalf of the CIBIS II Investigators. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation 2001, 103, 1428–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcalister, F.A.; Wiebe, N.; Ezekowitz, J.A.; Leung, A.A.; Armstrong, P.W. Meta-Analysis: β-Blocker Dose, Heart Rate Reduction, and Death in Patients with Heart Failure. Ann. Intern. Med. 2009, 150, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Ford, I.; Steg, P.G.; Tendera, M.; Robertson, M.; Ferrari, R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet 2008, 372, 817–821. [Google Scholar] [CrossRef]
- Böhm, M.; Swedberg, K.; Komajda, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010, 376, 886–894. [Google Scholar] [CrossRef]
- Hori, M.; Okamoto, H. Heart rate as a target of treatment of chronic heart failure. J. Cardiol. 2012, 60, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, A.M.; Tian, D.; Vudathaneni, V.; Shevchuk, O.L.; Ferrick, N.J.; Frishman, W. Wearable cardioverter defibrillators. Cardiol. Rev. 2016, 24, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Altibi, A.M.; Erqou, S.; Zmaili, M.A.; Saleh, A.; Al-Adham, R.; Ayoub, K.; Baghal, M.; Alkukhun, L.; Barakat, A.F.; et al. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2019, 5, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, C.G.; Maier, L.S.; Emoto, K.; Zirille, F.M.; Mirro, M.J. Achieving Guideline-Directed Heart Rate ControlEarly Posthospitalization. Am. J. Cardiol. 2019, 123, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
| Total number of patients | 4509 |
| Number of patients who died | 88 (2%) |
| Age, years | 59 ± 13 |
| Male gender | 3611 (80%) |
| Length of use, median (IQR) (days) | 66 [IQR 43–90] |
| Diagnosis | |
| Congestive heart failure | 1587 (35.2%) |
| PCI 1/CABG 2/MI 3 | 1493 (33.1%) |
| Myocarditis | 306 (6.8%) |
| ICD 4 explant | 197 (4.4%) |
| Others | 1054 (23.4%) |
| Daytime BOU 1 | Daytime EOU 2 | Nighttime BOU | Nighttime EOU | |
|---|---|---|---|---|
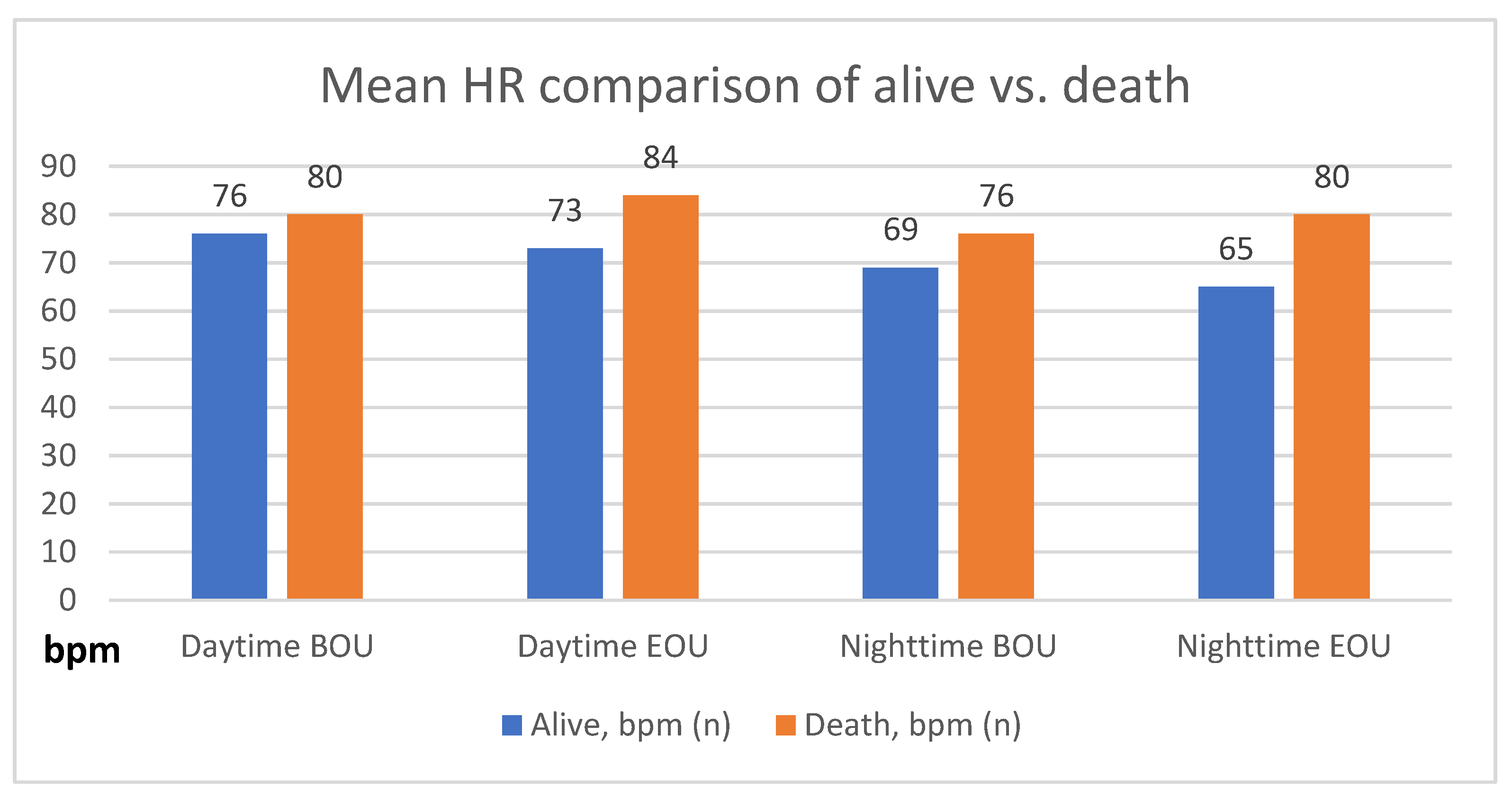
| Alive, bpm (n) | 76 ± 13 (4300) | 73 ± 13 (4178) | 69 ± 13 (4259) | 65 ± 12 (4120) |
| Dead, bpm (n) | 80 ± 15 (85) | 84 ± 20 (86) | 76 ± 14 (80) | 80 ± 20 (78) |
| p-Value | <0.01 | <0.0001 | <0.0001 | <0.0001 |
| HR Threshold | Daytime BOU | Daytime EOU | Nighttime BOU | Nighttime EOU | |
|---|---|---|---|---|---|
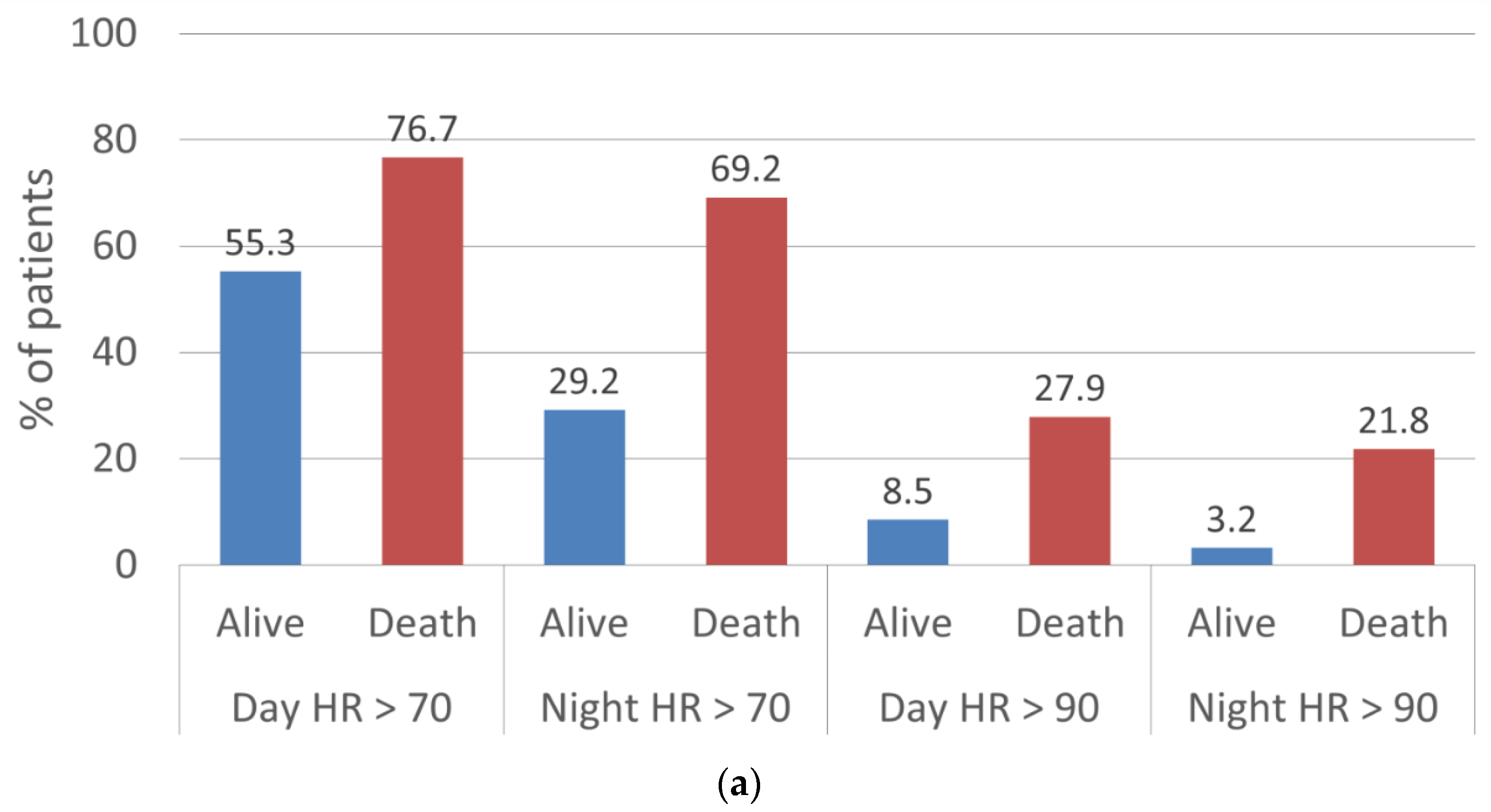
| Alive, n (%) | 70 bpm | 2823 (65.7) | 2310 (55.3) | 1888 (44.3) | 1204 (29.2) |
| 90 bpm | 572 (13.3) | 354 (8.5) | 280 (6.6) | 130 (3.2) | |
| Dead, n (%) | 70 bpm | 63 (74.1) | 66 (76.7) | 51 (63.8) | 54 (69.2) |
| 90 bpm | 21 (24.7) | 24 (27.9) | 11 (13.8) | 17 (21.8) |
| Trend | HR Threshold | n | BOU, bpm | EOU, bpm | Death, n (%) | p-Value |
|---|---|---|---|---|---|---|
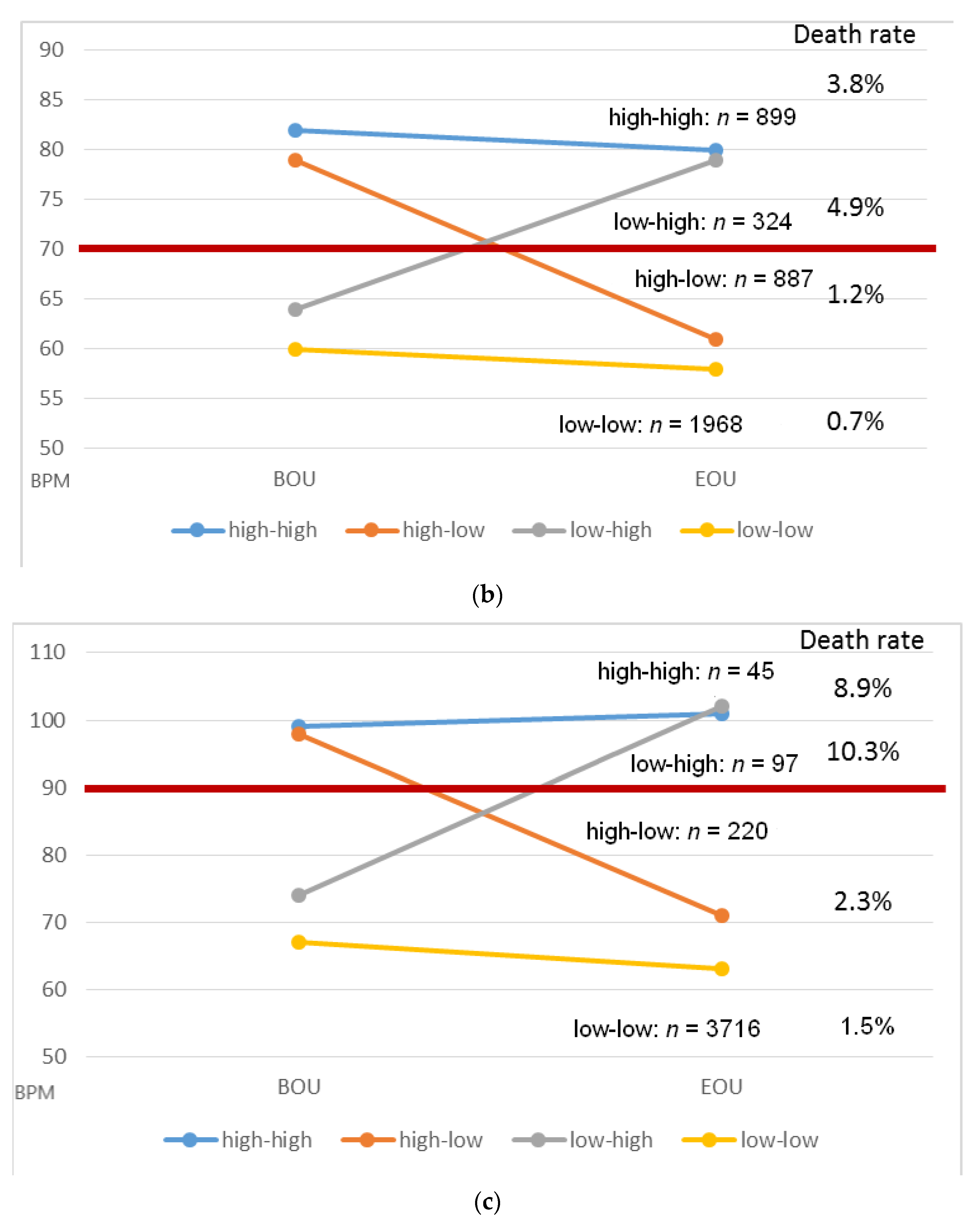
| High–high | 70 bpm | 899 | 82 ± 10 | 80 ± 10 | 34 (3.8) | <0.0001 |
| 90 bpm | 45 | 99 ± 9 | 101 ± 12 | 4 (8.9) | 0.40 | |
| High–low | 70 bpm | 887 | 79 ± 9 | 61 ± 6 | 11 (1.2) | <0.0001 |
| 90 bpm | 220 | 98 ± 8 | 71 ± 11 | 5 (2.3) | <0.0001 | |
| Low–high | 70 bpm | 324 | 64 ± 5 | 79 ± 11 | 16 (4.9) | <0.0001 |
| 90 bpm | 97 | 74 ± 10 | 102 ± 14 | 10 (10.3) | <0.0001 | |
| Low–low | 70 bpm | 1968 | 60 ± 7 | 57 ± 7 | 13 (0.7) | <0.0001 |
| 90 bpm | 3716 | 67 ± 10 | 63 ± 10 | 55 (1.5) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hain, A.; Busch, N.; Waezsada, S.E.; Hutter, J.; Kahle, P.; Kuniss, M.; Neumann, T.; Masuda, T.; Esser, H.O.; Hamm, C.; et al. High Resting Heart Rates Are Associated with Early Posthospitalization Mortality in Low Ejection Fraction Patients. J. Clin. Med. 2022, 11, 2901. https://doi.org/10.3390/jcm11102901
Hain A, Busch N, Waezsada SE, Hutter J, Kahle P, Kuniss M, Neumann T, Masuda T, Esser HO, Hamm C, et al. High Resting Heart Rates Are Associated with Early Posthospitalization Mortality in Low Ejection Fraction Patients. Journal of Clinical Medicine. 2022; 11(10):2901. https://doi.org/10.3390/jcm11102901
Chicago/Turabian StyleHain, Andreas, Nikolai Busch, Said Elias Waezsada, Julie Hutter, Patrick Kahle, Malte Kuniss, Thomas Neumann, Tsyuoshi Masuda, Horst O. Esser, Christian Hamm, and et al. 2022. "High Resting Heart Rates Are Associated with Early Posthospitalization Mortality in Low Ejection Fraction Patients" Journal of Clinical Medicine 11, no. 10: 2901. https://doi.org/10.3390/jcm11102901
APA StyleHain, A., Busch, N., Waezsada, S. E., Hutter, J., Kahle, P., Kuniss, M., Neumann, T., Masuda, T., Esser, H. O., Hamm, C., & Sperzel, J. (2022). High Resting Heart Rates Are Associated with Early Posthospitalization Mortality in Low Ejection Fraction Patients. Journal of Clinical Medicine, 11(10), 2901. https://doi.org/10.3390/jcm11102901






