Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series
Abstract
:1. Introduction
2. Materials and Methods
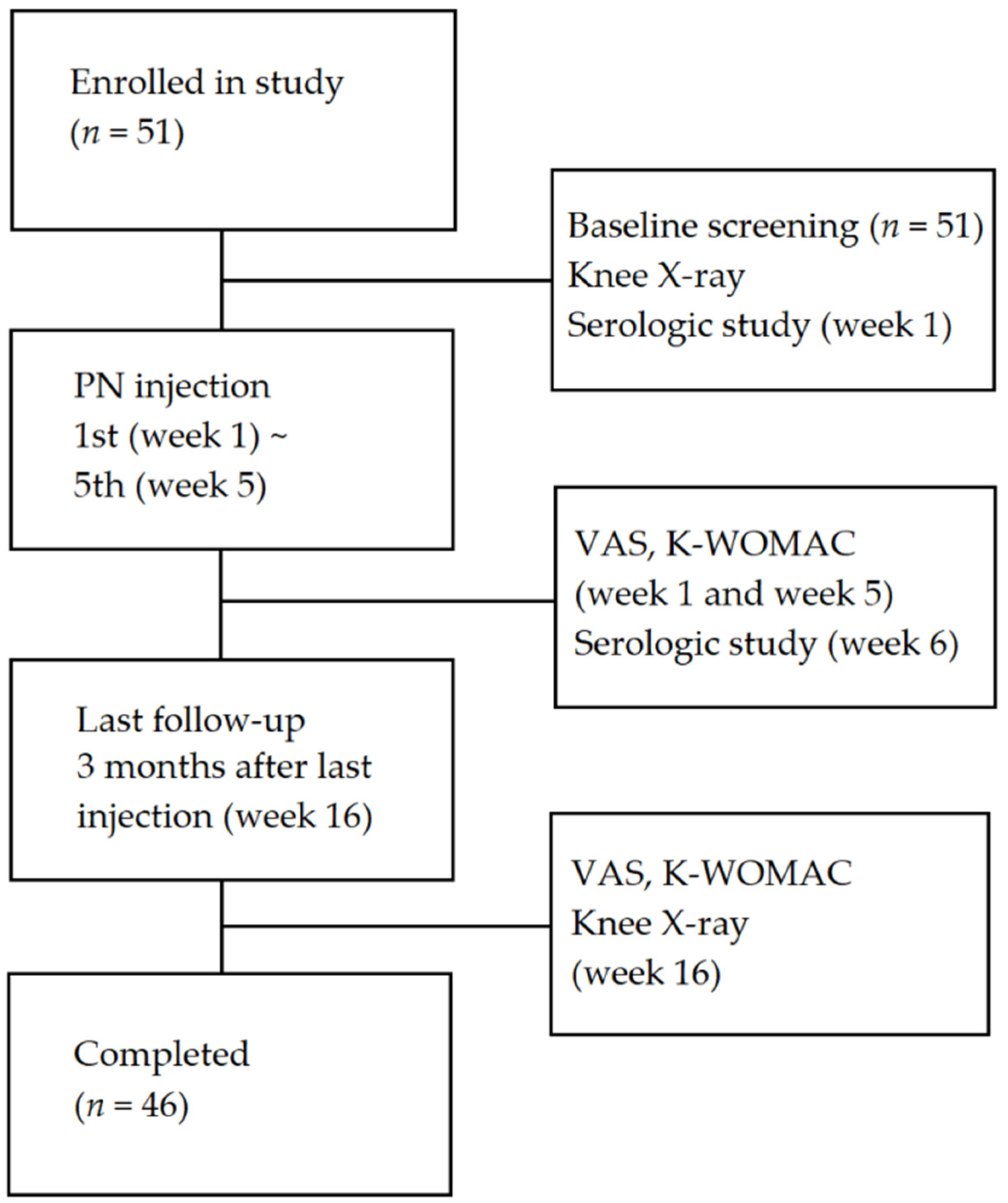
2.1. Study Design
2.2. Materials
2.3. Study Population
2.4. Experimental Intervention
2.4.1. Outcomes Measurements
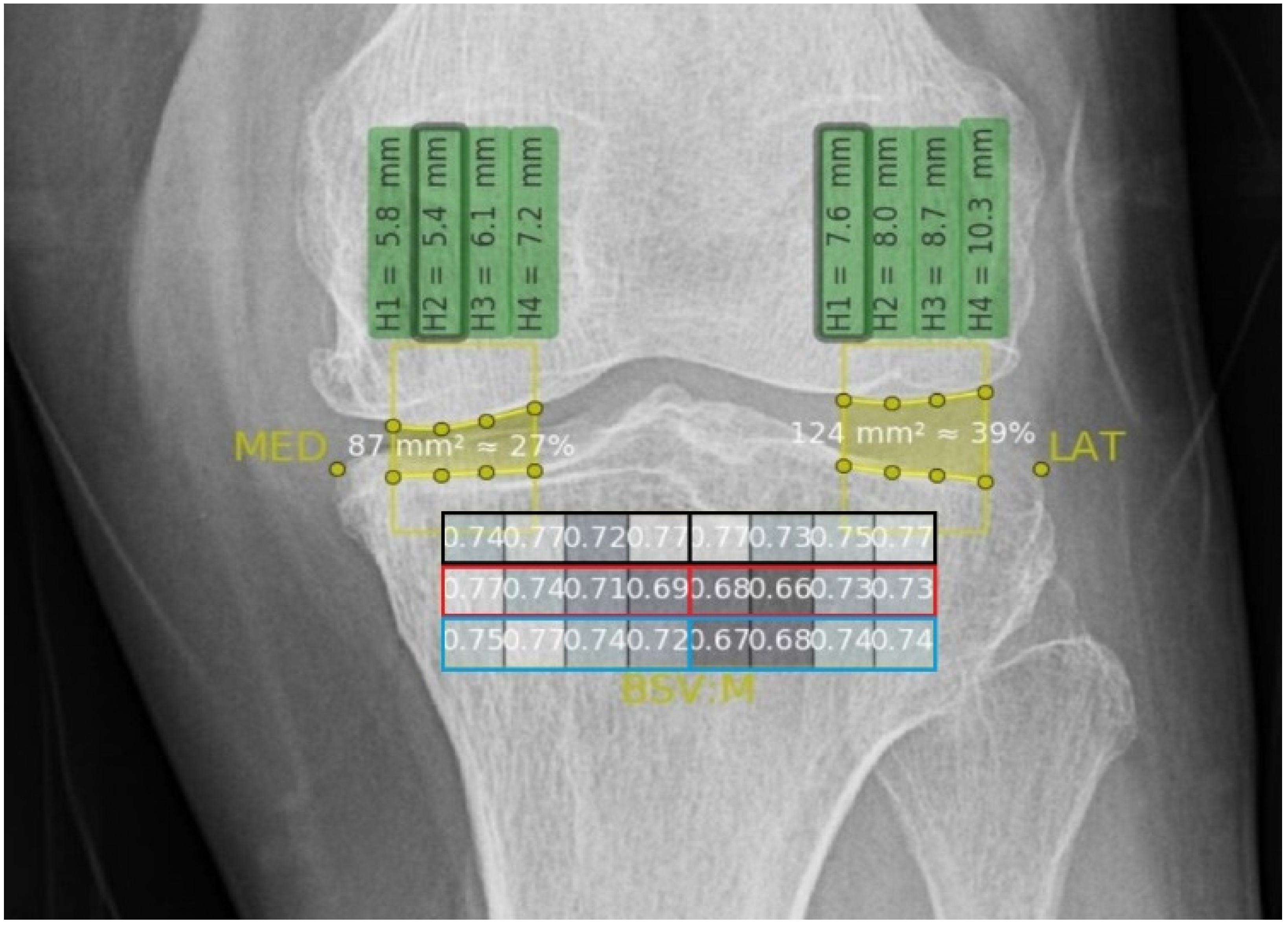
2.4.2. Texture Analysis
2.5. Rationale for Sample Size Determination
2.6. Statistical Analysis
3. Results
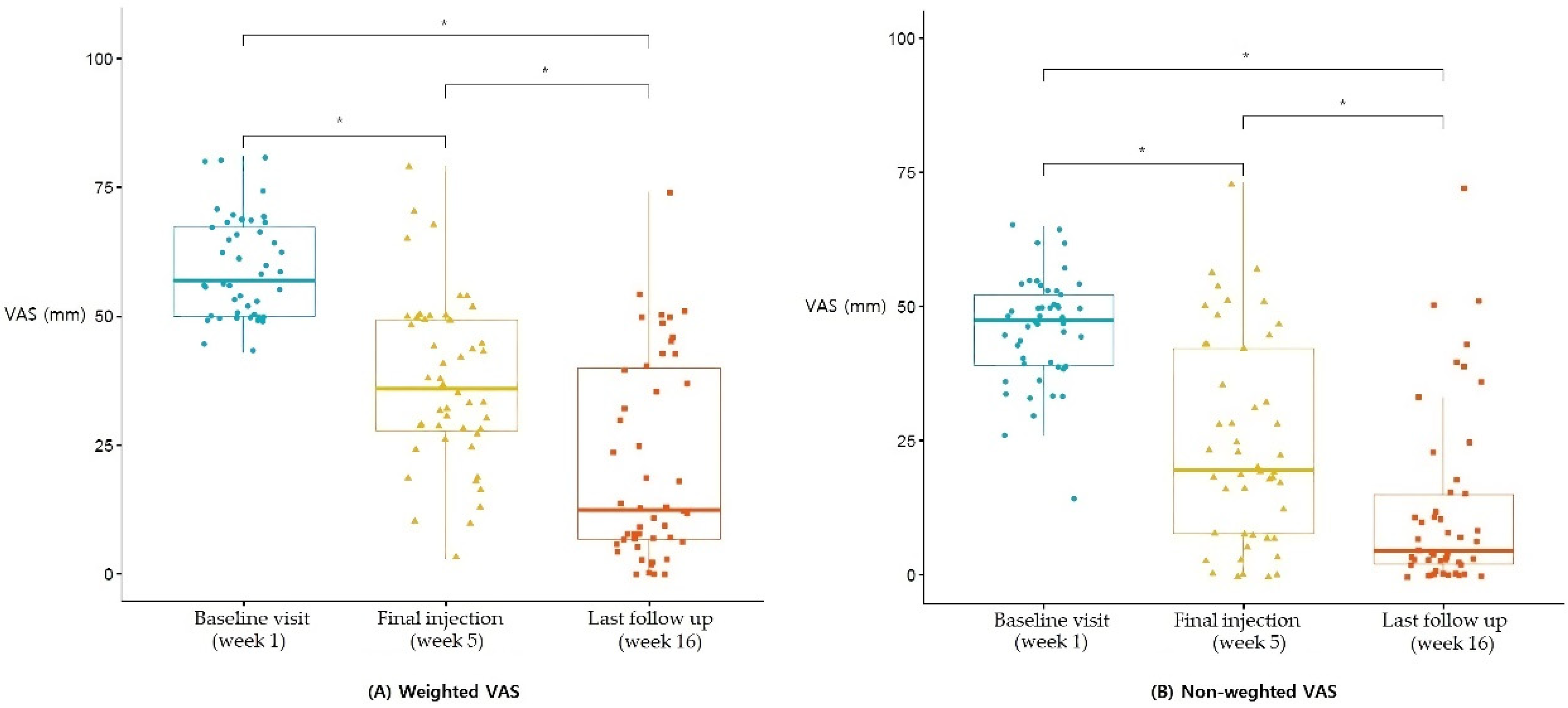
3.1. VAS
3.2. K-WOMAC
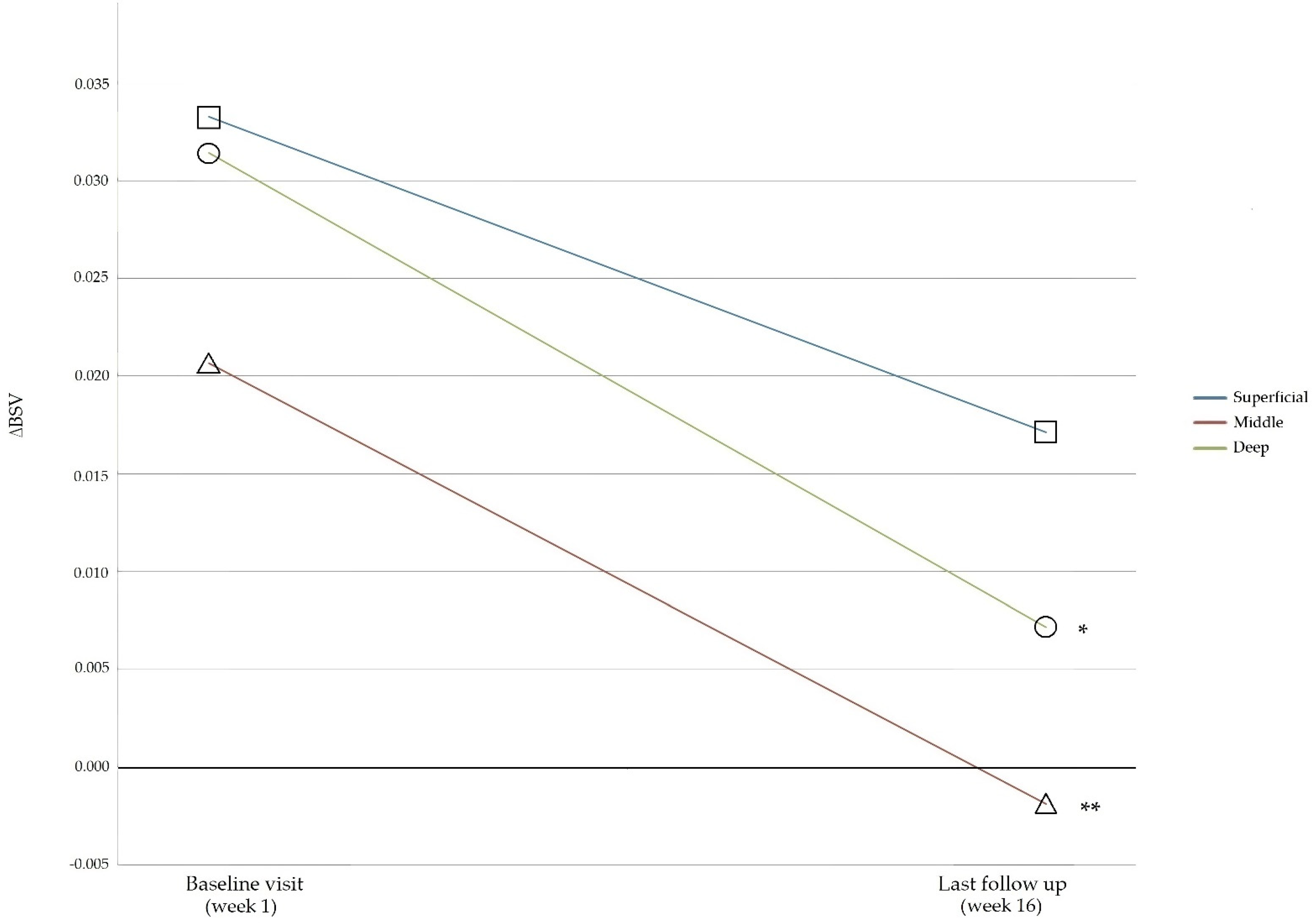
3.3. BSVs
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| (1) Male and female adults aged ≥40 and <80 | (1) Intra-articular injection to the knee within 6 months prior to screening |
| (2) Ability to understand and comply with the study procedures and visit schedule | (2) History of a knee surgery |
| (3) Ability to understand and comply with the study procedures and visit schedule | (3) Chronic inflammatory diseases such as rheumatoid arthritis |
| (4) Diagnosis of arthritis according to the American College of Rheumatology (ACR) classification criteria at the screening visit | (4) Use of non-steroidal anti-inflammatory drugs (NSAIDs) within 48 h prior to screening |
| (5) Kellgren–Lawrence Grade of I–IV for the severity of arthritis based on a knee X-ray at the screening visit | (5) Use of immunosuppressants such as cyclosporine A or azathioprine within 6 weeks prior to screening |
| (6) Visual Analogue Scale (VAS) at rest score of ≥40 mm at the screening visit | (6) Orthopedic diseases that may affect or interfere with the therapeutic effect |
| (7) Women of childbearing potential who have agreed to use a contraception method throughout the study | (7) Habitual use of psychotropic or narcotic analgesics for ≥1 week within 8 weeks prior to screening |
| (8) Psychological or psychiatric disorders that may affect a subject’s participation in the study | |
| (9) More severe pain in other areas of the body than in the knee bone and joint at the screening visit | |
| (10) Ligament instability of Grade II or higher upon physical examination at the screening visit (Grade 0 = no; Grade I = 0–5 mm; Grade II = 5–10 mm; Grade III ≥ 10 mm) | |
| (11) Participated in other intervention studies within 30 days prior to screening |
References
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, L.; Sangeorzan, E.; Chheda, N.; Lambright, D. Older Adults without Radiographic Knee Osteoarthritis: Knee Alignment and Knee Range of Motion. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Lee, E.B. Clinical Manifestations and Differential Diagnosis of Arthritides. Korean J. Med. 2012, 83, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Lo, G.H.; LaValley, M.; McAlindon, T.; Felson, D. Intra-articular Hyaluronic Acid in Treatment of Knee Osteoarthritis: A meta-analysis. JAMA 2003, 290, 3115–3121. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Y.; Yin, J.M.; Gao, J.J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.Q.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 607764. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Kraus, V.B.; Feng, S.; Wang, S.; White, S.; Ainslie, M.; Brett, A.; Holmes, A.; Charles, H.C. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Care Res. 2009, 60, 3711–3722. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Cicuttini, F.; Wolski, M.; Stachowiak, G.; Wluka, A. Trabecular bone texture detected by plain radiography is associated with an increased risk of knee replacement in patients with osteoarthritis: A 6 year prospective follow up study. Osteoarthr. Cartil. 2014, 22, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almhdie-Imjabbar, A.; Podsiadlo, P.; Ljuhar, R.; Jennane, R.; Nguyen, K.-L.; Toumi, H.; Saarakkala, S.; Lespessailles, E. Trabecular bone texture analysis of conventional radiographs in the assessment of knee osteoarthritis: Review and viewpoint. Arthritis Res. Ther. 2021, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Feng, S.; Wang, S.; White, S.; Ainslie, M.; Le Graverand, M.-P.H.; Brett, A.; Eckstein, F.; Hunter, D.J.; Lane, N.E.; et al. Subchondral Bone Trabecular Integrity Predicts and Changes Concurrently With Radiographic and Magnetic Resonance Imaging-Determined Knee Osteoarthritis Progression. Arthritis Care Res. 2013, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, J.W.; Murray, P.J.; Low, S.B.L.; Kasmai, B.; Johnson, G.; Donell, S.T.; Toms, A.P. Quantitative analysis of tibial subchondral bone: Texture analysis outperforms conventional trabecular microarchitecture analysis. J. Magn. Reson. Imaging 2016, 43, 1159–1170. [Google Scholar] [CrossRef]
- Thomson, J.; O’Neill, T.; Felson, D.; Cootes, T. Automated Shape and Texture Analysis for Detection of Osteoarthritis from Radiographs of the Knee; Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Germany, 2015; Volume 9350, pp. 127–134. [Google Scholar] [CrossRef]
- Bayramoglu, N.; Tiulpin, A.; Hirvasniemi, J.; Nieminen, M.; Saarakkala, S. Adaptive segmentation of knee radiographs for selecting the optimal ROI in texture analysis. Osteoarthr. Cartil. 2020, 28, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Pradelli, L.; Sinigaglia, T.; Migliore, A.; Checchia, G.A.; Franceschi, F.; Frediani, B.; Iannone, F.; Romanini, E. Non-Surgical Treatment of Knee Osteoarthritis: Multidisciplinary Italian Consensus on Best Practice. Ther. Clin. Risk Manag. 2021, 17, 507–530. [Google Scholar] [CrossRef]
- Vincent, K.R.; Vincent, H.K. Resistance Exercise for Knee Osteoarthritis. PM&R 2012, 4, S45–S52. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef]
- Billesberger, L.M.; Fisher, K.M.; Qadri, Y.J.; Boortz-Marx, R.L. Procedural Treatments for Knee Osteoarthritis: A Review of Current Injectable Therapies. Pain Res. Manag. 2020, 2020, 3873098. [Google Scholar] [CrossRef]
- Cudejko, T.; Van Der Esch, M.; Schrijvers, J.; Richards, R.; van den Noort, J.C.; Wrigley, T.; Van Der Leeden, M.; Roorda, L.D.; Lems, W.; Harlaar, J.; et al. The immediate effect of a soft knee brace on dynamic knee instability in persons with knee osteoarthritis. Rheumatology 2018, 57, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Cherian, J.J.; Kapadia, B.H.; Bhave, A.; McElroy, M.J.; Cherian, C.; Harwin, S.F.; Mont, M.A. Use of Transcutaneous Electrical Nerve Stimulation Device in Early Osteoarthritis of the Knee. J. Knee Surg. 2015, 28, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J.; Bock, F.; Stöve, J.; Jerosch, J.; Flechtenmacher, J. Pharmacological treatment of knee osteoarthritis: Special considerations of the new German guideline. Orthop. Rev. 2018, 10, 7782. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Li, C.-D.; Wang, L.; Ren, S.; Yu, F.-B.; Li, J.-G.; Ma, J.-X.; Ma, X.-L. Function scores of different surgeries in the treatment of knee osteoarthritis: A PRISMA-compliant systematic review and network-meta analysis. Medicine 2018, 97, e10828. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, L.S.; Marelli, B.M.; Crapanzano, C.; De Martinis, S.E.; Gala, L.; Ferraro, M.; Marelli, N.; Albisetti, W. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: The efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee 2014, 21, 661–668. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surgery Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Zazgyva, A.; Gergely, I.; Russu, O.M.; Roman, C.; Pop, T.S. Polynucleotides Versus Soium Hyaluronate in The Local Treatment of Knee Osteoarthritis. Acta Med. Transilv. 2013, 2, 260–263. [Google Scholar]
- Dallari, D.; Sabbioni, G.; Del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Fini, M. Efficacy of Intra-articular Polynucleotides Associated With Hyaluronic Acid Versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Clin. J. Sport Med. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Meccariello, L.; Cioffi, S.; Franzese, R.; Cioffi, R.; Petrucci, C.; Errico, G.; Olivieri, M.; Mugnaini, M. Intraarticular Knee Joint Injection: Hyaluronic acid vs Polynucleotide. Euromediterranean Biomed. J. 2013, 8, 35–41. [Google Scholar] [CrossRef]
- Zandieh, S.; Haller, J.; Bernt, R.; Hergan, K.; Rath, E. Fractal analysis of subchondral bone changes of the hand in rheumatoid arthritis. Medicine 2017, 96, e6344. [Google Scholar] [CrossRef]
- Ali, M.; Brogren, E.; Atroshi, I. Assessment of a novel computer software in diagnosing radiocarpal osteoarthritis on plain radiographs of patients with previous distal radius fracture. Osteoarthr. Cartil. Open 2020, 2, 100112. [Google Scholar] [CrossRef]
- Nehrer, S.; Ljuhar, R.; Steindl, P.; Simon, R.; Maurer, D.; Ljuhar, D.; Bertalan, Z.; Dimai, H.P.; Goetz, C.; Paixao, T. Automated Knee Osteoarthritis Assessment Increases Physicians’ Agreement Rate and Accuracy: Data from the Osteoarthritis Initiative. CARTILAGE 2019, 13, 957S–965S. [Google Scholar] [CrossRef] [PubMed]
- Hirvasniemi, J.; Klein, S.; Bierma-Zeinstra, S.; Vernooij, M.W.; Schiphof, D.; Oei, E.H.G. A machine learning approach to distinguish between knees without and with osteoarthritis using MRI-based radiomic features from tibial bone. Eur. Radiol. 2021, 31, 8513–8521. [Google Scholar] [CrossRef] [PubMed]
- Woloszynski, T.; Podsiadlo, P.; Stachowiak, G.W.; Kurzynski, M.; Lohmander, L.S.; Englund, M. Prediction of progression of radiographic knee osteoarthritis using tibial trabecular bone texture. Arthritis Care Res. 2012, 64, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E., Jr. Management of the Retired Athlete with Osteoarthritis of the Knee. CARTILAGE 2012, 3, 69S–76S. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A systematic review and network meta-analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Walsh, D.A.; McWilliams, D.; Turley, M.J.; Dixon, M.R.; Franses, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [Green Version]
- Pothuaud, L.; Benhamou, C.L.; Porion, P.; Lespessailles, E.; Harba, R.; Levitz, P. Fractal Dimension of Trabecular Bone Projection Texture Is Related to Three-Dimensional Microarchitecture. J. Bone Miner. Res. 2010, 15, 691–699. [Google Scholar] [CrossRef]
- Winzenrieth, R.; Michelet, F.; Hans, D. Three-Dimensional (3D) Microarchitecture Correlations with 2D Projection Image Gray-Level Variations Assessed by Trabecular Bone Score Using High-Resolution Computed Tomographic Acquisitions: Effects of Resolution and Noise. J. Clin. Densitom. 2013, 16, 287–296. [Google Scholar] [CrossRef]
- Intema, F.; Thomas, T.; Anderson, D.; Elkins, J.; Brown, T.; Amendola, A.; Lafeber, F.; Saltzman, C. Subchondral bone remodeling is related to clinical improvement after joint distraction in the treatment of ankle osteoarthritis. Osteoarthr. Cartil. 2011, 19, 668–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Cooper, G.M.; Gharaibeh, B.; Meszaros, L.B.; Li, G.; Usas, A.; Fu, F.H.; Huard, J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble flt-1. Arthritis Rheum 2009, 60, 1390–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kegelman, C.D.; Coulombe, J.C.; Jordan, K.M.; Horan, D.J.; Qin, L.; Robling, A.G.; Ferguson, V.L.; Bellido, T.M.; Boerckel, J.D. YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling. J. Bone Miner. Res. 2020, 35, 196–210. [Google Scholar] [CrossRef]
- Mazur, C.M.; Woo, J.J.; Yee, C.S.; Fields, A.J.; Acevedo, C.; Bailey, K.N.; Kaya, S.; Fowler, T.W.; Lotz, J.C.; Dang, A.; et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.-J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory Effect of DNA Polymeric Molecules in a Cell Model of Osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef]
- Bitto, A.; Polito, F.; Irrera, N.; D’Ascola, A.; Avenoso, A.; Nastasi, G.; Campo, G.M.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A(2A) receptor. Arthritis Care Res. 2011, 63, 3364–3371. [Google Scholar] [CrossRef]
- Lin, C.; Shao, Y.; Zeng, C.; Zhao, C.; Fang, H.; Wang, L.; Pan, J.; Liu, L.; Qi, W.; Feng, X.; et al. Blocking PI3K/AKT signaling inhibits bone sclerosis in subchondral bone and attenuates post-traumatic osteoarthritis. J. Cell. Physiol. 2018, 233, 6135–6147. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Muratore, O.; Cattarini, G.; Gianoglio, S.; Tonoli, E.L.; Saccà, S.; Ghiglione, D.; Venzano, D.; Ciurlo, C.; Lantieri, P.B.; Schito, G.C. A human placental polydeoxyribonucleotide (PDRN) may promote the growth of human corneal fibroblasts and iris pigment epithelial cells in primary culture. New Microbiol. 2003, 26, 13–26. [Google Scholar] [PubMed]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Saggini, R.; Di Stefano, A.; Cavezza, T.; Saladino, G.; Bellomo, R.G. Intrarticular treatment of osteoartropaty knee with polynucleotides: A pilot study with medium-term follow-up. J. Biol. Regul. Homeost. Agents 2013, 27, 543–549. [Google Scholar]
- Welker, M.E.; Kulik, G. Recent syntheses of PI3K/Akt/mTOR signaling pathway inhibitors. Bioorganic Med. Chem. 2013, 21, 4063–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janvier, T.; Jennane, R.; Toumi, H.; Lespessailles, E. Subchondral tibial bone texture predicts the incidence of radiographic knee osteoarthritis: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2017, 25, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- MacKay, J.; Murray, P.; Kasmai, B.; Johnson, G.; Donell, S.; Toms, A. Subchondral bone in osteoarthritis: Association between MRI texture analysis and histomorphometry. Osteoarthr. Cartil. 2017, 25, 700–707. [Google Scholar] [CrossRef] [Green Version]


| Variables | Male (n = 14) | Female (n = 37) | Total (n = 51) |
|---|---|---|---|
| Dropped out | 1 (20%) | 4 (80%) | 5 (100%) |
| Sex | 14 (27.5%) | 37 (72.5%) | 51 (100%) |
| Age (years) | |||
| Mean ± SD | 59.7 ± 9.7 | 63.9 ± 8.4 | 62.7 ± 8.9 |
| Median (min–max) | 58 (46–75) | 65 (44–77) | 64 (44–77) |
| Height (cm) | |||
| Mean ± SD | 167.5 ± 5.9 | 155.2 ± 6.1 | 158.7 ± 8.2 |
| Median (min–max) | 168 (156–176) | 156 (143–165) | 158 (143–176) |
| Weight (kg) | |||
| Mean ± SD | 71.1 ± 5.0 | 61.8 ± 8.8 | 64.4 ± 8.9 |
| Median (min–max) | 71.5 (60.3–71.7) | 62.0 (46.0–72.1) | 64.6 (46.0–72.1) |
| BMI (kg/m2) | |||
| Mean ± SD | 25.4 ± 2.7 | 25.7 ± 3.4 | 25.6 ± 3.2 |
| Median (min–max) | 58 (17.1–27.7) | 65 (21.1–27.4) | 25.6 (17.1–27.7) |
| Kellgren–Lawrence grade | |||
| I | 8 (15.7%) | 20 (39.2%) | 28 (54.9%) |
| II | 5 (9.8%) | 14 (27.5%) | 19 (37.3%) |
| III | 1 (2%) | 3 (5.9%) | 4 (7.8%) |
| Osteoarthritis site | |||
| Medial | 12 (23.5%) | 30 (58.8%) | 42 (82.4%) |
| Lateral | 1 (2%) | 4 (7.8%) | 5 (9.8%) |
| General | 1 (2%) | 3 (5.9%) | 4 (7.8%) |
| Coronal alignment | |||
| Varus | 9 (17.6%) | 26 (51.0%) | 35 (68.6%) |
| Valgus | 1 (2%) | 3 (5.9%) | 4 (7.8%) |
| Neutral | 4 (7.8%) | 8 (15.7%) | 12 (23.5%) |
| Injection side | |||
| Right | 9 (17.6%) | 21 (41.2%) | 24 (47.1%) |
| Left | 5 (9.8%) | 16 (31.4%) | 27 (52.9%) |
| Before PN Injection | p-Value | After PN Injection | p-Value | |||
|---|---|---|---|---|---|---|
| MC | LC | MC | LC | |||
| SL | 0.587 ± 0.029 | 0.553 ± 0.032 | <0.001 | 0.568 ± 0.017 | 0.550 ± 0.017 | 0.0021 |
| ML | 0.555 ± 0.032 | 0.535 ± 0.033 | <0.001 | 0.533 ± 0.018 | 0.535 ± 0.018 | 0.325 |
| DL | 0.552 ± 0.034 | 0.520 ± 0.033 | <0.001 | 0.525 ± 0.019 | 0.518 ± 0.016 | 0.322 |
| Before PN Injection | After PN Injection | ||
|---|---|---|---|
| Layer | ΔB | ΔA | p |
| Superficial | 0.015 (−0.430 to 0.400) | 0.020 (−0.43 to 0.40) | 0.0565 |
| Middle | 0.010 (−0.150 to 0.220) | 0.000 (−0.190 to 0.190) | 0.00078 * |
| Deep | 0.020 (−0.160 to 0.210) | 0.005 (−0.180 to 0.190) | 0.00081 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Kim, J.H.; Kim, M.W.; Kim, S.H.; Yong, S.Y. Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series. J. Clin. Med. 2022, 11, 2845. https://doi.org/10.3390/jcm11102845
Jang JY, Kim JH, Kim MW, Kim SH, Yong SY. Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series. Journal of Clinical Medicine. 2022; 11(10):2845. https://doi.org/10.3390/jcm11102845
Chicago/Turabian StyleJang, Ji Yoon, Ji Hyun Kim, Min Woo Kim, Sung Hoon Kim, and Sang Yeol Yong. 2022. "Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series" Journal of Clinical Medicine 11, no. 10: 2845. https://doi.org/10.3390/jcm11102845
APA StyleJang, J. Y., Kim, J. H., Kim, M. W., Kim, S. H., & Yong, S. Y. (2022). Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series. Journal of Clinical Medicine, 11(10), 2845. https://doi.org/10.3390/jcm11102845







