Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. UTI Definition
2.2. Data Source
2.3. Statistical Analysis
3. Results
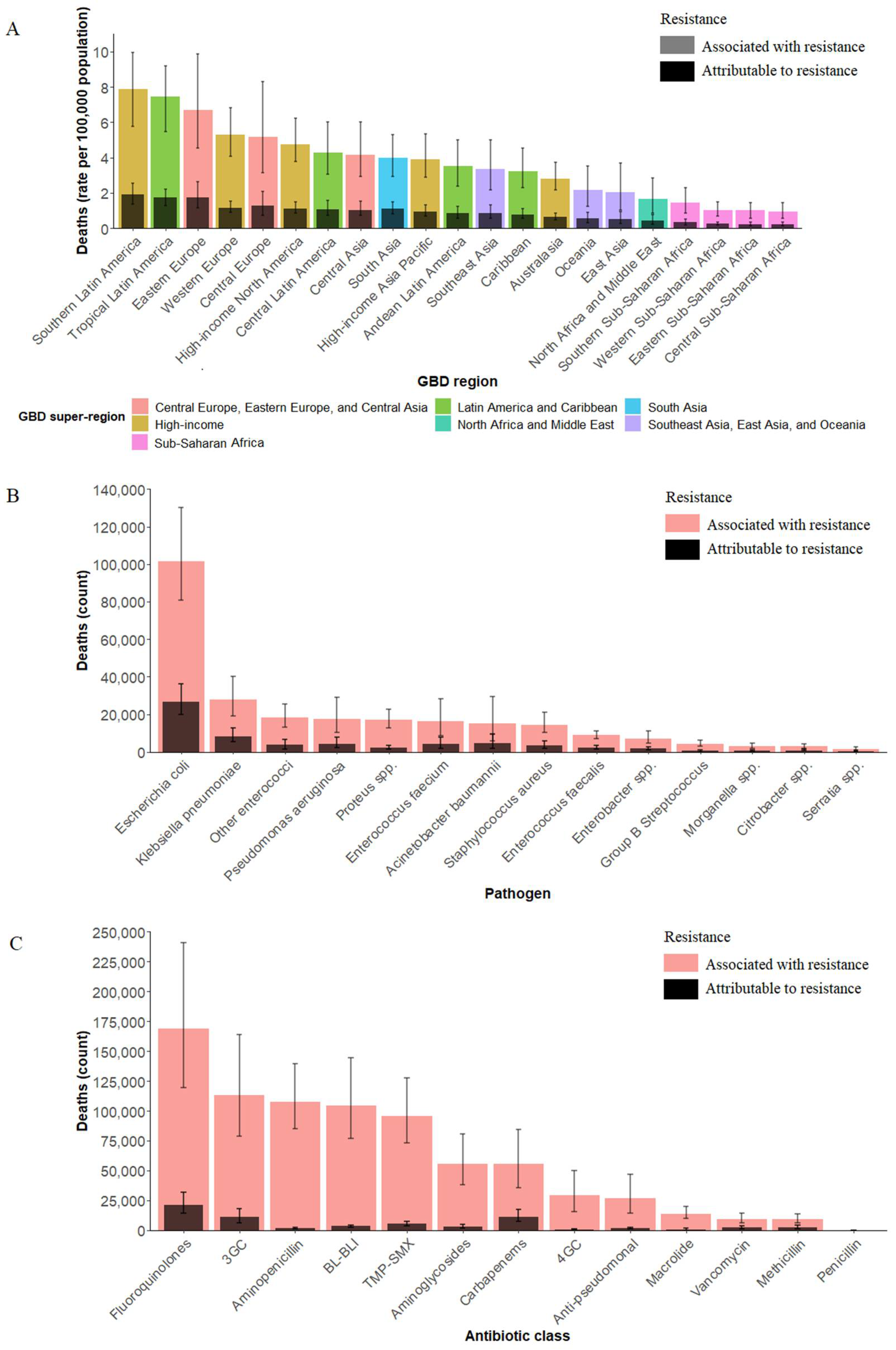
3.1. Global and Regional Burden of Bacterial AMR in UTI
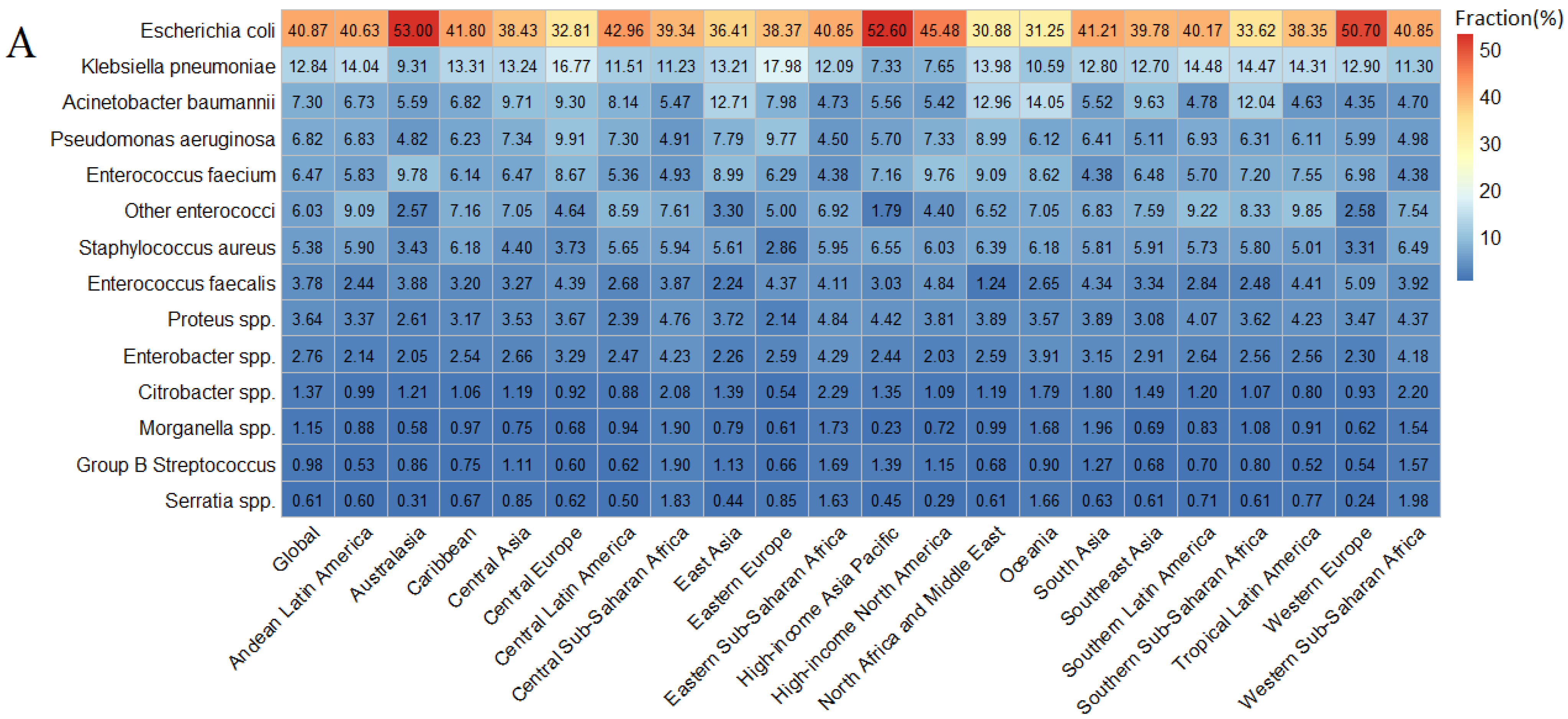
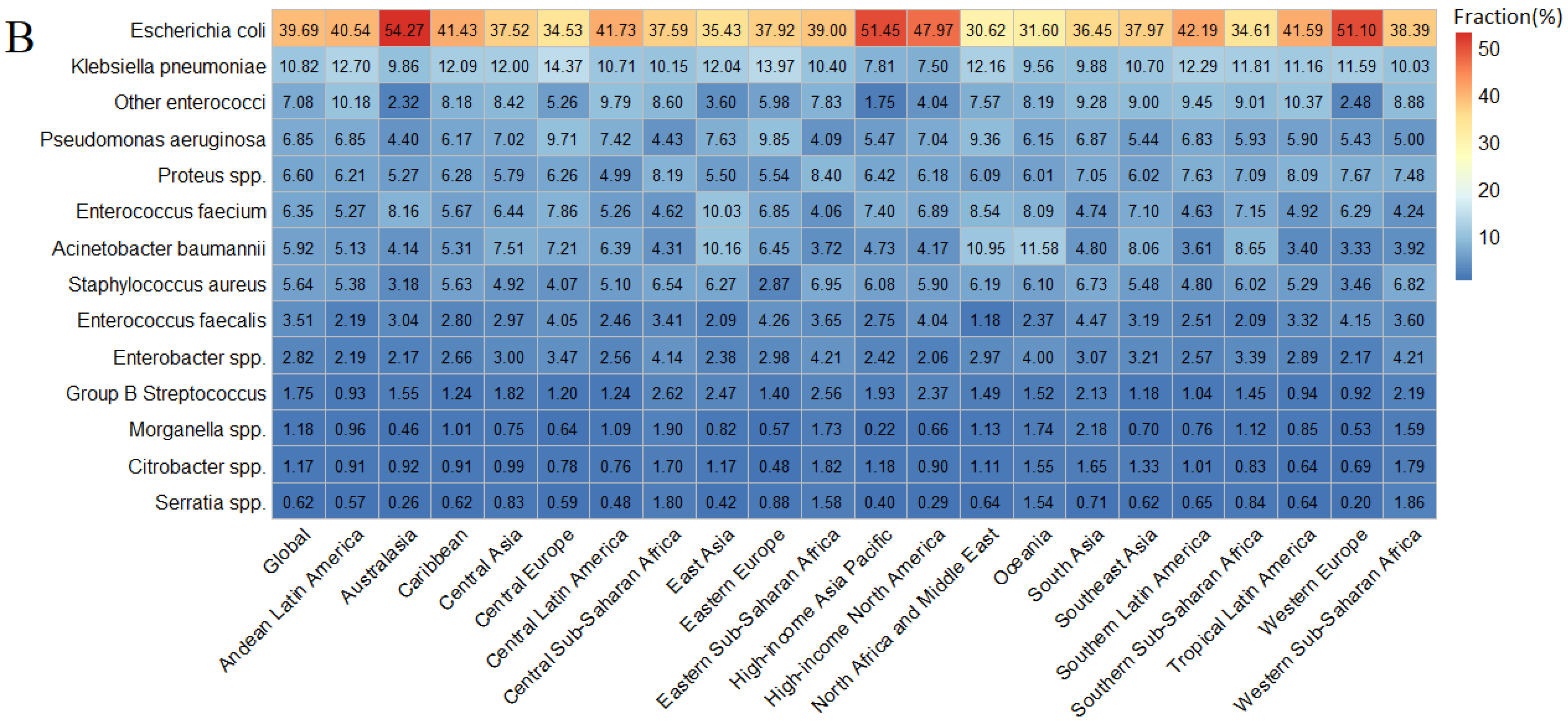
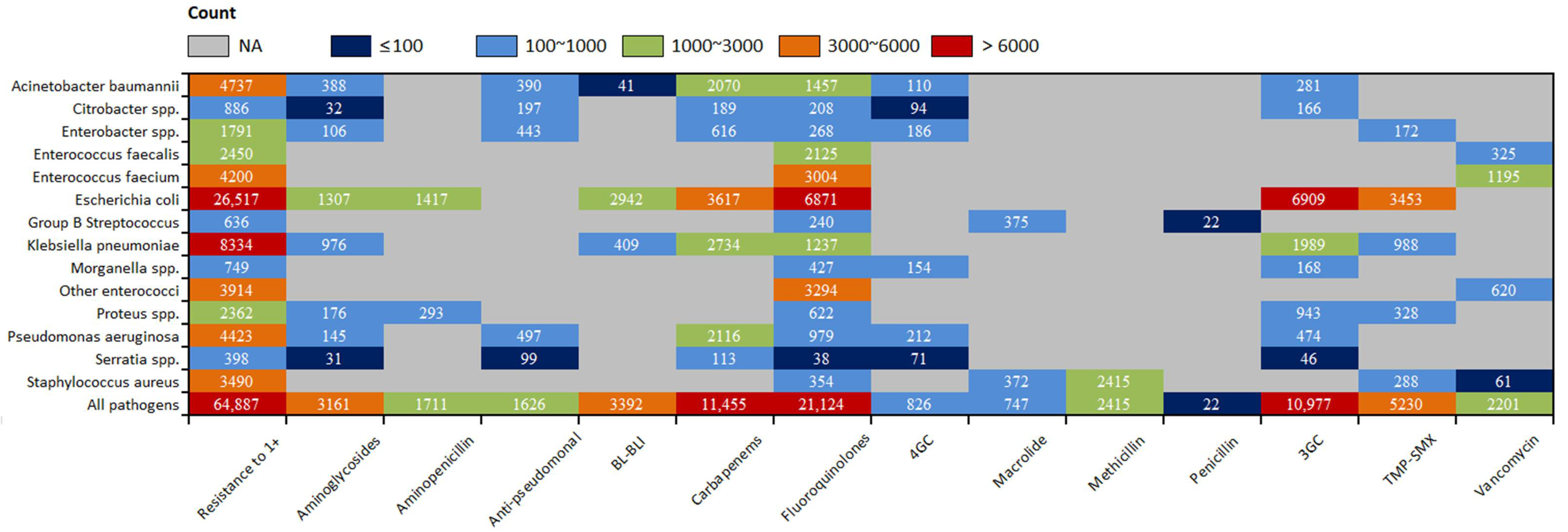
3.2. Pathogen-Specific Burden of Bacterial AMR in UTI
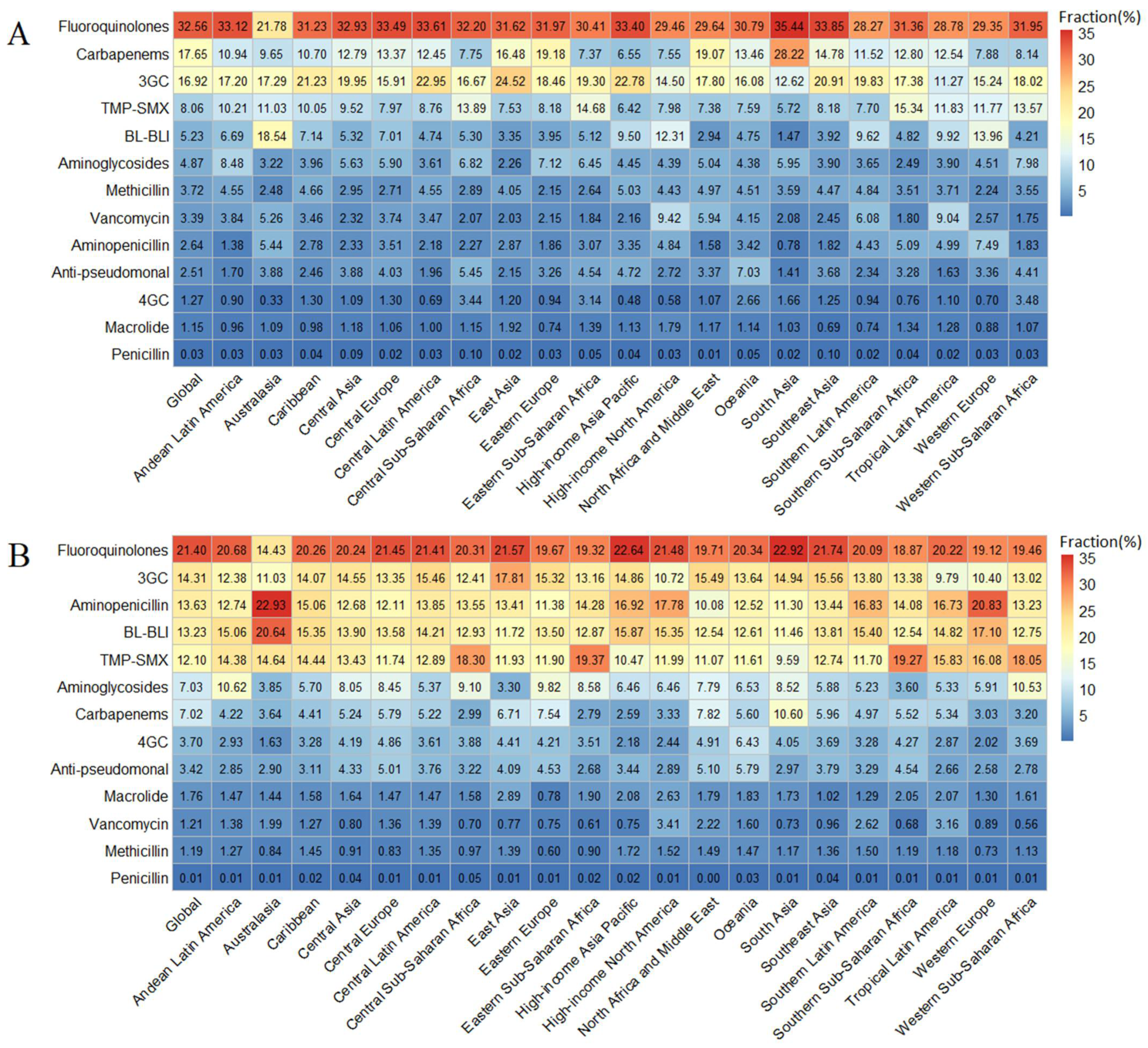
3.3. Antibiotic-Specific Burden of Bacterial AMR in UTI
3.4. Pathogen-Antibiotic Specific Burden of Bacterial AMR in UTI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 22 February 2022).
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 2022, 399, 606–607. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Johansen, T.E.B.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ceña, D.; Florencio, L.L.; Hernández-Barrera, V.; Fernandez-de-Las-Peñas, C.; de Miguel-Diez, J.; Martínez-Hernández, D.; Carabantes-Alarcón, D.; Jimenez-García, R.; Lopez-de-Andres, A.; Lopez-Herranz, M. Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018). J. Clin. Med. 2021, 10, 2332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, D.Q.; Zi, H.; Huang, Q.; Gu, J.M.; Li, L.Y.; Guo, X.P.; Li, F.; Fang, C.; Li, X.D.; et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil. Med. Res. 2021, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Cek, M.; Wagenlehner, F.; Naber, K.; Tenke, P.; van Ostrum, E.; Johansen, T.B. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J. Urol. 2014, 32, 791–801. [Google Scholar] [CrossRef]
- Choe, H.S.; Lee, S.J.; Cho, Y.H.; Çek, M.; Tandoğdu, Z.; Wagenlehner, F.; Bjerklund-Johansen, T.E.; Naber, K. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 2018, 24, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Multiple antimicrobial resistance and outcomes among hospitalized patients with complicated urinary tract infections in the US, 2013–2018: A retrospective cohort study. BMC Infect. Dis. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Anger, J.; Lee, U.; Ackerman, A.L.; Chou, R.; Chughtai, B.; Clemens, J.Q.; Hickling, D.; Kapoor, A.; Kenton, K.S.; Kaufman, M.R.; et al. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J. Urol. 2019, 202, 282–289. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F. EAU Guidelines on Urological Infections 2020. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urological-infections-2020.pdf (accessed on 22 February 2022).
- Fox, M.T.; Amoah, J.; Hsu, A.J.; Herzke, C.A.; Gerber, J.S.; Tamma, P.D. Comparative Effectiveness of Antibiotic Treatment Duration in Children with Pyelonephritis. JAMA Netw. Open 2020, 3, e203951. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; de Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU EANM ESTRO ESUR SIOG Guidelines on Prostate Cancer 2020. Available online: https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4-1.pdf (accessed on 22 February 2022).
- Bruyere, F.; Pilatz, A.; Boehm, A.; Pradere, B.; Wagenlehner, F.; Vallee, M. Associated measures to antibiotic prophylaxis in urology. World J. Urol. 2020, 38, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Maglietta, G.; di Costanzo, M.; Ceccoli, M.; Vergine, G.; la Scola, C.; Malaventura, C.; Falcioni, A.; Iacono, A.; Crisafi, A.; et al. Retrospective 8-Year Study on the Antibiotic Resistance of Uropathogens in Children Hospitalised for Urinary Tract Infection in the Emilia-Romagna Region, Italy. Antibiotics 2021, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, C.; Yan, Y.; Sun, L.; Li, H. Urinary Tract Infection Etiological Profiles and Antibiotic Resistance Patterns Varied Among Different Age Categories: A Retrospective Study from a Tertiary General Hospital During a 12-Year Period. Front. Microbiol. 2021, 12, 813145. [Google Scholar] [CrossRef]
- Sakeena, M.H.F.; Bennett, A.A.; McLachlan, A.J. Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: A systematic review. Int. J. Antimicrob. Agents 2018, 52, 771–782. [Google Scholar] [CrossRef]
- Kelesidis, T.; Falagas, M.E. Substandard/counterfeit antimicrobial drugs. Clin. Microbiol. Rev. 2015, 28, 443–464. [Google Scholar] [CrossRef] [Green Version]
- Monnier, A.A.; Schouten, J.; Tebano, G.; Zanichelli, V.; Huttner, B.D.; Pulcini, C.; Årdal, C.; Harbarth, S.; Hulscher, M.E.; Gyssens, I.C. Ensuring Antibiotic Development, Equitable Availability, and Responsible Use of Effective Antibiotics: Recommendations for Multisectoral Action. Clin. Infect. Dis. 2019, 68, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 22 February 2022).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 22 February 2022).
- Health Canada. Notice—Health Canada’s update to the Pathogens of Interest List and Ongoing Efforts to Support Innovative Human Therapeutic Products to Combat Antimicrobial Resistance (AMR). Available online: https://www.canada.ca/en/health-canada/programs/consultation-new-addition-pathogens-interest-list/document.html#a4 (accessed on 22 February 2022).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 22 February 2022).
- Zarocostas, J. New international group on antimicrobial resistance. Lancet 2020, 396, 1715. [Google Scholar] [CrossRef]
- Falcone, A.; Amantea, D.; Levato, A.; Arone, F.; Morrone, L.A.; Bagetta, D.; Florio, L.; Lista, M.R.; Bagetta, G. Outcomes of a pharmacoepidemiological survey on the antibiotic treatment of uncomplicated acute cystitis in community. Pharmacol. Res. 2006, 53, 193–196. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 22 February 2022).
- World Health Organization. Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/surveillance/central-asian-and-european-surveillance-of-antimicrobial-resistance-caesar (accessed on 22 February 2022).
- Frost, I.; Kapoor, G.; Craig, J.; Liu, D.; Laxminarayan, R. Status, challenges and gaps in antimicrobial resistance surveillance around the world. J. Glob. Antimicrob. Resist. 2021, 25, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Rupali, P.; Opintan, J.A.; Jaoko, W.; Feasey, N.A.; Peacock, S.J.; Ashley, E.A. Laboratory informatics capacity for effective antimicrobial resistance surveillance in resource-limited settings. Lancet Infect. Dis. 2021, 21, e170–e174. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Naber, K.G. Understanding clinical variables to improve empirical antibiotic therapy for UTI. Nat. Rev. Urol. 2019, 16, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.D.; Adepoju, O.; Stone, N.; Moudouni, D.K.; Nwaiwu, O.; Zhao, H.; Frentzel, E.; Mehr, D.; Garfinkel, S. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012, 12, 73. [Google Scholar] [CrossRef] [Green Version]
| Associated with Resistance | Attributable to Resistance | |||||||
|---|---|---|---|---|---|---|---|---|
| Deaths | DALYs | YLDs | YLLs | Deaths | DALYs | YLDs | YLLs | |
| Counts, thousands | ||||||||
| Global | 255.802 (182.553–363.908) | 5607.970 (4081.637–7991.093) | 167.469 (100.767–256.144) | 5440.501 (3910.668–7813.322) | 64.887 (45.859–94.345) | 1438.848 (1020.287–2101.201) | 33.512 (19.367–52.959) | 1405.336 (992.093–2061.045) |
| Central Europe, eastern Europe, and central Asia | 23.891 (16.045–35.879) | 516.223 (358.717–757.066) | 12.766 (7.650–19.945) | 503.457 (348.368–743.746) | 6.048 (4.001–9.321) | 130.323 (88.640–196.693) | 2.534 (1.442–4.126) | 127.789 (86.409–193.407) |
| High income | 53.856 (41.957–69.587) | 732.320 (556.679–995.742) | 25.261 (15.378–37.566) | 707.059 (527.611–971.935) | 12.361 (9.453–16.619) | 168.975 (125.217–240.207) | 4.573 (2.729–7.012) | 164.402 (120.670–234.943) |
| Latin America and Caribbean | 31.191 (24.059–40.852) | 614.994 (470.752–822.682) | 25.018 (15.092–38.827) | 589.975 (448.038–793.591) | 7.498 (5.584–10.170) | 148.274 (110.323–204.156) | 4.780 (2.789–7.585) | 143.494 (105.170–199.304) |
| North Africa and Middle East | 10.056 (5.100–17.225) | 243.098 (124.193–430.123) | 12.452 (7.259–20.035) | 230.645 (111.096–414.670) | 2.710 (1.365–4.784) | 65.167 (32.552–117.214) | 2.623 (1.455–4.473) | 62.544 (29.914–114.777) |
| South Asia | 72.124 (53.057–96.020) | 1953.222 (1487.744–2542.502) | 58.569 (33.299–92.863) | 1894.653 (1426.036–2486.039) | 20.067 (14.564–27.121) | 540.202 (396.373–722.421) | 12.534 (6.778–20.872) | 527.667 (385.580–706.749) |
| Southeast Asia, east Asia, and Oceania | 53.400 (30.379–88.532) | 1177.714 (689.684–1939.853) | 17.028 (9.800–27.123) | 1160.687 (676.277–1915.141) | 13.529 (7.563–22.868) | 298.833 (170.977–499.896) | 3.399 (1.815–5.750) | 295.434 (168.241–495.854) |
| Sub-Saharan Africa | 11.303 (6.960–15.950) | 370.421 (243.293–519.435) | 16.385 (8.521–29.816) | 354.036 (228.904–501.817) | 2.677 (1.663–3.798) | 87.042 (57.826–122.462) | 3.069 (1.531–5.798) | 83.974 (55.246–119.396) |
| ASR rates, per 100,000 | ||||||||
| Global | 3.310 (2.360–4.700) | 72.478 (52.752–103.278) | 2.160 (1.300–3.310) | 70.314 (50.542–100.980) | 0.839 (0.593–1.220) | 18.596 (13.186–27.156) | 0.433 (0.250–0.684) | 18.163 (12.822–26.637) |
| Central Europe, eastern Europe, and central Asia | 5.720 (3.840–8.590) | 123.580 (85.874–181.236) | 3.060 (1.830–4.770) | 120.523 (83.397–178.047) | 1.450 (0.958–2.230) | 31.198 (21.220–47.087) | 0.607 (0.345–0.988) | 30.592 (20.686–46.300) |
| High income | 4.970 (3.870–6.420) | 67.559 (51.355–91.860) | 2.330 (1.420–3.470) | 65.228 (48.674–89.664) | 1.140 (0.872–1.530) | 15.588 (11.552–22.160) | 0.422 (0.252–0.647) | 15.166 (11.132–21.674) |
| Latin America and Caribbean | 5.340 (4.120–6.990) | 105.239 (80.556–140.779) | 4.280 (2.580–6.640) | 100.958 (76.669–135.801) | 1.280 (0.955–1.740) | 25.373 (18.879–34.935) | 0.818 (0.477–1.300) | 24.555 (17.997–34.105) |
| North Africa and Middle East | 1.650 (0.838–2.830) | 39.936 (20.403–70.661) | 2.050 (1.190–3.290) | 37.891 (18.251–68.122) | 0.445 (0.224–0.786) | 10.706 (5.350–19.256) | 0.431 (0.239–0.735) | 10.275 (4.910–18.856) |
| South Asia | 4.000 (2.940–5.320) | 108.200 (82.414–140.843) | 3.240 (1.840–5.140) | 104.955 (78.996–137.715) | 1.110 (0.807–1.500) | 29.925 (21.957–40.019) | 0.694 (0.375–1.160) | 29.230 (21.359–39.151) |
| Southeast Asia, east Asia, and Oceania | 2.470 (1.410–4.100) | 54.542 (31.941–89.839) | 0.789 (0.454–1.260) | 53.754 (31.320–88.694) | 0.627 (0.350–1.060) | 13.840 (7.920–23.151) | 0.157 (0.084–0.266) | 13.682 (7.790–22.964) |
| Sub-Saharan Africa | 1.050 (0.645–1.480) | 34.355 (22.565–48.176) | 1.520 (0.790–2.770) | 32.836 (21.230–46.542) | 0.248 (0.154–0.352) | 8.070 (5.360–11.358) | 0.285 (0.142–0.538) | 7.790 (5.120–11.074) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Fan, H.; Zi, H.; Hu, H.; Li, B.; Huang, J.; Luo, P.; Zeng, X. Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. J. Clin. Med. 2022, 11, 2817. https://doi.org/10.3390/jcm11102817
Li X, Fan H, Zi H, Hu H, Li B, Huang J, Luo P, Zeng X. Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. Journal of Clinical Medicine. 2022; 11(10):2817. https://doi.org/10.3390/jcm11102817
Chicago/Turabian StyleLi, Xuhui, Hua Fan, Hao Zi, Hankun Hu, Binghui Li, Jiao Huang, Pengcheng Luo, and Xiantao Zeng. 2022. "Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019" Journal of Clinical Medicine 11, no. 10: 2817. https://doi.org/10.3390/jcm11102817
APA StyleLi, X., Fan, H., Zi, H., Hu, H., Li, B., Huang, J., Luo, P., & Zeng, X. (2022). Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. Journal of Clinical Medicine, 11(10), 2817. https://doi.org/10.3390/jcm11102817






